Revision of Carpal Tunnel Surgery
Abstract
:1. Introduction
2. Etiology of Failed Carpal Tunnel Release
2.1. Persistent Symptoms
2.1.1. Incomplete Release
2.1.2. Secondary Sites of Compression
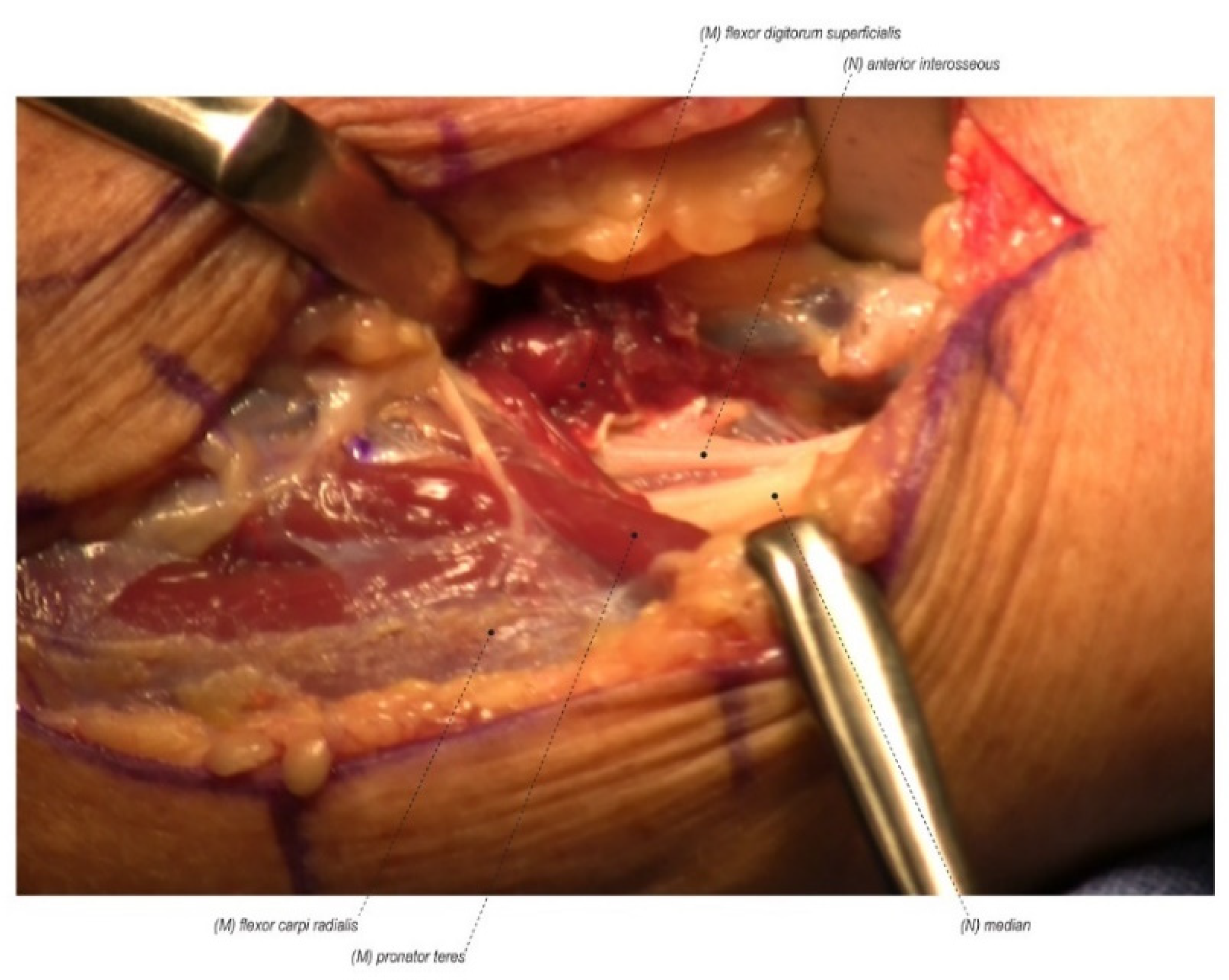
2.1.3. Wrong Diagnosis
2.2. Recurrent Symptoms
2.2.1. Scar Formation
2.2.2. Secondary Sites of Compression
2.3. New Symptoms
2.3.1. Iatrogenic Injury
2.3.2. Secondary Pathology
3. Approach and Assessment
4. Management
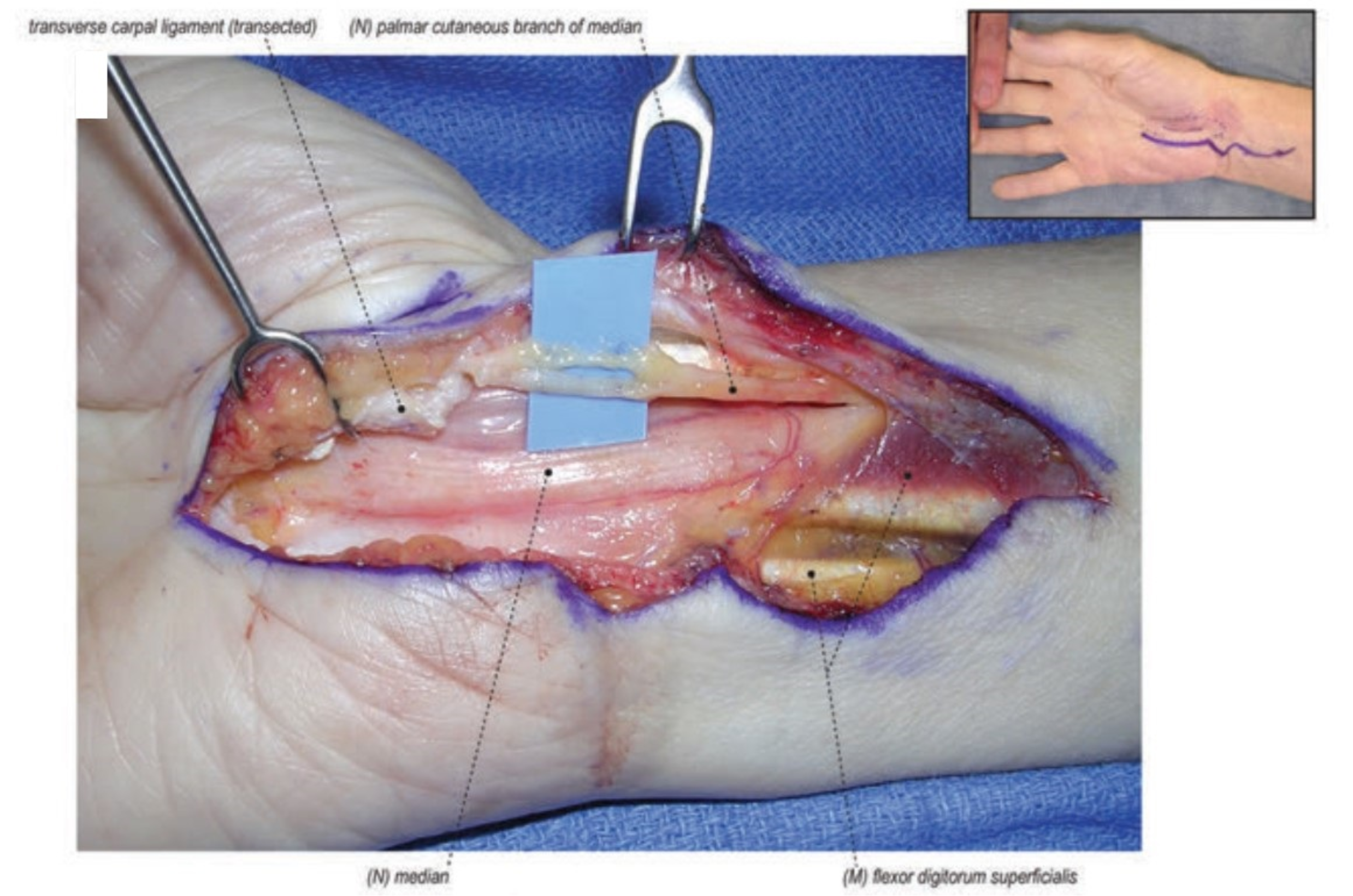
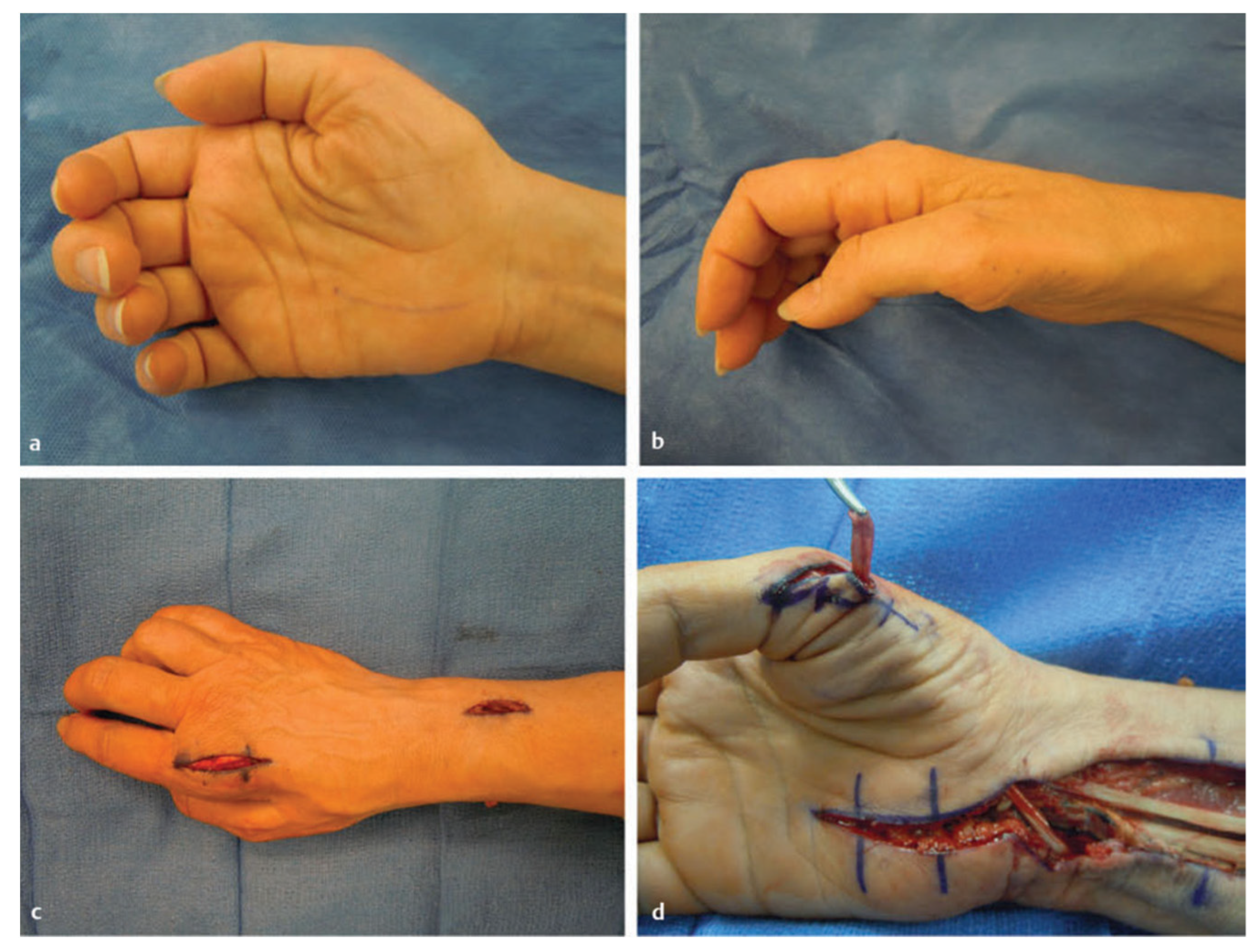
4.1. Repeat Carpal Tunnel Release
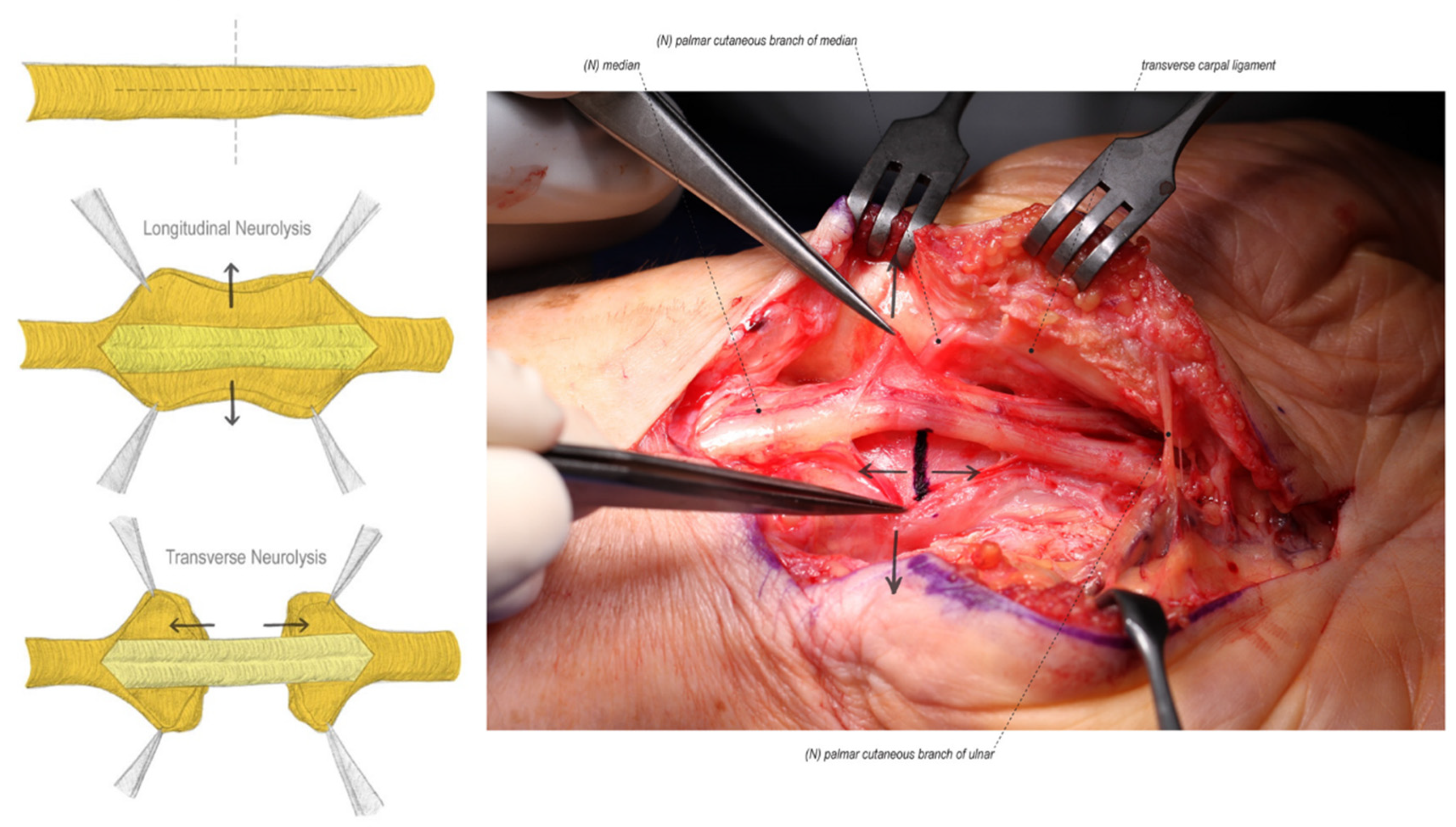
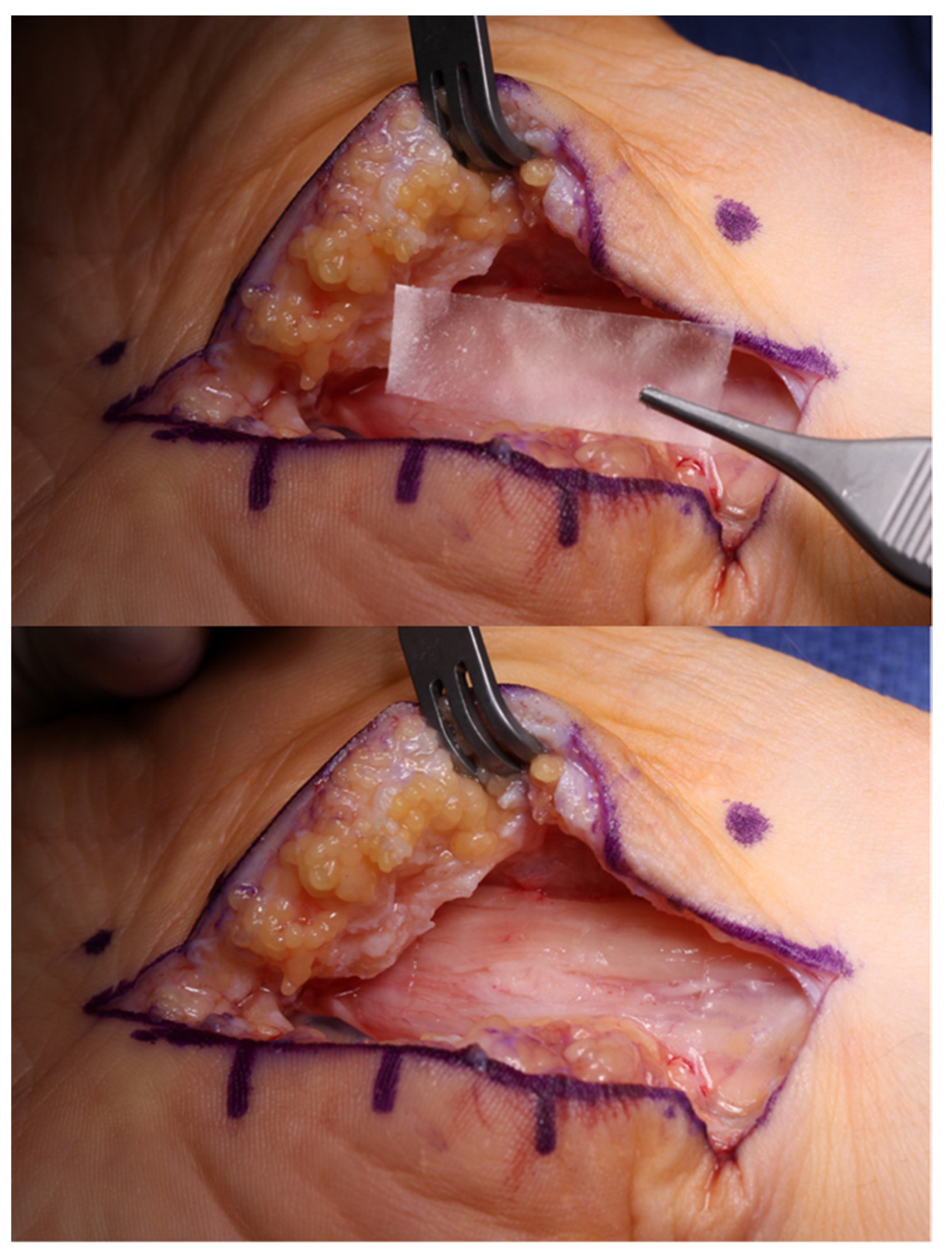
4.2. External and Internal Neurolysis
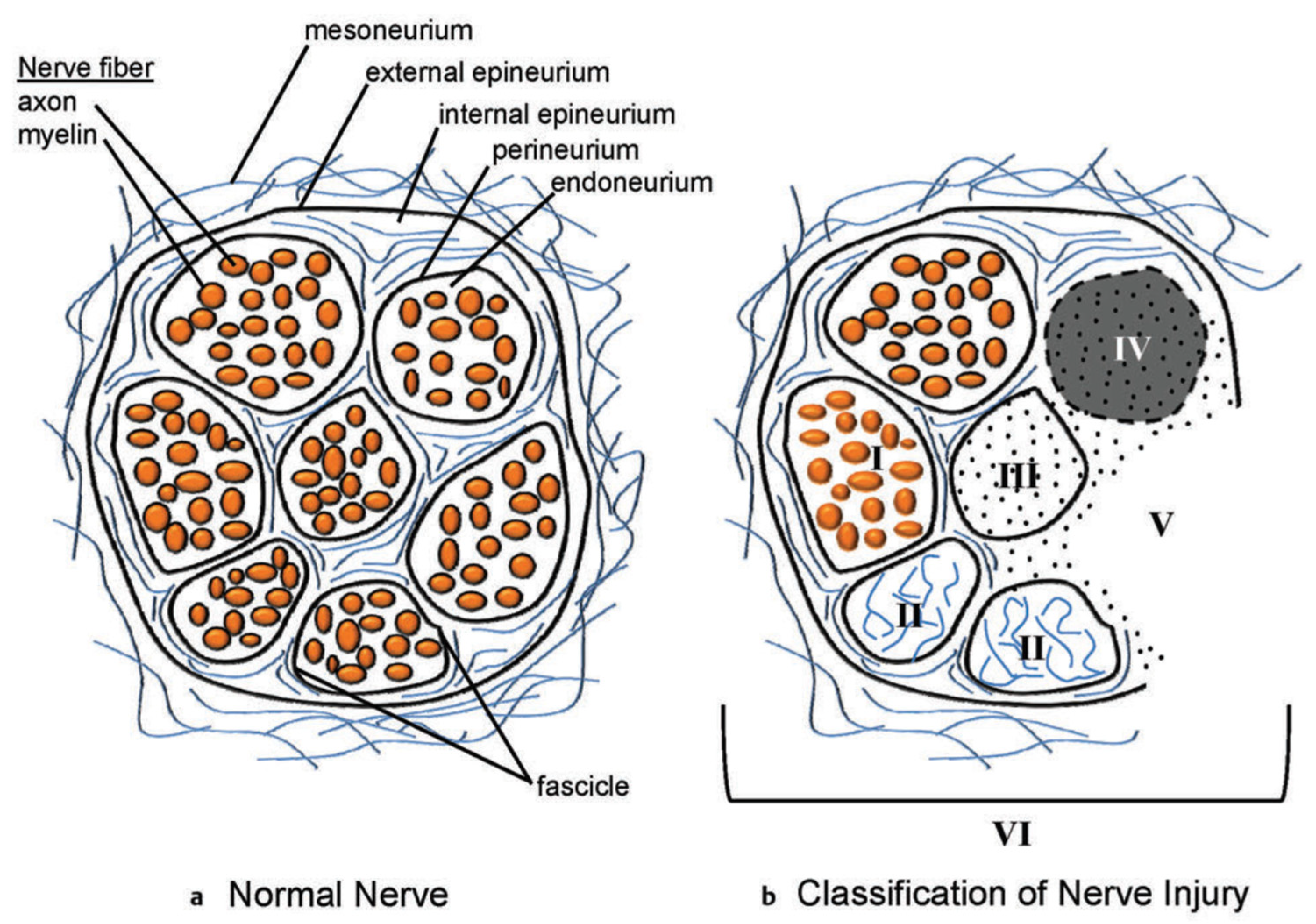
4.3. Neuroma or Nerve Injury Identification and Management
4.4. Nerve Coverage
4.5. Author’s Preference
4.6. Comprehensive Review of Nerve Coverage Options
4.7. Secondary Sites of Compression
4.8. Ancillary Procedures for Chronic Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Harris-Adamson, C.; Rempel, D.; Gerr, F.; Hegmann, K.; Silverstein, B.; Burt, S.; Garg, A.; Kapellusch, J.; Merlino, L.; et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: Pooled analysis of six prospective studies. Scand. J. Work Environ. Health 2013, 39, 495–505. [Google Scholar] [CrossRef] [PubMed]
- de Krom, M.C.; Knipschild, P.G.; Kester, A.D.; Thijs, C.T.; Boekkooi, P.F.; Spaans, F. Carpal tunnel syndrome: Prevalence in the general population. J. Clin. Epidemiol. 1992, 45, 373–376. [Google Scholar] [CrossRef]
- Tanaka, S.; Wild, D.K.; Seligman, P.J.; Halperin, W.E.; Behrens, V.J.; Putz-Anderson, V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: Analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am. J. Ind. Med. 1995, 27, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Sun, S.; Beard, C.M.; O’Fallon, W.M.; Kurland, L.T. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology 1988, 38, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Gelfman, R.; Melton, L.J.; Yawn, B.P.; Wollan, P.C.; Amadio, P.C.; Stevens, J.C. Long-term trends in carpal tunnel syndrome. Neurology 2009, 72, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, M.; Kim, S.H.; Szabo, R.M. Incidence of carpal tunnel release: Trends and implications within the United States ambulatory care setting. J. Hand Surg. Am. 2012, 37, 1599–1605. [Google Scholar] [CrossRef]
- Bromley, G.S. Minimal-incision open carpal tunnel decompression. J. Hand Surg. 1994, 19, 119–120. [Google Scholar] [CrossRef]
- Biyani, A.; Downes, E.M. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J. Hand Surg. 1993, 18, 331–334. [Google Scholar] [CrossRef]
- Chow, J.C.Y. Endoscopic release of the carpal ligament for carpal tunnel syndrome: Long-term results using the chow technique. Arthroscopy 1999, 15, 417–421. [Google Scholar] [CrossRef]
- Okutsu, I.; Ninomiya, S.; Takatori, Y.; Ugawa, Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy 1989, 5, 11–18. [Google Scholar] [CrossRef]
- Westenberg, R.F.; Oflazoglu, K.; de Planque, C.A.; Jupiter, J.B.; Eberlin, K.R.; Chen, N.C. Revision carpal tunnel release: Risk factors and rate of secondary surgery. Plast. Reconstr. Surg. 2020, 145, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.H.; Mackinnon, S.E. Secondary carpal tunnel surgery. Plast. Reconstr. Surg. 2001, 107, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Djerbi, I.; César, M.; Lenoir, H.; Coulet, B.; Lazerges, C.; Chammas, M. Revision surgery for recurrent and persistent carpal tunnel syndrome: Clinical results and factors affecting outcomes. Chir. Main 2015, 34, 312–317. [Google Scholar] [CrossRef]
- Jones, N.F.; Ahn, H.C.; Eo, S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast. Reconstr. Surg. 2012, 129, 683–692. [Google Scholar] [CrossRef]
- Stütz, N.; Gohritz, A.; van Schoonhoven, J.; Lanz, U. Revision surgery after carpal tunnel release—Analysis of the Pathology in 200 cases during a 2 year period. J. Hand Surg. 2006, 31, 68–71. [Google Scholar] [CrossRef]
- Adler, J.A.; Wolf, J.M. Proximal median nerve compression: Pronator syndrome. J. Hand Surg. 2020, 45, 1157–1165. [Google Scholar] [CrossRef]
- Lee, M.J.; LaStayo, P.C. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J. Orthop. Sports Phys. Ther. 2004, 34, 601–609. [Google Scholar] [CrossRef]
- Presciutti, S.; Rodner, C.M. Pronator syndrome. J. Hand Surg. Am. 2011, 36, 907–909. [Google Scholar] [CrossRef]
- Rodner, C.M.; Tinsley, B.A.; O’Malley, M.P. Pronator syndrome and anterior interosseous nerve syndrome. J. Am. Acad. Orthop. Surg. 2013, 21, 268–275. [Google Scholar] [CrossRef]
- Gross, P.T.; Tolomeo, E.A. Proximal median neuropathies. Neurol. Clin. 1999, 17, 425–445. [Google Scholar] [CrossRef]
- Johnson, R.K.; Spinner, M.; Shrewsbury, M.M. Median nerve entrapment syndrome in the proximal forearm. J. Hand Surg. Am. 1979, 4, 48–51. [Google Scholar] [CrossRef]
- El-Haj, M.; Ding, W.; Sharma, K.; Novak, C.; Mackinnon, S.E.; Patterson, J.M.M. Median nerve compression in the forearm: A clinical diagnosis. Hand 2021, 16, 586–591. [Google Scholar] [CrossRef]
- Abdalbary, S.A.; Abdel-Wahed, M.; Amr, S.; Mahmoud, M.; El-Shaarawy, E.A.A.; Salaheldin, S.; Fares, A. The Myth of Median Nerve in Forearm and Its Role in Double Crush Syndrome: A Cadaveric Study. Front. Surg. 2021, 8, 648779. [Google Scholar] [CrossRef] [PubMed]
- Upton, A.R.; McComas, A.J. The double crush in nerve entrapment syndromes. Lancet 1973, 2, 359–362. [Google Scholar] [CrossRef]
- Mackinnon, S.E. Double and multiple “crush” syndromes. Double and multiple entrapment neuropathies. Hand Clin. 1992, 8, 369–390. [Google Scholar] [CrossRef]
- Lo, S.F.; Chou, L.W.; Meng, N.H.; Chen, F.F.; Juan, T.T.; Ho, W.C.; Chiang, C.F. Clinical characteristics and electrodiagnostic features in patients with carpal tunnel syndrome, double crush syndrome, and cervical radiculopathy. Rheumatol. Int. 2012, 32, 1257–1263. [Google Scholar] [CrossRef]
- Marek, T.; Spinner, R.J.; Syal, A.; Wahood, W.; Mahan, M.A. Surgical treatment of lipomatosis of nerve: A systematic review. World Neurosurg. 2019, 128, 587–592.e2. [Google Scholar] [CrossRef]
- Rüsch, C.T.; Knirsch, U.; Weber, D.M.; Rohrbach, M.; Eichenberger, A.; Lütschg, J.; Weber, K.; Broser, P.J.; Stettner, G.M. Etiology of carpal tunnel syndrome in a large cohort of children. Children 2021, 8, 624. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Novak, C.B.; Landau, W.M. Clinical diagnosis of carpal tunnel syndrome. JAMA 2000, 284, 1924–1925. [Google Scholar] [CrossRef]
- Wiesman, I.M.; Novak, C.B.; Mackinnon, S.E.; Winograd, J.M. Sensitivity and specificity of clinical testing for carpal tunnel syndrome. Can. J. Plast. Surg. 2003, 11, 70–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthik, K.; Nanda, R.; Stothard, J. Recurrent Carpal Tunnel Syndrome––Analysis of the impact of patient personality in altering functional outcome following a vascularised hypothenar fat pad flap surgery. J. Hand Microsurg. 2012, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieske, L.; Ebersole, G.C.; Davidge, K.; Fox, I.; Mackinnon, S.E. Revision carpal tunnel surgery: A 10-year review of intraoperative findings and outcomes. J. Hand Surg. Am. 2013, 38, 1530–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duclos, L.; Sokolow, C. Management of true recurrent carpal tunnel syndrome: Is it worthwhile to bring vascularized tissue? Chir. Main 1998, 17, 113–117. [Google Scholar] [CrossRef]
- Rodrigues, R.L.; Shin, A.Y. Treatment options for recurrent carpal tunnel syndrome: Local flaps. Tech. Orthop. 2006, 21, 61–74. [Google Scholar] [CrossRef]
- DeQuattro, K.; Imboden, J.B. Neurologic manifestations of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2017, 43, 561–571. [Google Scholar] [CrossRef]
- Shiri, R. Arthritis as a risk factor for carpal tunnel syndrome: A meta-analysis. Scand. J. Rheumatol. 2016, 45, 339–346. [Google Scholar] [CrossRef]
- Sood, R.F.; Kamenko, S.; McCreary, E.; Sather, B.K.; Schmitt, M.; Peterson, S.L.; Lipira, A.B. Diagnosing systemic amyloidosis presenting as carpal tunnel syndrome: A risk nomogram to guide biopsy at time of carpal tunnel release. J. Bone Jt. Surg. Am. 2021, 103, 1284–1294. [Google Scholar] [CrossRef]
- Das, S.K.; Brown, H.G. In search of complications in carpal tunnel decompression. Hand 1976, 8, 243–249. [Google Scholar] [CrossRef]
- Conolly, W.B. Pitfalls in carpal tunnel decompression. Aust. N. Z. J. Surg. 1978, 48, 421–425. [Google Scholar] [CrossRef]
- Louis, D.S.; Greene, T.L.; Noellert, R.C. Complications of carpal tunnel surgery. J. Neurosurg. 1985, 62, 352–356. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.I.; Lichtman, D.M.; Hanlon, J.J.; Wilson, J.N. Complications of surgical release for carpal tunnel syndrome. J. Hand Surg. Am. 1978, 3, 70–76. [Google Scholar] [CrossRef]
- Cartotto, R.C.; McCabe, S.; Mackinnon, S.E. Two devastating complications of carpal tunnel surgery. Ann. Plast. Surg. 1992, 28, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Watchmaker, G.P.; Weber, D.; Mackinnon, S.E. Avoidance of transection of the palmar cutaneous branch of the median nerve in carpal tunnel release. J. Hand Surg. Am. 1996, 21, 644–650. [Google Scholar] [CrossRef]
- Buckmiller, J.F.; Rickard, T.A. Isolated compression neuropathy of the palmar cutaneous branch of the median nerve. J. Hand Surg. Am. 1987, 12, 97–99. [Google Scholar] [CrossRef]
- Inglis, A.E. Two unusual operative complications in the carpal-tunnel syndrome. A report of two cases. J. Bone Jt. Surg. Am. 1980, 62, 1208–1209. [Google Scholar] [CrossRef]
- Seradge, H.; Seradge, E. Piso-triquetral pain syndrome after carpal tunnel release. J. Hand Surg. Am. 1989, 14, 858–862. [Google Scholar] [CrossRef]
- Gartsman, G.M.; Kovach, J.C.; Crouch, C.C.; Noble, P.C.; Bennett, J.B. Carpal arch alteration after carpal tunnel release. J. Hand Surg. Am. 1986, 11, 372–374. [Google Scholar] [CrossRef]
- Bloem, J.J.; Pradjarahardja, M.C.; Vuursteen, P.J. The post-carpal tunnel syndrome. Causes and prevention. Neth. J. Surg. 1986, 38, 52–55. [Google Scholar]
- Durkan, J.A. A new diagnostic test for carpal tunnel syndrome. JBJS 1991, 73, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Kuschner, S.H.; Ebramzadeh, E.; Johnson, D.; Brien, W.W.; Sherman, R. Tinel’s sign and Phalen’s test in carpal tunnel syndrome. Orthopedics 1992, 15, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Mackinnon-Patterson, B.; Beck, J.L.; Mackinnon, S.E. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J. Hand Surg. Am. 2008, 33, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Davidge, K.M.; Gontre, G.; Tang, D.; Boyd, K.U.; Yee, A.; Damiano, M.S.; Mackinnon, S.E. The “hierarchical” Scratch Collapse Test for identifying multilevel ulnar nerve compression. Hand 2015, 10, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.D.; Eisen, A. Tinel’s sign and the carpal tunnel syndrome. Br. Med. J. 1978, 2, 1125–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunderland, S.; Bradley, K.C. Rate of advance of Hoffmann-Tinel sign in regenerating nerves; further observations. AMA Arch. Neurol. Psychiatry 1952, 67, 650–654. [Google Scholar] [CrossRef]
- Berger, M.J.; Regan, W.R.; Seal, A.; Bristol, S.G. Reliability of the “Ten Test” for assessment of discriminative sensation in hand trauma. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1411–1416. [Google Scholar] [CrossRef]
- Zhang, D.; Earp, B.E.; Blazar, P. Utility of “baseline” electrodiagnostic studies for carpal tunnel release. J. Hand Surg. Eur. Vol. 2019, 44, 273–277. [Google Scholar] [CrossRef]
- Watson, J.C. The electrodiagnostic approach to carpal tunnel syndrome. Neurol. Clin. 2012, 30, 457–478. [Google Scholar] [CrossRef]
- Osiak, K.; Mazurek, A.; Pękala, P.; Koziej, M.; Walocha, J.A.; Pasternak, A. Electrodiagnostic studies in the surgical treatment of carpal tunnel syndrome—A systematic review. J. Clin. Med. 2021, 10, 2691. [Google Scholar] [CrossRef]
- Carità, E.; Donadelli, A.; Laterza, M.; Perazzini, P.; Tamburin, S.; Zanette, G. High-resolution ultrasound in the diagnosis of failed carpal tunnel decompression: A study of 35 cases. J. Hand Surg. Eur. Vol. 2022, 17531934211068636. [Google Scholar] [CrossRef]
- Kluge, S.; Langer, M.; Schelle, T. Sonographic Diagnosis of Carpal Tunnel Syndrome. Hand Clin. 2022, 38, 35–53. [Google Scholar] [CrossRef]
- Botchu, R.; Khan, A.; Jeyapalan, K. Pictorial essay: Role of ultrasound in failed carpal tunnel decompression. Indian J. Radiol. Imaging 2012, 22, 31–34. [Google Scholar] [CrossRef]
- Campagna, R.; Pessis, E.; Feydy, A.; Guerini, H.; Le Viet, D.; Corlobe, P.; Drape, J.L. MRI assessment of recurrent carpal tunnel syndrome after open surgical release of the median nerve. AJR Am. J. Roentgenol. 2009, 193, 644–650. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Tahir, A.; Stevenson, S.; Barnes, D.E.; Spratt, J.D.; Erdmann, M.W.H. The role of MRI in the diagnosis of recurrent/persistent carpal tunnel syndrome: A radiological and intra-operative correlation. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1250–1252. [Google Scholar] [CrossRef]
- Wu, H.T.H.; Schweitzer, M.E.; Culp, R.W. Potential MR signs of recurrent carpal tunnel syndrome: Initial experience. J. Comput. Assist. Tomogr. 2004, 28, 860–864. [Google Scholar] [CrossRef]
- Luria, S.; Waitayawinyu, T.; Trumble, T.E. Endoscopic revision of carpal tunnel release. Plast. Reconstr. Surg. 2008, 121, 2029–2034. [Google Scholar] [CrossRef]
- Fried, S.M.; Nazarian, L.N. Ultrasound-Guided Hydroneurolysis of the Median Nerve for Recurrent Carpal Tunnel Syndrome. Hand 2019, 14, 413–421. [Google Scholar] [CrossRef]
- Hunter, J.M. Recurrent carpal tunnel syndrome, epineural fibrous fixation, and traction neuropathy. Hand Clin. 1991, 7, 491–504. [Google Scholar] [CrossRef]
- Pizzillo, M.F.; Sotereanos, D.G.; Tomaino, M.M. Recurrent carpal tunnel syndrome: Treatment options. J. South. Orthop. Assoc. 1999, 8, 28–36. [Google Scholar]
- Curtis, R.M.; Eversmann, W.W. Internal neurolysis as an adjunct to the treatment of the carpal-tunnel syndrome. J. Bone Jt. Surg. Am. 1973, 55, 733–740. [Google Scholar] [CrossRef]
- Rhoades, C.E.; Mowery, C.A.; Gelberman, R.H. Results of internal neurolysis of the median nerve for severe carpal-tunnel syndrome. J. Bone Jt. Surg. Am. 1985, 67, 253–256. [Google Scholar] [CrossRef]
- Wadstroem, J.; Nigst, H. Reoperation for carpal tunnel syndrome. A retrospective analysis of forty cases. Ann. Chir. Main 1986, 5, 54–58. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Pfeffer, G.B.; Galbraith, R.T.; Szabo, R.M.; Rydevik, B.; Dimick, M. Results of treatment of severe carpal-tunnel syndrome without internal neurolysis of the median nerve. J. Bone Jt. Surg. Am. 1987, 69, 896–903. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; McCabe, S.; Murray, J.F.; Szlai, J.P.; Kelly, L.; Novak, C.; Kin, B.; Burke, G.M. Internal neurolysis fails to improve the results of primary carpal tunnel decompression. J. Hand Surg. Am. 1991, 16, 211–218. [Google Scholar] [CrossRef]
- Sunderland, S. Nerves and Nerve Injuries, 1st ed.; Williams and Wilkins Co.: Philadelphia, PA, USA, 1968. [Google Scholar]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Dvali, L.; Mackinnon, S. Nerve repair, grafting, and nerve transfers. Clin. Plast. Surg. 2003, 30, 203–221. [Google Scholar] [CrossRef]
- Mackinnon, S.E. Surgical management of the peripheral nerve gap. Clin. Plast. Surg. 1989, 16, 587–603. [Google Scholar] [CrossRef]
- Poppler, L.H.; Davidge, K.; Lu, J.C.Y.; Armstrong, J.; Fox, I.K.; Mackinnon, S.E. Alternatives to sural nerve grafts in the upper extremity. Hand 2015, 10, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Ducic, I.; Yoon, J.; Buncke, G. Chronic postoperative complications and donor site morbidity after sural nerve autograft harvest or biopsy. Microsurgery 2020, 40, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Hallgren, A.; Björkman, A.; Chemnitz, A.; Dahlin, L.B. Subjective outcome related to donor site morbidity after sural nerve graft harvesting: A survey in 41 patients. BMC Surg. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.S.; Barbosa, R.A.; Siqueira, M.G.; Soares, M.S.; Heise, C.O.; Foroni, L.; Teixeira, M.J. Morbidity following sural nerve harvesting: A prospective study. Clin. Neurol. Neurosurg. 2012, 114, 1149–1152. [Google Scholar] [CrossRef]
- IJpma, F.F.A.; Nicolai, J.P.A.; Meek, M.F. Sural nerve donor-site morbidity: Thirty-four years of follow-up. Ann. Plast. Surg. 2006, 57, 391–395. [Google Scholar] [CrossRef]
- Poppler, L.H.; Parikh, R.P.; Bichanich, M.J.; Rebehn, K.; Bettlach, C.R.; Mackinnon, S.E.; Moore, A.M. Surgical interventions for the treatment of painful neuroma: A comparative meta-analysis. Pain 2018, 159, 214–223. [Google Scholar] [CrossRef]
- Vernadakis, A.J.; Koch, H.; Mackinnon, S.E. Management of neuromas. Clin. Plast. Surg. 2003, 30, 247–268. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Dellon, A.L.; Hudson, A.R.; Hunter, D.A. Alteration of neuroma formation by manipulation of its microenvironment. Plast. Reconstr. Surg. 1985, 76, 345–353. [Google Scholar] [CrossRef]
- Dahlin, L.B.; Lekholm, C.; Kardum, P.; Holmberg, J. Coverage of the median nerve with free and pedicled flaps for the treatment of recurrent severe carpal tunnel syndrome. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2002, 36, 172–176. [Google Scholar] [CrossRef]
- Rose, E.H.; Norris, M.S.; Kowalski, T.A.; Lucas, A.; Flegler, E.J. Palmaris brevis turnover flap as an adjunct to internal neurolysis of the chronically scarred median nerve in recurrent carpal tunnel syndrome. J. Hand Surg. Am. 1991, 16, 191–201. [Google Scholar] [CrossRef]
- Peters, B.R.; Pripotnev, S.; Chi, D.; Mackinnon, S.E. Complete foot drop with normal electrodiagnostic studies: Sunderland “Zero” ischemic conduction block of the common peroneal nerve. Ann. Plast. Surg. 2021. Available online: https://journals.lww.com/annalsplasticsurgery/Abstract/9000/Complete_Foot_Drop_With_Normal_Electrodiagnostic.96047.aspx (accessed on 10 December 2021). [CrossRef]
- Rebowe, R.; Rogers, A.; Yang, X.; Kundu, S.C.; Smith, T.L.; Li, Z. Nerve repair with nerve conduits: Problems, solutions, and future directions. J. Hand Microsurg. 2018, 10, 61–65. [Google Scholar] [CrossRef]
- Moore, A.M.; Kasukurthi, R.; Magill, C.K.; Farhadi, H.F.; Borschel, G.H.; Mackinnon, S.E. Limitations of conduits in peripheral nerve repairs. Hand 2009, 4, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Wulle, C. The synovial flap as treatment of the recurrent carpal tunnel syndrome. Hand Clin. 1996, 12, 379–388. [Google Scholar] [CrossRef]
- Koncilia, H.; Kuzbari, R.; Worseg, A.; Tschabitscher, M.; Holle, J. The lumbrical muscle flap: Anatomic study and clinical application. J. Hand Surg. Am. 1998, 23, 111–119. [Google Scholar] [CrossRef]
- Milward, T.M.; Stott, W.G.; Kleinert, H.E. The abductor digiti minimi muscle flap. Hand 1977, 9, 82–85. [Google Scholar] [CrossRef]
- Dellon, A.L.; Mackinnon, S.E. The pronator quadratus muscle flap. J. Hand Surg. Am. 1984, 9, 423–427. [Google Scholar] [CrossRef]
- Strickland, J.W.; Idler, R.S.; Lourie, G.M.; Plancher, K.D. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J. Hand Surg. Am. 1996, 21, 840–848. [Google Scholar] [CrossRef]
- Mathoulin, C.; Bahm, J.; Roukoz, S. Pedicled hypothenar fat flap for median nerve coverage in recalcitrant carpal tunnel syndrome. Hand Surg. 2000, 5, 33–40. [Google Scholar] [CrossRef]
- Tham, S.K.; Ireland, D.C.; Riccio, M.; Morrison, W.A. Reverse radial artery fascial flap: A treatment for the chronically scarred median nerve in recurrent carpal tunnel syndrome. J. Hand Surg. Am. 1996, 21, 849–854. [Google Scholar] [CrossRef]
- Dy, C.J.; Aunins, B.; Brogan, D.M. Barriers to epineural scarring: Role in treatment of traumatic nerve injury and chronic compressive neuropathy. J. Hand Surg. Am. 2018, 43, 360–367. [Google Scholar] [CrossRef]
- Lee, J.Y.; Parisi, T.J.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Does the addition of a nerve wrap to a motor nerve repair affect motor outcomes? Microsurgery 2014, 34, 562–567. [Google Scholar] [CrossRef]
- Kokkalis, Z.T.; Mavrogenis, A.F.; Ballas, E.G.; Papagelopoulos, P.J.; Soucacos, P.N. Collagen nerve wrap for median nerve scarring. Orthopedics 2015, 38, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Soltani, A.M.; Allan, B.J.; Best, M.J.; Mir, H.S.; Panthaki, Z.J. Revision decompression and collagen nerve wrap for recurrent and persistent compression neuropathies of the upper extremity. Ann. Plast. Surg. 2014, 72, 572–578. [Google Scholar] [CrossRef]
- Magill, C.K.; Tuffaha, S.H.; Yee, A.; Luciano, J.P.; Hunter, D.A.; Mackinnon, S.E.; Borschel, G.H. The short- and long-term effects of Seprafilm on peripheral nerves: A histological and functional study. J. Reconstr. Microsurg. 2009, 25, 345–354. [Google Scholar] [CrossRef]
- Felder, J.M.; Hill, E.J.R.; Power, H.A.; Hasak, J.; Mackinnon, S.E. Cross-palm nerve grafts to enhance sensory recovery in severe ulnar neuropathy. Hand 2020, 15, 526–533. [Google Scholar] [CrossRef]
- de Roode, C.P.; James, M.A.; McCarroll, H.R. Abductor digit minimi opponensplasty: Technique, modifications, and measurement of opposition. Tech. Hand Up. Extrem. Surg. 2010, 14, 51–53. [Google Scholar] [CrossRef]
- Matsuki, H.; Nakatsuchi, Y.; Momose, T. Opponensplasty using the extensor indicis proprius tendon for severe carpal tunnel syndrome in 40 patients. J. Hand Surg. Eur. Vol. 2021, 17531934211045956. [Google Scholar] [CrossRef]
- Burkhalter, W.; Christensen, R.C.; Brown, P. Extensor indicis proprius opponensplasty. J. Bone Jt. Surg. Am. 1973, 55, 725–732. [Google Scholar] [CrossRef]
- Waitayawinyu, T.; Numnate, W.; Boonyasirikool, C.; Niempoog, S. Outcomes of endoscopic carpal tunnel release with ring finger flexor digitorum superficialis opponensplasty in severe carpal tunnel syndrome. J. Hand Surg. Am. 2019, 44, 1095.e1–1095.e7. [Google Scholar] [CrossRef]
- Anderson, G.A.; Lee, V.; Sundararaj, G.D. Opponensplasty by extensor indicis and flexor digitorum superficialis tendon transfer. J. Hand Surg. Br. 1992, 17, 611–614. [Google Scholar] [CrossRef]
- Rymer, B.; Thomas, P.B.M. The Camitz transfer and its modifications: A review. J. Hand Surg. Eur. Vol. 2016, 41, 632–637. [Google Scholar] [CrossRef]
- Chadderdon, R.C.; Gaston, R.G. Low median nerve transfers (Opponensplasty). Hand Clin. 2016, 32, 349–359. [Google Scholar] [CrossRef]
- Foucher, G.; Malizos, C.; Sammut, D.; Braun, F.M.; Michon, J. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release. A series of 73 cases. J. Hand Surg. Br. 1991, 16, 56–60. [Google Scholar] [CrossRef]
- Hattori, Y.; Doi, K.; Sakamoto, S.; Kumar, K.; Koide, S. Camitz tendon transfer using flexor retinaculum as a pulley in advanced carpal tunnel syndrome. J. Hand Surg. Am. 2014, 39, 2454–2459. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pripotnev, S.; Mackinnon, S.E. Revision of Carpal Tunnel Surgery. J. Clin. Med. 2022, 11, 1386. https://doi.org/10.3390/jcm11051386
Pripotnev S, Mackinnon SE. Revision of Carpal Tunnel Surgery. Journal of Clinical Medicine. 2022; 11(5):1386. https://doi.org/10.3390/jcm11051386
Chicago/Turabian StylePripotnev, Stahs, and Susan E. Mackinnon. 2022. "Revision of Carpal Tunnel Surgery" Journal of Clinical Medicine 11, no. 5: 1386. https://doi.org/10.3390/jcm11051386
APA StylePripotnev, S., & Mackinnon, S. E. (2022). Revision of Carpal Tunnel Surgery. Journal of Clinical Medicine, 11(5), 1386. https://doi.org/10.3390/jcm11051386




