“In Less than No Time”: Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy
Abstract
:1. Introduction
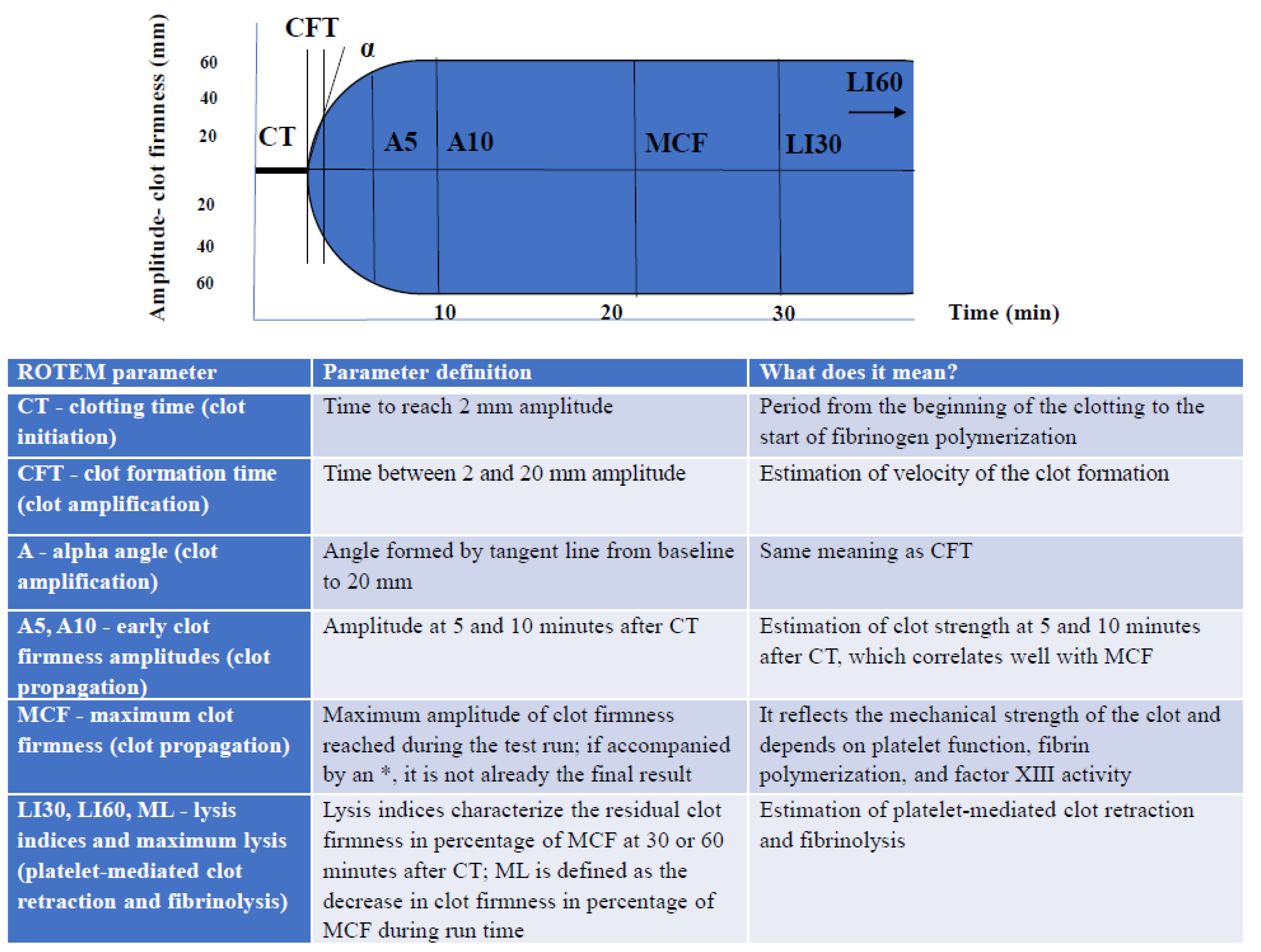
2. ROTEM Device
3. Parenteral Anticoagulation
3.1. Unfractionated Heparin
3.2. Low-Molecular-Weight Heparins
3.3. Fondaparinux
3.4. Direct Thrombin Inhibitors (Argatroban and Bivalirudin)
4. Oral Anticoagulation: Antivitamin K Antagonists (VKAs) and Direct Oral Anticoagulants (DOACs)
4.1. Antivitamin K Antagonists (VKAs)
4.1.1. ROTEM Monitoring in VKA Therapy
4.1.2. ROTEM Monitoring in VKA Reversal
4.2. Direct Oral Anticoagulants (DOACs)
4.2.1. ROTEM Monitoring in DOACs Therapy
4.2.2. ROTEM Monitoring in DOAC Reversal
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guyatt, G.H.; Akl, E.A.; Crowther, M.; Gutterman, D.D.; Schünemann, H.J.; American College of Chest Physician Evidence-Based Clinical Practice Guidelines. Executive Summary: Antithrombotic Therapy and prevention of thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. 2), 7S–47S. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Outes, A.; Suarez-Gea, M.L.; Lecumberri, R.; Terleira-Fernandez, A.I.; Vargas-Catrillon, E. Direct acting oral anticoagulants: Pharmacology, indications, management, and future perspectives. Eur. J. Haematol. 2015, 95, 389–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onishi, A.; St Ange, K.; Dordick, J.S.; Linhardt, R.J. Heparin and anticoagulation. Front. Biosci. 2016, 21, 1372–1392. [Google Scholar]
- Mann, K.G.; Butenas, S.; Brummel, K. the dynamic of thrombin formation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 17–25. [Google Scholar] [CrossRef]
- Shojania, A.M.; Tetreault, J.; Turnbull, G. The variations between heparin sensitivity of different lots of activated partial thromboplastin time reagent produced by the same manufactured. Am. J. Clin. Pathol. 1988, 89, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funk, D.M.A. Coagulation assays and anticoagulant monitoring. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 460–465. [Google Scholar] [CrossRef]
- Babin, J.L.; Traylor, K.L.; Witt, D.M. Laboratory monitoring of low-molecular-weight heparin and fondaparinux. Semin. Thromb. Hemost. 2017, 43, 261–269. [Google Scholar]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Riddez, L.; Samama, C.-M.; et al. The European guidelines on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Ganter, M.T.; Hofer, C.K. Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth. Analg. 2008, 106, 1366–1375. [Google Scholar] [CrossRef] [Green Version]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Perez Calatayud, A.A.; Kim, T.-Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anaesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schochl, N.; Nienaber, U.; Hofer, G.; Voelckel, W.; Jambor, C.; Scharbert, G.; Kozek-Langenecker, S.; Solomon, C. Goal-directed coagulation management of major trauma patients using rotation thromboelastometry (ROTEM)-guided administration of fibrinogen and prothrombin complex concentrate. Crit. Care 2010, 14, R55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simurda, T.; Asselta, R.; Zolkova, J.; Brunclikova, M.; Dobtotova, M.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Congenital afibrinogenemia and hypofibrinogenemia: Laboratory and genetic testing in rare bleeding disorders with life-threating clinicval manifestations and challenging management. Diagnostics 2021, 11, 2140. [Google Scholar] [CrossRef]
- Haas, T.; Spielmann, N.; Mauch, J.; Speer, O.; Schmugge, M.; Weiss, M. Reproducibility of thromboelastometry (ROTEM ®): Point-of care versus hospital laboratory performance. Scand. J. Clin. Lab. Investig. 2012, 72, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Hartert, H. Blutgerinnungsstudien mit der Thromboelastographie, einem neuen Untersuchungsverfahren. Klin. Wschr. 1948, 26, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Schenk, B.; Görlinger, K.; Treml, B.; Tauber, H.; Fries, D.; Niederwanger, C.; Oswald, E.; Bachler, M. A comparison of the new ROTEM sigma with its predecessor, the ROTEM delta. Anaesthesia 2019, 74, 348–356. [Google Scholar] [CrossRef]
- Sucker, C.; Zotz, R.B.; Görlinger, K.; Hartmann, M. Rotational thromboelastometry for the bedside monitoring of recombinant hirudin. Acta Anaesthesiol. Scand. 2008, 52, 358–362. [Google Scholar] [CrossRef]
- De Denus, S.; Spinler, S.A. Clinical monitoring of direct thrombin inhibitors using the ecarin clotting time. Pharmacotherapy 2002, 22, 433–435. [Google Scholar] [CrossRef] [Green Version]
- Adamzik, M.; Langemeier, T.; Frey, U.H.; Görlinger, K.; Saner, F.; Eggebrecht, H.; Peters, J.; Hartmann, M. Comparison of thromboelastometry with simple acute physiology score II and sequential organ failure assessment scores for the prediction of 30-day survival: A cohort study. Shock 2011, 35, 339–342. [Google Scholar] [CrossRef]
- Zipplerle, J.; Schlim, C.J.; Holnthoner, W.; Husa, A.M.; Nurnberger, S.; Redl, H.; Schochl, H. A novel coagulation assay incorporating adherent endothelial cells in thromboelastometry. Thromb. Haemost. 2013, 109, 869–877. [Google Scholar] [CrossRef]
- Adamzik, M.; Eggmann, M.; Frey, U.H.; Görlinger, K.; Bröcker-Preuss, M.; Marggraf, G.; Saner, F.; Eggebrecht, H.; Peters, J.; Hartmann, M. Comparison of thromboelastometry with procalcitonin, interleukin 6, C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit. Care 2010, 14, R178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, M.C.; Meijers, J.C.; Vroom, M.B.; Juffermans, N.P. Utility of thromboelastography and/or thromboelastometry in adult with sepsis: A systematic review. Crit. Care 2014, 18, R30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, N.A.; Harrison, N.K.; Sabra, A.; Lawrence, M.J.; Noble, S.; Davidson, S.J.; Evans, V.J.; Morris, R.H.K.; Hawkins, K.; Williams, P.R.; et al. Application of ROTEM to assess hypercoagulability in patients with lung cancer. Thromb. Res. 2015, 135, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Meesters, M.I.; Koch, A.; Kuiper, G.; Zacharowski, K.; Boer, C. Instability of the non-activated rotational thromboelastometry assay (NATEM) in citrate stored blood. Thromb. Res. 2015, 136, 481–483. [Google Scholar] [CrossRef]
- Clatzis, A.; Spannagl, M.; Gempeler-Messina, P.; Kolde, U.H.J.; Schramm, W.; Haas, S. The prothrombinase-induced clotting test: A new technique for the monitoring of anticoagulant. Haemostasis 2000, 30 (Suppl. 2), 172–174. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Gunay, N.S. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 1999, 25 (Suppl 3), 5–16. [Google Scholar]
- Hirsh, J. Heparin. N. Engl. J. Med. 1991, 324, 1565–1574. [Google Scholar]
- Eikelboom, J.W.; Hirsh, J. Monitoring unfractioned heparin with the aPTT: Time for a fresh look. Thromb. Haemost. 2006, 96, 2475–2479. [Google Scholar]
- Levine, M.N.; Hirsh, J.; Gent, M.; Turpie, A.G.; Cruckshank, M.; Weitz, J.; Anderson, D.; Johnson, M. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large doses of heparin. Arch. Intern. Med. 1994, 154, 49–56. [Google Scholar] [CrossRef]
- Baluwala, I.; Favaloro, E.J.; Pasalic, L. Therapeutic monitoring of unfractioned heparin-trials and tribulation. Expert Rev. Hematol. 2017, 10, 595–605. [Google Scholar] [CrossRef]
- Hattersley, P.G. Progress report: The activated coagulation time of whole blood (ACT). Am. J. Clin. Pathol. 1976, 66, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, B.S.; Korpman, R.A.; Huse, W.M.; Briggs, B.D. Heparin therapy during extracorporeal circulation. I. Problems inherent in existing heparin protocols. J. Thorac. Cardiovasc. Surg. 1975, 69, 674–684. [Google Scholar] [CrossRef]
- Nilsson, S.; Appelblad, M.; Svenmarker, S. Can we rely on the activated clotting time to measure heparin anticoagulation? A clinical evaluation of two ACT monitors. J. Extra Corpor. Technol. 2020, 52, 212–217. [Google Scholar] [PubMed]
- Murray, D.J.; Bronsnahan, W.J.; Pennell, B.; Kapalanski, D.; Weiler, J.M.; Olson, J. Heparin detection by the activated coagulation time: A comparison of the sensitivity of coagulation tests and heparin assays. J. Cardiothorac. Vasc. Anesth. 1997, 11, 24–28. [Google Scholar] [CrossRef]
- Ortmann, E.; Rubino, A.; Altemimi, B.; Collier, T.; Besser, M.W.; Klein, A.A. Validation of viscoelastic coagulation tests during cardiopulmonary bypass. J. Thromb. Haemost. 2015, 13, 1207–1216. [Google Scholar] [CrossRef]
- Gronchi, F.; Perret, A.; Ferrari, E.; Marcucci, C.M.; Flèche, J.; Crosset, M.; Schoettker, P.; Marcucci, C. Validation of rotational thromboelastometry during cardiopulmonary bypass. A prospective, observational in-vivo study. Eur. J. Anaesthesiol. 2014, 31, 68–75. [Google Scholar] [CrossRef]
- Najafi, A.; Etezadi, F.; Pourfakhr, P.; Imani, F.; Khajavi, R.M.; Moharari, R.S. Comparison of aPTT and CT of the ROTEM test to monito heparin anti-coagulation effect in ICU patients: An observational study. Acta Med. Iran. 2015, 53, 643–646. [Google Scholar]
- Prakash, S.; Wiersema, U.F.; Bihari, S.; Roxby, D. Discordance between ROTEM clotting time and conventional tests during unfractioned heparin-based anticoagulation in intensive care patients on extracorporeal membrane oxygenation. Anesth. Int. Care 2016, 44, 85–92. [Google Scholar]
- Ogawa, S.; Szlam, F.; Chen, E.P.; Nishimura, T.; Kim, H.; Roback, J.D.; Levy, J.H.; Tanaka, K.A. A comparative evaluation of rotational thromboelastometry and standard coagulation tests in hemodilution-induced coagulation changes after cardiac surgery. Transfusion 2021, 52, 14–22. [Google Scholar] [CrossRef]
- Heilmann, C.; Geisen, U.; Beyerdorf, F.; Nakamura, L.; Benk, C.; Trummer, G.; Berchtold-Henz, M.; Schlensak, C.; Zieger, B. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Int. Care Med. 2021, 38, 62–68. [Google Scholar] [CrossRef]
- Gravlee, G.P.; Case, L.D.; Angert, K.C.; Rogers, A.T.; Miller, G.S. Variability of the activated coagulation time. Anesth. Analg. 1988, 67, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Ottensen, S.; Stormorken, H.; Hatteland, K. The value of activated coagulation time in monitoring heparin therapy during extracorporeal circulation. Scand. J. Thorac. Cardiovasc. Surg. 1984, 18, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Taneja, R.; Marwaha, G.; Sinha, P.; Quantz, M.; Stitt, L.; Gao, R.; Subramanian, S.; Schaus, M.; Keeney, M.; Chin-Yee, I.; et al. Elevated activated partial thromboplastin time does not correlate with heparin rebound following cardiac surgery. Can. J. Anaesth. 2009, 56, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, J.; Kodaka, M.; Nishiyama, K.; Hirasaki, Y.; Ozaki, M.; Komori, M. Reappearance of circulating heparin in whole blood heparin concentration-based management does not correlate with postoperative bleeding after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1003–1007. [Google Scholar] [CrossRef]
- Mittermayr, M.; Velik-Salchner, C.; Stalzer, B.; Margreiter, J.; Klingler, A.; Streif, W.; Fries, D.; Innerhofer, P. Detection of protamine and heparin after termination of cardiopulmonary bypass by thrombelastometry (ROTEM): Results of a pilot study. Anesth. Analg. 2009, 108, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, M.; Margreiter, J.; Velik-Salchner, C.; Klingler, A.; Streif, W.; Fries, D.; Innerhofer, P. Effects of protamine and heparin can be detected and easily differentiated by modified thromboelastography (Rotem): An in vitro study. Br. J. Anaesth. 2005, 95, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Hanke, A.A.; Severloh, I.; Flöricke, F.; Weber, C.F.; Lang, T. Interaction of heparin and protamine in presence of overdosage: In vitro study. Asian Cardiovasc. Thorac. Ann. 2021, 29, 5–9. [Google Scholar] [CrossRef]
- Schaden, E.; Jilch, S.; Hacker, S.; Schober, A.; Kozek-Langenecker, S. Monitoring of unfractioned heparin with rotational thromboelastometry using the prothrombinase-induced clotting time reagent (PiCT®). Clin. Chim. Acta 2012, 414, 202–205. [Google Scholar] [CrossRef]
- Casu, B.; Torrington, K.G. Structural characterization of low molecular weight heparins. Semin. Thromb. Hemost. 1999, 25 (Suppl. 3), 17–25. [Google Scholar]
- Hirsh, J.; Levine, M.N. Low molecular weight heparin. Blood 1992, 79, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. 2), e24S–e43S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitz, J. Low molecular weight heparin. N. Engl. J. Med. 1997, 237, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Dorffler-Melly, J.; de Jonge, E.; Pont, A.C.; Meijers, J.; Vroom, M.B.; Buller, H.R.; Levi, M. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet 2002, 359, 849–850. [Google Scholar] [CrossRef]
- Al Dieri, R.; Alban, S.; Beguin, S.; Hemker, H.C. Fixed dosage of low-molecular-weight heparins causes large individual variation in coagulability, only partly correlated to body weight. J. Thromb. Haemost. 2006, 4, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Casini, A.; Stasko, J.; Udecek, J.; Skornova, I.; Vilar, R.; Neerman-Arbex, M.; Kubisz, P. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb. Res. 2020, 188, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gouin-Thibault, I.; Pautas, E.; Siguret, V. Safety profile of different low-molecular weight heparins used at therapeutic dose. Drug Saf. 2005, 28, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Duplaga, B.A.; Rivers, C.W.; Nutescu, E. Dosing and monitoring of low molecular-weight heparins. Pharmacotherapy 2001, 21, 218–234. [Google Scholar] [CrossRef]
- Houbouyan, L.; Boutière, B.; Contant, G.; Dautzenberg, M.D.; Fievet, P.; Potron, G.; Vassault, A.; Gourmelin, Y. Validation protocol of analytical hemostasis systems: Measurement of anti-Xa activity of low-molecular-weight heparins. Clin. Chem. 1996, 42, 1223–1230. [Google Scholar] [CrossRef]
- Bounameaux, H.; De Moerloose, P. Is laboratory monitoring of low-molecular-weight heparin therapy necessary? No. J. Thromb. Haemost. 2004, 2, 551–554. [Google Scholar] [CrossRef]
- Leizorovicz, A.; Bara, L.; Samama, M.M.; Haugh, M.C. Factor Xa inhibition: Correlation between the plasma levels of anti-Xa activity and occurrence of thrombosis and haemorrhage. Haemostasis 1993, 23 (Suppl. 1), 89–98. [Google Scholar] [CrossRef]
- Kovacs, M.J.; Keeney, M.; MacKinnon, K.; Boyle, E. Three different chromogenic methods do not give equivalent anti-Xa levels for patients on therapeutic low molecular weight or unfractioned heparin. Clin. Lab. Haemost. 1999, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Limitations of the laboratory monitoring of heparin therapy. Thromb. Haemost. 2002, 87, 163–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhenc-Gelas, M.; Jestin-Le Guernic, C.; Vitoux, J.F.; Kher, A.; Aiach, M.; Fiessinger, J.N.; Fragmin study group. Adjusted versus fixed doses of the low-molecular weight heparin fragmin in the treatment of deep vein thrombosis. Thromb. Haemost. 1992, 71, 698–702. [Google Scholar]
- Artim-Esen, B.; Pericleous, C.; Mackie, I.; Ripoll, V.M.; Latchman, D.; Isenberg, D.; Rahman, A.; Ioannou, Y.; Giles, I. Anti-factor Xa antibodies in patients with antiphospholipid syndrome and their effects upon coagulation assays. Arthritis Res. Ther. 2015, 1, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandiver, J.W.; Vondracek, T.G. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy 2012, 32, 546–558. [Google Scholar] [CrossRef]
- Schaden, E.; Schober, A.; Hacker, S.; Spiss, C.; Chiari, A.; Kozek-Langenecker, S. Determination of enoxaparin with rotational thromboelastometry using the prothrombinase-induced clotting time reagent. Blood Coagul. Fibrinol. 2010, 21, 256–261. [Google Scholar] [CrossRef]
- Schoen, P.; Lindhout, T.; Franssen, J.; Hemker, H.C. Low molecular weight heparin-catalyzed inactivation of factor Xa and thrombin by antithrombin III-effect of platelet factor 4. Thromb. Haemost. 1991, 66, 435–441. [Google Scholar] [CrossRef]
- Bendetowicz, A.V.; Kai, H.; Knebel, R.; Caplain, H.; Hemker, H.C.; Lindhout, T.; Béguin, S. The effect of subcutaneous injection of unfractioned and low molecular weight heparin on thrombin generation in platelet rich plasma- a study in human volunteers. Thromb. Haemost. 1994, 72, 705–712. [Google Scholar]
- Thomas, O.; Larsson, A.; Tynngard, N.; Schott, U. Thromboelastometry versus free-oscillation rheometry and enoxaparin versus tinzaparin: An in-vitro study comparing two viscoelastic haemostatic tests’ dose-responses to two low molecular weight heparins at the time of withdrawing epidural catheters from ten patients after major surgery. BMC Anesthesiol. 2015, 15, 170. [Google Scholar]
- Thomas, O.; Lybeck, E.; Strandberg, K.; Tyngard, N.; Schott, U. Monitoring low molecular weight heparins at therapeutic levels: Dose-responses of and correlations and differences between aPTT, anti-factor Xa and thrombin generation assays. PLoS ONE 2015, 73, 457–465. [Google Scholar] [CrossRef]
- Mousa, S.A.; Bozarth, J.; Barrett, J.S. Pharmacodynamic proprieties of the low molecular weight heparin, tinzaparin: Effect of molecular weight distribution on plasma tissue factor pathway inhibitor in healthy human subjects. J. Clin. Pharmacol. 2003, 43, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Feuring, M.; Wehling, M.; Schultz, A. Dalteparin dose-dependently increases ROTEM ® thromboelastometry parameters only at supratherapeutic anti-factor Xa levels: An in vitro study. Clin. Exp. Pharmacol. Physiol. 2011, 38, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Von Tempelhooff, G.F.; Schelkunov, O.; Demirhan, A.; Tsikouras, P.; Rath, W.; Velten, E.; Csorba, R. Thromboelastometric results and platelet function during pregnancy in women receiving low molecular weight heparin with a history of recurrent/late abortion- A retrospective analysis. Clin. Hemor. Microcirc. 2015, 61, 99–110. [Google Scholar]
- Cvirn, G.; Wagner, T.; Juergens, G.; Koestenberg, M. Effects of nadroparin, enoxaparin, and unfractionated heparin on endogenous factor Xa and IIa formation and on thromboelastometry profiles. Blood Coagul. Fibrinol. 2009, 20, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jilma-Stohlawetz, P.; Fritsche-Polanz, S.; Quehenberg, P.; Schorgenhofer, C.; Bartko, J.; Ristl, R.; Jilma, B. Evaluation of between-, within- and day-to-day variation of coagulation measured by rotational thromboelastometry (ROTEM). Scand. J. Clin. Lab. Investig. 2017, 77, 651–657. [Google Scholar] [CrossRef]
- Christensen, T.D.; Vad, H.; Pedersen, S.; Hornbech, K.; Zois, N.E.; Licht, P.B.; Nybo, M.; Hvas, A.-M. Coagulation profile in patients undergoing video-assisted thoracoscopic lobectomy: A randomized, controlled trial. PLoS ONE 2017, 15, e0171809. [Google Scholar] [CrossRef] [Green Version]
- Stanciakova, L.; Dobrotova, M.; Holly, P.; Zolkova, J.; Vadelova, L.; Skornova, I.; Ivankova, J.; Bolek, T.; Samos, M.; Grendar, M.; et al. How can rotational thromboelastometry as a point-of-care method be useful for the management of secondary thromboprophylaxis in high-risk regnant patients? Diagnostics 2021, 11, 828. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Chakroun, T.; Depasse, F.; Arzoglou, P.; Samama, M.M.; Elalamy, I. The role of platelets and recombinant factor VIIa on thrombin generation, platelet activation and clot formation. Thromb. Haemost. 2004, 91, 977–985. [Google Scholar]
- Chakroun, T.; Gerotziafas, G.T.; Seghatchian, J.; Samama, M.M.; Hatmi, M.; Elalamy, I. The influence of fibrin polymerization and platelet mediated contractile forces on citrated whole blood thromboelastography profile. Thromb. Haemost. 2006, 95, 822–828. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Chakroun, T.; Samama, M.M.; Elalamy, I. In vitro comparison of the effect of fondaparinux and enoxaparin on whole blood tissue factor-triggered thromboelastography profile. Thromb. Haemost. 2004, 92, 1296–1302. [Google Scholar] [CrossRef]
- Sorensen, B.; Johansen, P.; Christiansen, K.; Woelke, M.; Ingerslev, J. Whole blood coagulation thromboelastographic profiles employing minimal tissue factor activation. J. Thromb. Haemost. 2003, 1, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Parhami-Seren, B.; Butenas, S.; Krudysz-Amblo, J.; Mann, K.G. Immunologic quantitation of tissue factor. J. Thromb. Haemost. 2006, 4, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.; Gerasimidis, T.; Verdy, E.; Elalamy, I.; Samama, M.M.; Gerotziafas, G.T. Inhibition of clot formation process by treatment with the low molecular weight heparin nadroparin in patients with carotid artery disease undergoing angioplasty and stenting. A thromboelastography study on whole blood. Thromb. Haemost. 2007, 97, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ӧrlander, F.; Schott, U. Monitoring of low molecular weight heparin thromboprophylaxis with alternative methods to anti-factor Xa. Anesth. Med. Pract. J. 2021, 5, 136. [Google Scholar]
- Mehta, S.R.; Granger, C.B.; Eikelboom, J.W.; Bassand, J.P.; Wallentin, L.; Faxon, D.P.; Peters, R.J.G.; Budaj, A.; Afzal, R.; Chrolavicius, S.; et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: Results from the OASIS-5 trial. J. Am. Coll. Cardiol. 2007, 50, 1742–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turpie, A.G.; Gallus, A.S.; Hoek, J.A. Pentasaccharide Investigators. A synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N. Engl. J. Med. 2001, 344, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Bauer, K.A.; Donati, M.B.; Gould, M.; Samama, M.M.; Weitz, J.I. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 141S–159S. [Google Scholar] [CrossRef]
- Young, G.; Yonekawa, K.E.; Nakagawa, P.A.; Blain, R.C.; Lovejoy, A.E.; Nugent, D.J. Recombinant activated factor VII effectively reverses the anticoagulant effects of heparin, enoxaparin, fondaparinux, argatroban, and bivalirudin ex vivo as measured using thromboelastography. Blood Coagul. Fibrinolysis 2007, 18, 547–553. [Google Scholar] [CrossRef]
- Gatt, A.; van Veen, J.J.; Wolley, A.M.; Kitchen, S.; Cooper, P.; Makris, M. Thrombin generation assays are superior to traditional tests in assessing anticoagulation reversal in vitro. Thromb. Haemost. 2008, 100, 350–355. [Google Scholar] [CrossRef]
- Eller, T.; Busse, J.; Dittrich, M.; Flieder, T.; Alban, S.; Knabbe, C.; Birschmann, I. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin. Chem. Lab. Med. 2014, 52, 835–844. [Google Scholar] [CrossRef]
- Godier, A.; Durand, M.; Emmerich, J.; Dizier, B.; Lecompte, T.; Samama, C.M. Efficacy of prothrombin complex concentrate to reverse the anticoagulant effect of the pentasaccharide fondaparinux in a rabbit model. Thromb. Haemost. 2011, 105, 161–168. [Google Scholar] [PubMed]
- Linkins, L.A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. American College of Chest Physicians. Treatment and prevention of heparin induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 9th ed. Chest 2012, 141 (Suppl. 2), e495S–e530S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.; Wenham, T. Heparin-induced thrombocytopenia. Postgrad. Med. J. 2018, 94, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.K.; Hursting, M.J. The pharmacokinetics and pharmacodynamics of argatroban: Effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy 2000, 20, 318–329. [Google Scholar] [CrossRef]
- Hursting, M.J.; Joffrion, J.L.; Brooks, R.L.; Swan, S.K. Effect of renal function on the pharmacokinetics and pharmacodynamics of argatroban (a direct thrombin inhibitor). Blood 1996, 88 (Suppl. 1). [Google Scholar]
- Guy, S.; Kitchen, S.; Maclean, R.; Van Veen, J.J. Limitation of the activated partial thromboplastin time as a monitoring method of the direct thrombin inhibitor argatroban. Int. J. Lab. Hematol. 2015, 37, 834–843. [Google Scholar] [CrossRef]
- Keyl, C.; Zimmer, E.; Bek, M.J.; Wiessner, M.; Trenk, D. Argatroban pharmacokinetics and pharmacodynamics in critically ill cardiac surgical patients with suspected heparin-induced thrombocytopenia. Thromb. Haemost. 2016, 115, 1081–1089. [Google Scholar]
- Seidel, H.; Kolde, H.J. Monitoring of Argatroban and Lepirudin: What is the Input of Laboratory Values in “Real Life”? Clin. Appl. Thromb. Hemost. 2018, 24, 287–294. [Google Scholar] [CrossRef]
- Guy, S.; Kitchen, S.; Makris, M.; Maclean, R.; Saccullo, G.; Vanveen, J.J. Caution in Using the Activated Partial Thromboplastin Time to Monitor Argatroban in COVID-19 and Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT). Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211066945. [Google Scholar] [CrossRef]
- Williamson, D.R.; Boulager, I.; Tardif, M.; Albert, M.; Grégoire, G. Argatroban dosing in intensive care patients with acute renal failure and liver dysfunction. Pharmacotherapy 2004, 24, 409–414. [Google Scholar] [CrossRef]
- Warkentin, T.E. Anticoagulant failure in coagulopathic patients: PTT confounding and other pitfalls. Expert Opin. Drug Saf. 2014, 13, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, M.; Rundgren, M.; Schott, U. An evaluation of monitoring possibilities of argatroban using rotational thromboelastometry and activated partial thromboplastin time. Acta Anaesth. Scand. 2010, 54, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Schaden, E.; Schober, A.; Hacker, S.; Kozek-Langenecker, S. Ecarin modified rotational thromboelastometry: A point—of-care applicable alternative to monitor the direct thrombin inhibitor argatroban. Wien. Klin. Wochenschr. 2013, 125, 156–159. [Google Scholar] [CrossRef]
- Beiderlinden, M.; Werner, P.; Bahlmann, A.; Kemper, J.; Brezina, T.; Schäfer, M.; Görlinger, K.; Seidel, H.; Kienbaum, P.; Treschan, T.A.; et al. Monitoring of argatroban and lepirudin anticoagulation in critically ill patients by conventional laboratory parameters and rotational thromboelastometry- a prospectively controlled randomized double-blind clinical trial. BMC Anesthesiol. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warkentin, T.E.; Koster, A. Bivalirudin: A review. Expert Opin. Pharmacother. 2005, 6, 1349–1371. [Google Scholar] [CrossRef] [PubMed]
- Irving, F.; Adrian, D.; Peter, L.; Jane, E.; Elizabeth, L.; Don, H.; Tim, M.; John, W.; John, M. Anticoagulant activity of HirulogTM, a direct thrombin inhibitor, in humans. Thromb. Haemost. 1993, 69, 157–163. [Google Scholar]
- Terauya, J.; Hensch, L.; Bruzdoski, K.; Adachi, I.; Rochy Hui, S.-K.; Kostousov, V. Monitoring bivalirudin therapy in children on extracorporeal circulatory support device: Thromboelastometry versus routine coagulation testing. Thromb. Res. 2020, 186, 54–57. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. ESC Scientific Document Group- 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- Pengo, V.; Pegoraro, C.; Cucchini, U.; Iliceto, S. Worldwide management of oral anticoagulant therapy: The ISAM study. J. Thromb. Thrombol. 2006, 21, 73–77. [Google Scholar] [CrossRef]
- Witt, D.M.; Clark, N.P.; Kaatz, S.; Schnurr, T.; Ansell, J.E. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J. Thromb. Thrombol. 2016, 41, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Aronis, K.N.; Hylek, E.M. Evidence gaps in the era of non-vitamin K oral anticoagulants. J. Am. Heart Assoc. 2018, 7, e007338. [Google Scholar] [CrossRef] [Green Version]
- Haykal, T.; Adam, S.; Bala, A.; Zayed, Y.; Deliwala, S.; Kerbage, J.; Ponnapalli, A.; Malladi, S.; Samji, V.; Ortel, T.L. Thromboprophylaxis for orthopedic surgery: An updated meta-analysis. Thromb. Res. 2021, 199, 43–53. [Google Scholar] [CrossRef]
- Holbrook, A.M.; Pereira, J.A.; Labinis, R.; McDonald, H.; Douketis, J.D.; Crowther, M.; Wells, P.S. Systematic overview of warfarin and its drug and food interactions. Arch. Int. Med. 2005, 165, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Ansell, J.; Hirsh, J.; Hylek, E.; Jacobson, A.; Crowther, M.; Palareti, G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physician evidence-based clinical practice guidelines (8th Edition). Chest 2008, 133, 160S–198S. [Google Scholar] [CrossRef]
- Barcellona, D.; Vannini, M.L.; Fenu, L.; Balestrieri, C.; Marongiu, F. Warfarin or acenocoumarol: Which is better in the management of oral anticoagulant? Thromb. Haemost. 1998, 80, 899–902. [Google Scholar] [CrossRef]
- Horsti, J. Comparison of Quick and Owren prothrombin time with regard to the harmonization of the International Normalized Ratio (INR) system. Clin. Chem. Lab. Med. 2002, 40, 399–403. [Google Scholar] [CrossRef]
- Haraldsson, H.M.; Onundarson, P.T.; Einarsdottir, K.A.; Gudmundsdottir, B.R.; Petursson, M.K.; Palsson, K.; Kristinsson, A. Performance of prothrombin-proconvertin time as a monitoring test of oral anticoagulation therapy. Am. J. Clin. Pathol. 1997, 107, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, F.; Rosentaal, F.; Vanderbroucke, J.; Briet, E. Bleeding complications in oral anticoagulant therapy. Arch. Int. Med. 1993, 153, 1557–1562. [Google Scholar] [CrossRef]
- Schmidt, D.E.; Holmstrom, M.; Majeed, A.; Naslin, D.; Wallén, H.; Agren, A. Detection of elevated INR by thromboelastometry and thromboelastography in warfarin treated patients and healthy controls. Thromb. Res. 2015, 135, 1007–1011. [Google Scholar] [CrossRef]
- Schmidt, D.E.; Chaireti, R.; Bruzelius, M.; Holmstrom, M.; Antovic, J.; Agren, A. Correlation of thromboelastography and thrombin generation assays in warfarin-treated patients. Thromb. Res. 2019, 178, 34–40. [Google Scholar] [CrossRef]
- Nilsson, C.U.; Strandberg, K.; Reinstrup, P. Warfarin monitoring with viscoelastic haemostatic assays, thrombin generation, coagulation factors and correlations of Owren and Quick prothrombin time. Scan. J. Clin. Lab. Investig. 2018, 78, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, B.R.; Francis, C.W.; Bjornsdottir, A.M.; Nellbring, M.; Onundarson, P.T. Critical role of factors II and X during coumadin anticoagulation and their combined measurement with a new Fiix-prothrombin time. Thromb. Res. 2012, 130, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Beguin, S.; Hemker, H.C. The relative importance of the factors II, VII, IX and X for the prothrombinase activity in plasma of orally anticoagulated patients. Thromb. Haemost. 1989, 62, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Rumph, B.; Bolliger, D.; Narang, N.; Molinaro, R.J.; Levy, J.H.; Szlam, F.; Tanaka, K.A. In vitro comparative study of hemostatic components in warfarin-treated and fibrinogen-deficient plasma. J. Cardiothorac. Vasc. Anesth. 2010, 24, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Rossetto, V.; Campello, E.; Bulato, C.; Radu, C.M.; Simioni, P. Thrombin generation and thromboelastometry in monitoring the in-vitro reversal of warfarin: Comparison between 3-factor and 4-factor prothrombin complex concentrates. Blood Coagul. Fibrinol. 2020, 31, 127–131. [Google Scholar] [CrossRef]
- Bonderski, V.A.; Portillo, J.; Sharp, L.; Rech, M. Thromboelastometry-guided anticoagulation reversal in a patient with ventricular assist device with intracranial hemorrhage. Am. J. Emerg. Med. 2020, 41, e5–e265. [Google Scholar] [CrossRef]
- Blasi, A.; Muñoz, G.; de Soto, I.; Mellado, R.; Taura, P.; Rios, J.; Balust, J.; Beltran, J. Reliability of thromboelastometry for detecting the safe coagulation threshold in patients taking acenocoumarol after elective heart valve replacement. Thromb. Res. 2015, 136, 669–672. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Olgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Pratical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.; Cattaneo, D.; Giannetti, L.; Bottino, R.; Laezza, N.; Apripaldi, U.; Clementi, E. Pharmacokinetics of direct oral anticoagulant in patients with atrial fibrillation and extreme obesity. Clin. Ther. 2021, 43, e255–e263. [Google Scholar] [CrossRef]
- Brinkman, H.J. Global assay and management of oral anticoagulation. Thromb. J. 2015, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Caturano, A.; Galiero, R.; Pafundi, P.C. Atrial fibrillation and stroke. A review on the use of vitamin K antagonists and novel oral anticoagulants. Medicina 2019, 55, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosselin, R.C.; Adcock, D.M. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J. Thromb. Haemost. 2016, 14, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillarp, A.; Baghaei, F.; Fagerberg Blixter, I.; Gustafsson, K.M.; Stigendal, L.; Sten-Linder, M.; Strandberg, K.; Lindahl, T.L. Effect of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J. Thromb. Haemost. 2011, 9, 133–139. [Google Scholar] [CrossRef]
- Barrett, Y.C.; Wang, Z.; Frost, C.; Shenker, A. Clinical laboratory measurement of direct factor Xa inhibitors: Anti-Xa assay is preferable to prothrombin time assay. Thromb. Haemost. 2010, 104, 1263–1271. [Google Scholar]
- Lippi, G.; Ardissino, D.; Quintavalla, R.; Cervellin, G. Urgent monitoring of direct oral anticoagulants in patients with atrial fibrillation: A tentative approach based on routine laboratory tests. J. Thromb. Thrombol. 2014, 38, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Noguez, J.H.; Ritchie, J.C. Quantification of the oral anticoagulants dabigatran, rivaroxaban, apixaban, and warfarin in plasma using ultra-performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS). Methods Mol. Biol. 2016, 1383, 21–27. [Google Scholar]
- Hanada, K.; Matsumoto, S.I.; Shibata, S.; Matsubara, H.; Tsukimura, Y.; Takahashi, H. Quantitative LC/MSMS method for determination of edoxaban, a Xa inhibitor and its pharmacokinetic application in patients after knee arthroplasty. Biomed. Chromatogr. 2018, 32, e4213. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, E.A.; Kyriakou, E.; Ikonomidis, I.; Katogiannis, K.; Papadakis, I.; Douramani, P.; Kopterides, P.; Kapsimali, V.; Lekakis, J.; Tsangaris, I.; et al. Comparative assessment of the anticoagulant activity of rivaroxaban and dabigatran in patients with nonvalvular atrial fibrillation. Medicine 2016, 95, e3037. [Google Scholar] [CrossRef]
- Vedovati, M.C.; Mosconi, M.G.; Isidori, F.; Agnelli, G.; Becattini, C. Global thromboelastometry in patients receiving direct oral anticoagulants: The RO-DOA study. J. Thromb. Thrombol. 2020, 49, 251–258. [Google Scholar] [CrossRef]
- Korpallova, B.; Samos, M.; Skornova, I.; Bolek, T.; Zolkova, J.; Vadelova, L.; Kubisz, P.; Galajda, P.; Stasko, J.; Mokan, M. Assessing the hemostasis with thromboelastometry in direct oral anticoagulants-treated patients with atrial fibrillation. Thromb. Res. 2020, 191, 38–41. [Google Scholar] [CrossRef]
- Levy, J.H.; Ageno, W.; Chen, N.C.; Crowther, M.; Verhamme, P.; Weitz, J.I. Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Henskens, Y.M.C.; Gulpen, A.J.W.; van Oerle, R.; Wetzels, R.; Verhezen, P.; Spronk, H.; Schalla, S.; Crijns, H.I.; Ten Cate, H.; Ten Cate-Hoek, A. Detecting clinically relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Thromb. J. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Taune, V.; Wallen, H.; Agren, A.; Gryfelt, G.; Sjovik, C.; Wintler, A.M.; Malmstrom, R.E.; Wikman, A.; Skeppholm, M. Whole blood coagulation assays ROTEM and T-TAS to monitor dabigatran treatment. Thromb. Res. 2017, 153, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Thom, J.; Wood, A.; Phillips, M.; Muhammad, S.; Baker, R. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb. Haemost. 2014, 111, 989–995. [Google Scholar]
- Klages, M.; Raimann, F.J.; Philipp, A.L.; Lindhoff-Last, E.; Zacharowski, K.; Mutlak, H. Direct oral anticoagulant in point-of-care monitoring: An ex-vivo study. Minerva Anestesiol. 2021, 87, 514–522. [Google Scholar] [CrossRef]
- Comuth, W.J.; Henriksen, L.O.; van de Kerkhof, D.; Husted, S.E.; Kristensen, S.D.; de Maat, M.P.M.; Munster, A.-M.B. Comprehensive characteristics of the anticoagulant activity of dabigatran in relation to its plasma concentration. Thromb. Res. 2018, 164, 32–39. [Google Scholar] [CrossRef]
- Sokol, J.; Nehaj, F.; Ivankova, J.; Mokan, M.; Zolkova, J.; Lisa, L.; Linekova, L.; Mokan, M.; Stasko, J. Impact of dabigatran treatment on rotation thromboelastometry. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620983902. [Google Scholar] [CrossRef]
- Körber, M.K.; Langer, E.; Kohr, M.; Wernecke, K.D.; Korte, W.; von Heymann, C. In vitro and ex vivo measurement of prophylatic dabigatran concentrations with a new ecarin-based thromboelastometry test. Transfus. Med. Hemother. 2017, 44, 100–105. [Google Scholar] [CrossRef]
- Schäfer, S.T.; Wiederkehr, T.; Kammerer, T.; Acevedo, A.C.; Feil, K.; Kellert, L.; Gorlinger, K.; Hinske, L.C.; Groene, P. Real-time detection, and differentiation of direct oral anticoagulants (rivaroxaban and dabigatran) using modified thromboelastometric reagents. Thromb. Res. 2020, 190, 103–111. [Google Scholar] [CrossRef]
- Taune, V.; Skeppholm, M.; Agren, A.; Gryfelt, G.; Malmstrom, R.E.; Van Ryn, J.; Wallén, H. Rapid determination of anticoagulant effects of dabigatran in whole blood with rotational thromboelastometry and thrombin-based trigger. J. Thromb. Haemost. 2018, 16, 2462–2470. [Google Scholar] [CrossRef] [Green Version]
- Seyve, L.; Richarme, C.; Polack, B.; Marlu, R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int. J. Lab. Hematol. 2018, 40, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Mahamad, S.; Chaudhry, H.; Nisenbaum, R.; McFarlan, A.; Rizoli, S.; Ackery, L.; Sholzberg, M. Exploring the effect of factor Xa inhibitors on rotational thromboelastometry: A case series of bleeding patients. J. Thromb. Thrombol. 2019, 47, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Escolar, G.; Fernandez-Gallego, V.; Arellano-Rodrigo, E.; Roquer, J.; Reverter, J.C.; Sanz, V.V.; Molina, P.; Lopez-Vilchez, I.; Diaz-Ricart, M.; Galan, A.M. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates significance of studies in vitro with circulating human blood. PLoS ONE 2013, 8, e78696. [Google Scholar] [CrossRef]
- Adelmann, D.; Wiegele, A.; Wohlgemuth, R.K.; Koch, S.; Frantal, S.; Quehenberger, P.; Scharbert, G.; Kozek-Langenecker, S.; Schaden, E. Measuring the activity of apixaban and rivaroxaban with rotational thromboelastometry. Thromb. Res. 2014, 134, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Pailleret, C.; Jourdi, G.; Siguret, V.; Gouin-Thibault, I.; Gandrille, S.; Stefanian, A.; Curis, E.; Golmard, J.-L.; Gaussem, P.; Le Bonniec, B.; et al. Modified ROTEM for the detection of rivaroxaban and apixaban anticoagulant activity in whole blood. Eur. J. Anaesthesiol. 2019, 35, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, E.; Katogiannis, K.; Ikonomidis, I.; Giallouros, G.; Nikolopuolos, G.K.; Rapti, E.; Taichert, M.; Pantavou, K.; Gialeraki, A.; Kousathana, F.; et al. Laboratory assessment of the anticoagulant activity of apixaban in patients with nonvalvular atrial fibrillation. Clin. Appl. Thromb. Hemost. 2018, 24, 194S–201S. [Google Scholar] [CrossRef] [Green Version]
- Schenk, B.; Wurtinger, P.; Streif, W.; Sturm, W.; Fries, D.; Bachler, M. Ex vivo reversal of effects of rivaroxaban evaluated using thromboelastometry and thrombin generation assay. Br. J. Anaesth. 2016, 117, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Casutt, M.; Konrad, C.; Schuepfer, G. Effect of rivaroxaban on blood coagulation using the viscoelastic coagulation test ROTEM. Anaesthesist 2012, 61, 948–953. [Google Scholar] [CrossRef]
- Chojnowski, K.; Gorski, T.; Robak, M.; Trelinski, J. Effects of rivaroxaban therapy on ROTEM coagulation parameters in patients with venous thromboembolism. Adv. Clin. Exp. Med. 2015, 24, 995–1000. [Google Scholar] [CrossRef]
- Oswald, E.; Velik-Salchnera, C.; Innerhofera, P.; Taubera, H.; Auckenthalerb, T.; Ulmerc, H.; Streifd, W. Results of rotational thromboelastometry, coagulation activation markers and thrombin generation assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: A prospective cohort study. Blood Coagul. Fibrinol. 2015, 26, 136–144. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Voith, B.; Zuehlsdorf, M.; Wensing, G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-79-39, an oral, direct factor Xa inhibitor. Clin. Pharmacol. Ther. 2005, 78, 412–421. [Google Scholar] [CrossRef]
- Perzborn, E.; Heitmeier, S.; Laux, V.; Buchmuller, A. Reversal of rivaroxaban-induced anticoagulation with prothrombin complex concentrate, activated prothrombin complex concentrate and recombinant activated factor VII in vitro. Thromb. Res. 2014, 133, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Fontana, P.; Alberio, L.; Angelillo-Scherrer, A.; Asmis, L.A.; Korte, W.; Mendez, A.; Schmid, P.; Stricker, H.; Studt, J.-D.; Tsakiris, D.A.; et al. Impact of rivaroxaban on point-of-care assays. Thromb. Res. 2017, 153, 65–70. [Google Scholar] [CrossRef]
- Havrdova, M.; Saari, T.I.; Jalonen, J.J.; Peltoniemi, M.; Kurkela, M.; Vahlberg, T.; Tienhaara, A.; Backman, J.Y.; Olkkola, K.T.; Schramko, A.; et al. Relationship of edoxaban plasma concentration of blood coagulation in healthy volunteers using standard laboratory tests and viscoelastic analysis. J. Clin. Pharm. 2021, 61, 522–530. [Google Scholar] [CrossRef]
- Honickel, M.; Braunschweig, T.; van Ryn, J.; ten Cate, H.; Spronk, H.M.H.; Roissant, R.; Grottke, O. Prothrombin complex concentrate is effective in treating the anticoagulant effects of dabigatran in a porcine polytrauma model. Anesthesiology 2015, 123, 1350–1361. [Google Scholar] [CrossRef]
- Grottke, O.; van Ryn, J.; Spronk, H.M.; Rossaint, R. Prothrombin complex concentrates and a specific antidote to dabigatran are effective ex-vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit. Care 2014, 18, R27. [Google Scholar] [CrossRef] [Green Version]
- Dinkelaar, J.; Patiwael, S.; Harenberg, J.; Leyte, A.; Brinkman, H.J. Global coagulation tests: Their applicability for measuring direct factors Xa and thrombin inhibition and reversal of anticoagulation by prothrombin complex concentrate. Clin. Chem. Lab. Med. 2014, 52, 1615–1623. [Google Scholar] [CrossRef]
- Honickel, M.; Treutler, S.; van Ryn, J.; Tillmann, S.; Roissant, R.; Grottke, O. Reversal of dabigatran anticoagulation ex vivo: Porcine study comparing prothrombin complex concentrates and idarucizumab. Thromb. Haemost. 2015, 113, 728–740. [Google Scholar] [CrossRef]
- Akman, N.; Braunschweig, T.; Honickel, M.; Schütt, K.; Schöchl, H.; Stoppe, C.; Rossaint, R.; Grottke, O. Reversal of dabigatran by intraosseous or intravenous idarucizumab in a porcine polytrauma model. Br. J. Anaesth. 2018, 120, 978–987. [Google Scholar] [CrossRef]
- Takeshita, S.; Tanaka, K.A.; Sawa, T.; Sanda, M.; Mizobe, T.; Ogawa, S. Whole blood point-of-care testing for incomplete reversal with idarucizumab in supratherapeutic dabigatran. Anesth. Analg. 2020, 130, 535–541. [Google Scholar] [CrossRef]
- Schulz, N.H.; Tran, H.Y.Y.; Bjornsen, S.; Henriksson, C.E.; Sandset, P.M.; Holme, P.A. The reversal effect of prothrombin complex concentrate (PCC), activated PCC and recombinant activated factor VII against anticoagulation of Xa inhibitor. Thromb. J. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körber, M.K.; Langer, E.; Ziemer, S.; Perzborn, E.; Gericke, C.; von Heymann, C. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: An in vitro study. Clin. Appl. Thromb. Hemost. 2014, 20, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Körber, M.K.; Langer, E.; Kaufner, L.; Sander, M.; von Heymann, C. In vitro reversal of supratherapeutic rivaroxaban levels with coagulation factor concentrates. Blood Transf. 2016, 14, 481–486. [Google Scholar]
- Bar, J.; David, A.; Khader, T.; Mulcare, M.; Tedeschi, C. Assessing coagulation by rotational thromboelastometry (ROTEM) in rivaroxaban-anticoagulated blood using hemostatic agents. Prehosp. Dis. Med. 2017, 32, 580–587. [Google Scholar] [CrossRef]
- Schenk, B.; Goerke, S.; Helbok, R.; Fries, D.; Bachler, M. Four-factor prothrombin complex concentrate improves thrombin generation and prothrombin time in patients with bleeding complications related to rivaroxaban: A single-center pilot trial. Thromb. J. 2018, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.C.; Gouin-Thibault, I.; Siguret, V.; Mordohay, A.; Samama, C.-M.; Gaussem, P.; Le Bonniec, B.; Godier, A. Multimodal assessment of non-specific hemostatic agents for apixaban reversal. J. Thromb. Haemost. 2015, 115, 426–436. [Google Scholar] [CrossRef]
- Schmidt, K.; Kruger, K.; Langer, E.; Schmutzler, M.; Johnen, E.; Wernecke, K.D.; von Heymann, C.; Körber, M.K. Reversal of apixaban induced alterations in haemostasis by different coagulation factor concentrates in patients after hip or knee replacement surgery. Blood Transf. 2019, 17, 157–162. [Google Scholar]
- Schäfer, S.T.; Otto, A.-C.; Acevedo, A.-C.; Görlinger, K.; Massberg, S.; Kammerer, T.; Groene, P. Point-of-care detection, and differentiation of anticoagulant therapy-development of thromboelastometry-guided decision-making support algorithms. Thromb. J. 2021, 19, 63. [Google Scholar] [CrossRef]

| Type of Drug | Clinical Indications | Route of Administration | Target | Peak Onset | Half-Life Elimination | Standard Coagulation Tests | Specific Coagulation Tests |
|---|---|---|---|---|---|---|---|
| UFH | VTE (prophylaxis and therapy) | Subcutaneous or intravenous | Factor IIa and Xa | 2–4 h | 1–2 h | aPTT | Anti-FXa assay |
| ACT | |||||||
| LMWH | VTE (prophylaxis and therapy) | Subcutaneous | Factor Xa (Factor IIa) (molecular weight dependent) | 3–5 h | 4–5 h | None | Anti-FXa assay |
| Fondaparinux | VTE (prophylaxis and therapy) | Subcutaneous | Factor Xa | 2 h | 15–17 h | None | Anti-FXa assay |
| Argatroban | HIT | Intravenous | Factor IIa | 1–3 h | 45 min | PT | ECT |
| aPTT | |||||||
| Bivalirudin | PCI | Intravenous | Factor IIa | 1–2 h | 25 min | PT | ECT |
| aPTT | |||||||
| VKA | NVAF, AHV, VTE | Oral | Factors II, VII, IX, X | 36–42 h | 5–7 days | PT ↑ | None |
| INR | |||||||
| Dabigatran | NVAF, VTE | Oral | Factor IIa | 2 h | 14–17 h | PT ↑ | dTT, ECT |
| aPTT ↑↑ | |||||||
| Rivaroxaban | NVAF, VTE | Oral | Factor Xa | 2–4 h | 7–11 h | PT ↑↑ | Anti-FXa assay calibrated |
| aPTT ↑ | |||||||
| Apixaban | NVAF, VTE | Oral | Factor Xa | 1–4 h | 12 h | PT (↑) | Anti-FXa assay calibrated |
| aPTT (↑) | |||||||
| Edoxaban | NVAF, VTE | Oral | Factor Xa | 1–2 h | 10–14 h | PT (↑) | Anti-FXa assay calibrated |
| aPTT ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavoni, V.; Gianesello, L.; Conti, D.; Ballo, P.; Dattolo, P.; Prisco, D.; Görlinger, K. “In Less than No Time”: Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy. J. Clin. Med. 2022, 11, 1407. https://doi.org/10.3390/jcm11051407
Pavoni V, Gianesello L, Conti D, Ballo P, Dattolo P, Prisco D, Görlinger K. “In Less than No Time”: Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy. Journal of Clinical Medicine. 2022; 11(5):1407. https://doi.org/10.3390/jcm11051407
Chicago/Turabian StylePavoni, Vittorio, Lara Gianesello, Duccio Conti, Piercarlo Ballo, Pietro Dattolo, Domenico Prisco, and Klaus Görlinger. 2022. "“In Less than No Time”: Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy" Journal of Clinical Medicine 11, no. 5: 1407. https://doi.org/10.3390/jcm11051407
APA StylePavoni, V., Gianesello, L., Conti, D., Ballo, P., Dattolo, P., Prisco, D., & Görlinger, K. (2022). “In Less than No Time”: Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy. Journal of Clinical Medicine, 11(5), 1407. https://doi.org/10.3390/jcm11051407






