Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy
Abstract
:1. Introduction
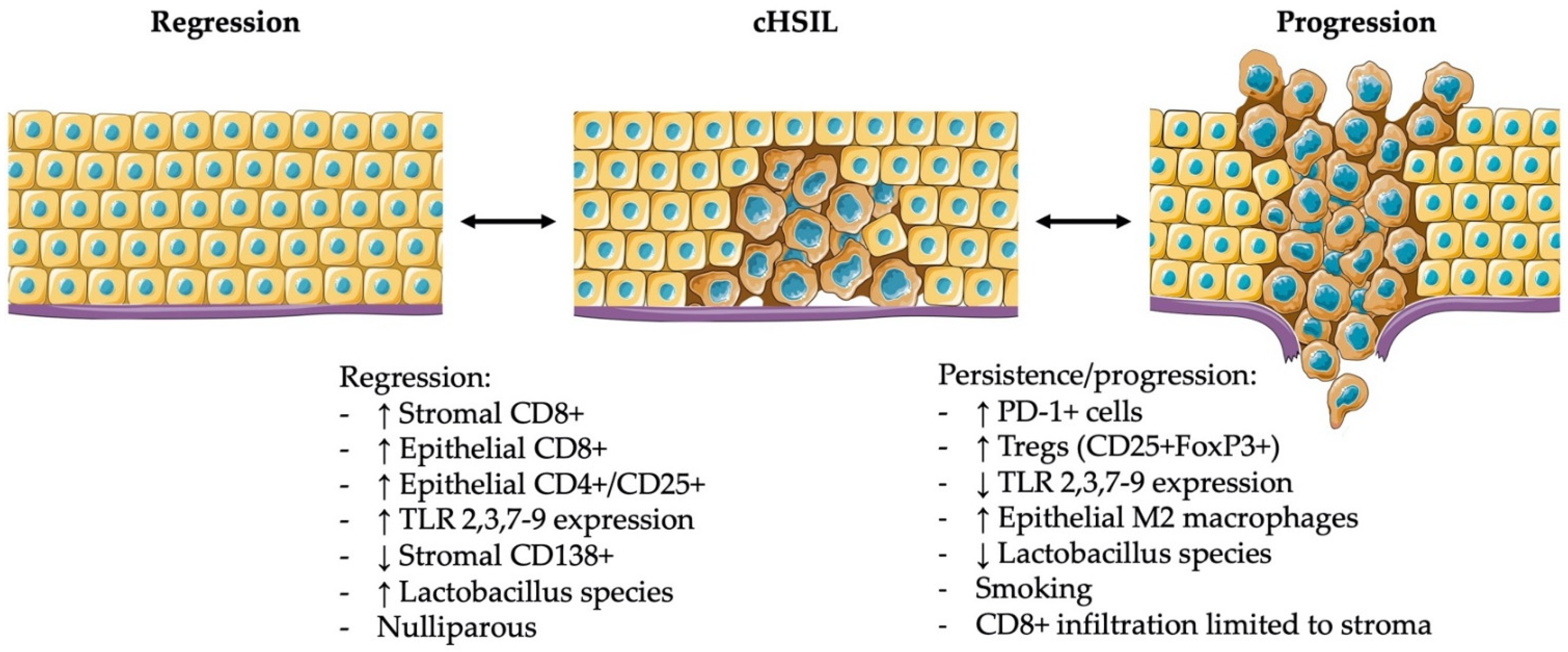
2. The Importance of Immune Infiltrates in the Microenvironment of cLSIL and cHSIL in the Natural Course of the Disease
2.1. Innate Immune Responses in cLSIL and cHSIL
2.1.1. Antigen Presenting Cells
2.1.2. Macrophages
2.2. Adaptive Immune Responses in cLSIL and cHSIL
2.2.1. T Cells
2.2.2. Immune Evading Mechanisms during Disease Progression and Persistence
3. Importance of Clinical Parameters and HPV Genotype in the Natural History of Disease in Relation to the Immune System
4. Modulation of the Local Microenvironment by Immunotherapy
4.1. Imiquimod
4.2. Therapeutic HPV Vaccination
4.3. Other Topical Treatments
5. Conclusions
6. Author Commentary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khieu, M.; Butler, S. High Grade Squamous Intraepithelial Lesion. In Definitions; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Woodman, C.B.J.; Collins, S.I.; Young, L.S. The Natural History of Cervical HPV Infection: Unresolved Issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Mustafa, R.A.; Schünemann, H.J.; Arbyn, M.; Blumenthal, P.D.; Cain, J.; Chirenje, M.; Denny, L.; de Vuyst, H.; Eckert, L.O.; et al. World Health Organization Guidelines for Treatment of Cervical Intraepithelial Neoplasia 2-3 and Screen-and-Treat Strategies to Prevent Cervical Cancer. Int. J. Gynecol. Obstet. 2016, 132, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Koutsky, L. Epidemiology of Genital Human Papillomavirus Infection. Am. J. Med. 1997, 102, 3–8. [Google Scholar] [CrossRef]
- Baseman, J.G.; Koutsky, L.A. The Epidemiology of Human Papillomavirus Infections. J. Clin. Virol. 2005, 32, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.F.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural History of Cervicovaginal Papillomavirus Infection in Young Women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Lindsay, L.; Pimenta, J.M.; Poole, C.; Jenkins, D.; Smith, J.S. Persistent Human Papillomavirus Infection and Cervical Neoplasia: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2008, 168, 123–137. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, S.H.; Palefsky, J.M. Human Immunodeficiency Virus and Human Papilloma Virus—Why HPV-Induced Lesions Do Not Spontaneously Resolve and Why Therapeutic Vaccination Can Be Successful. J. Transl. Med. 2009, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human Papillomavirus and Cervical Cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Bosch, F.X.; Burchell, A.N.; Schiffman, M.; Giuliano, A.R.; de Sanjose, S.; Bruni, L.; Tortolero-Luna, G.; Kjaer, S.K.; Muñoz, N. Epidemiology and Natural History of Human Papillomavirus Infections and Type-Specific Implications in Cervical Neoplasia. Vaccine 2008, 26, K1–K16. [Google Scholar] [CrossRef]
- Wheeler, C.M. Natural History of Human Papillomavirus Infections, Cytologic and Histologic Abnormalities, and Cancer. Obstet. Gynecol. Clin. N. Am. 2008, 35, 519–536. [Google Scholar] [CrossRef]
- Clifford, G.; Franceschi, S.; Diaz, M.; Muñoz, N.; Villa, L.L. Chapter 3: HPV Type-Distribution in Women with and without Cervical Neoplastic Diseases. Vaccine 2006, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kjær, S.K.; Frederiksen, K.; Munk, C.; Iftner, T. Long-Term Absolute Risk of Cervical Intraepithelial Neoplasia Grade 3 or Worse Following Human Papillomavirus Infection: Role of Persistence. J. Natl. Cancer Inst. 2010, 102, 1478–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snijders, P.J.F.; Steenbergen, R.D.M.; Heideman, D.A.M.; Meijer, C.J.L.M. HPV-Mediated Cervical Carcinogenesis: Concepts and Clinical Implications. J. Pathol. 2006, 208, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.L.M.; Shah, K.V. The Causal Relation between Human Papillomavirus and Cervical Cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Loopik, D.L.; Bentley, H.A.; Eijgenraam, M.N.; Inthout, J.; Bekkers, R.L.M.; Bentley, J.R. The Natural History of Cervical Intraepithelial Neoplasia Grades 1, 2, and 3: A Systematic Review and Meta-Analysis. J. Low. Genit. Tract Dis. 2021, 25, 221–231. [Google Scholar] [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.A.O.; Aaltonen, R.; Cárdenas, J.; Hernándes; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; et al. Clinical Course of Untreated Cervical Intraepithelial Neoplasia Grade 2 under Active Surveillance: Systematic Review and Meta-Analysis. BMJ 2018, 360, k499. [Google Scholar] [CrossRef] [Green Version]
- Kyrgiou, M.; Athanasiou, A.; Kalliala, I.E.J.; Paraskevaidi, M.; Mitra, A.; Martin-Hirsch, P.P.L.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Obstetric Outcomes after Conservative Treatment for Cervical Intraepithelial Lesions and Early Invasive Disease. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Spracklen, C.; Harland, K.; Stegmann, B.; Saftlas, A. Cervical Surgery for Cervical Intraepithelial Neoplasia and Prolonged Time to Conception of a Live Birth: A Case-Control Study. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 960–965. [Google Scholar] [CrossRef]
- Crane, J.M.G. Pregnancy Outcome after Loop Electrosurgical Excision Procedure: A Systematic Review. Obstet. Gynecol. 2003, 102, 1058–1062. [Google Scholar] [CrossRef]
- Loopik, D.L.; van Drongelen, J.; Bekkers, R.L.M.; Voorham, Q.J.M.; Melchers, W.J.G.; Massuger, L.F.A.G.; van Kemenade, F.J.; Siebers, A.G. Cervical Intraepithelial Neoplasia and the Risk of Spontaneous Preterm Birth: A Dutch Population-Based Cohort Study with 45,259 Pregnancy Outcomes. PLoS Med. 2021, 18, e1003665. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.O.; Possati-Resende, J.C.; Salcedo, M.P.; Schmeler, K.M.; Accorsi, G.S.; Fregnani, J.H.T.G.; Antoniazzi, M.; Pantano, N.P.; Santana, I.V.V.; Matsushita, G.M.; et al. Topical Imiquimod for the Treatment of High-Grade Squamous Intraepithelial Lesions of the Cervix: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Polterauer, S.; Natter, C.; Rahhal, J.; Hefler, L.; Tempfer, C.B.; Heinze, G.; Stary, G.; Reinthaller, A.; Speiser, P. Treatment of Cervical Intraepithelial Neoplasia with Topical Imiquimod: A Randomized Controlled Trial. Obstet. Gynecol. 2012, 120, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, C.; Fischer, A.; Clad, A. Treatment of Vulvar Intraepithelial Neoplasia with Topical 5% Imiquimod Cream. Int. J. Gynecol. Obstet. 2013, 120, 266–270. [Google Scholar] [CrossRef]
- Cokan, A.; Pakiž, M.; Serdinšek, T.; Dovnik, A.; Kodrič, T.; Repše Fokter, A.; Kavalar, R.; But, I. Comparison of Conservative Treatment of Cervical Intraepithelial Lesions with Imiquimod with Standard Excisional Technique Using LLETZ: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 5777. [Google Scholar] [CrossRef]
- Hendriks, N.; Koeneman, M.M.M.; van de Sande, A.J.; Penders, C.G.; Piek, J.; Kooreman, L.F.; van Kuijk, S.M.; Hoosemans, L.; Sep, S.J.; de Vos van Steenwijk, P.J.; et al. Topical Imiquimod Treatment of High-Grade Cervical Intraepithelial Neoplasia (TOPIC-3): A Non-Randomized Multicentre Study. J. Immunother. 2022; accepted. [Google Scholar] [CrossRef]
- Cancer Council Australia Cervical Cancer Screening Guidelines Working Party. National Cervical Screening Program: Guidelines for the Management of Screen-Detected Abnormalities, Screening in Specific Populations and Investigation of Abnormal Vaginal Bleed. Available online: https://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Screening (accessed on 21 January 2022).
- Arbyn, M.; Herbert, A.; Schenck, U.; Nieminen, P.; Jordan, J.; Mcgoogan, E.; Patnick, J.; Bergeron, C.; Baldauf, J.-J.; Klinkhamer, P.; et al. European Guidelines for Quality Assurance in Cervical Cancer Screening: Recommendations for Collecting Samples for Conventional and Liquid-Based Cytology*. Cytopathology 2007, 18, 133–139. [Google Scholar] [CrossRef]
- Arbyn, M.; Anttila, A.; Jordan, J.; Ronco, G.; Schenck, U.; Segnan, N.; Wiener, H.; Herbert, A.; von Karsa, L. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition—Summary Document. Ann. Oncol. 2010, 21, 448–458. [Google Scholar] [CrossRef]
- Cervical Screening: Programme and Colposcopy Management. Available online: https://www.gov.uk/government/publications/cervical-screening-programme-and-colposcopy-management (accessed on 21 January 2022).
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [Green Version]
- Petry, K.U.; Köchel, H.; Bode, U.; Schedel, I.; Niesert, S.; Glaubitz, M.; Maschek, H.; Kühnle, H. Human Papillomavirus Is Associated with the Frequent Detection of Warty and Basaloid High-Grade Neoplasia of the Vulva and Cervical Neoplasia among Immunocompromised Women. Gynecol. Oncol. 1996, 60, 30–34. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Paramsothy, P.; Cu-Uvin, S.; Duerr, A. Vulvar, Vaginal, and Perianal Intraepithelial Neoplasia in Women with or at Risk for Human Immunodeficiency Virus. Obstet. Gynecol. 2006, 107, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; Ma, W.; Santegoets, S.J.A.M.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; van Ham, V.J.; van Unen, V.; Koning, F.; van Egmond, S.I.; et al. Intratumoral HPV16-Specific T Cells Constitute a Type I–Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vos Van Steenwijk, P.J.; Piersma, S.J.; Welters, M.J.P.; van der Hulst, J.M.; Fleuren, G.; Hellebrekers, B.W.J.; Kenter, G.G.; van der Burg, S.H. Surgery Followed by Persistence of High-Grade Squamous Intraepithelial Lesions Is Associated with the Induction of a Dysfunctional HPV16-Specific T-Cell Response. Clin. Cancer Res. 2008, 14, 7188–7195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.L.; Sterling, J.; Damay, I.; Coleman, N.; Crawford, R.; van der Burg, S.H.; Stanley, M. Characterising the Local Immune Responses in Cervical Intraepithelial Neoplasia: A Cross-Sectional and Longitudinal Analysis. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1616–1622. [Google Scholar] [CrossRef]
- Du, H.; Xu, T.; Cui, M. CGAS-STING Signaling in Cancer Immunity and Immunotherapy. Biomed. Pharmacother. 2021, 133, 110972. [Google Scholar] [CrossRef]
- Monnier-Benoit, S.; Mauny, F.; Riethmuller, D.; Guerrini, J.S.; Cǎpîlna, M.; Félix, S.; Seillès, E.; Mougin, C.; Prétet, J.L. Immunohistochemical Analysis of CD4+ and CD8+ T-Cell Subsets in High Risk Human Papillomavirus-Associated Pre-Malignant and Malignant Lesions of the Uterine Cervix. Gynecol. Oncol. 2006, 102, 22–31. [Google Scholar] [CrossRef]
- Bottley, G.; Watherston, O.G.; Hiew, Y.L.; Norrild, B.; Cook, G.P.; Blair, G.E. High-Risk Human Papillomavirus E7 Expression Reduces Cell-Surface MHC Class I Molecules and Increases Susceptibility to Natural Killer Cells. Oncogene 2008, 27, 1794–1799. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.M.-L.; Man, S.; Evans, A.S.; Borysiewicz, L.K. Infiltration of Cervical Cancer Tissue with Human Papilloma Virus-Specific Cytotoxic T-Lymphocytes. Immunol. Lett. 1997, 56, 455. [Google Scholar] [CrossRef]
- Um, S.J.; Rhyu, J.W.; Kim, E.J.; Jeon, K.C.; Hwang, E.S.; Park, J.S. Abrogation of IRF-1 Response by High-Risk HPV E7 Protein in Vivo. Cancer Lett. 2002, 179, 205–212. [Google Scholar] [CrossRef]
- Woo, Y.L.; van den Hende, M.; Sterling, J.C.; Coleman, N.; Crawford, R.A.F.; Kwappenberg, K.M.C.; Stanley, M.A.; van der Burg, S.H. A Prospective Study on the Natural Course of Low-Grade Squamous Intraepithelial Lesions and the Presence of HPV16 E2-, E6- And E7-Specific T-Cell Responses. Int. J. Cancer 2010, 126, 133–141. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, A.; Weinberg, V.; Darragh, T.; Smith-McCune, K. Evolving Immunosuppressive Microenvironment during Human Cervical Carcinogenesis. Mucosal Immunol. 2008, 1, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.H.; Norval, M.; Howie, S.E.M. Expression of Major Histocompatibility Class II Antigens by Langerhans’ Cells in Cervical Intraepithelial Neoplasia. J. Clin. Pathol. 1988, 41, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, S.K.; Jenkins, D.; Maddox, P.; Campion, M.; Singer, A. Subpopulations of Langerhans’ Cells in Cervical Neoplasia. BJOG Int. J. Obstet. Gynaecol. 1987, 94, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Viac, J.; Guérin-Reverchoni, I.; Chardonnet, Y.; Brémond, A. Langerhans Cells and Epithelial Cell Modifications in Cervical Intraepithelial Neoplasia: Correlation with Human Papillomavirus Infection. Immunobiology 1990, 180, 328–338. [Google Scholar] [CrossRef]
- Mota, F.F.; Rayment, N.B.; Kanan, J.H.; Singer, A.; Chain, B.M. Differential Regulation of HLA-DQ Expression by Keratinocytes and Langerhans Cells in Normal and Premalignant Cervical Epithelium. Tissue Antigens 1998, 52, 286–293. [Google Scholar] [CrossRef]
- Al-Saleh, W.; Giannini, S.L.; Jacobs, N.; Moutschen, M.; Doyen, J.; Boniver, J.; Delvenne, P. Correlation of T-Helper Secretory Differentiation and Types of Antigen- Presenting Cells in Squamous Intraepithelial Lesions of the Uterine Cervix. J. Pathol. 1998, 184, 283–290. [Google Scholar] [CrossRef]
- Utrera-Barillas, D.; Castro-Manrreza, M.; Castellanos, E.; Gutiérrez-Rodríguez, M.; Arciniega-Ruíz de Esparza, O.; García-Cebada, J.; Velazquez, J.R.; Flores-Reséndiz, D.; Hernández-Hernández, D.; Benítez-Bribiesca, L. The Role of Macrophages and Mast Cells in Lymphangiogenesis and Angiogenesis in Cervical Carcinogenesis. Exp. Mol. Pathol. 2010, 89, 190–196. [Google Scholar] [CrossRef]
- Hammes, L.S.; Tekmal, R.R.; Naud, P.; Edelweiss, M.I.; Kirma, N.; Valente, P.T.; Syrjänen, K.J.; Cunha-Filho, J.S. Macrophages, Inflammation and Risk of Cervical Intraepithelial Neoplasia (CIN) Progression-Clinicopathological Correlation. Gynecol. Oncol. 2007, 105, 157–165. [Google Scholar] [CrossRef]
- Chen, X.J.; Han, L.F.; Wu, X.G.; Wei, W.F.; Wu, L.F.; Yi, H.Y.; Yan, R.M.; Bai, X.Y.; Zhong, M.; Yu, Y.H.; et al. Clinical Significance of CD163+ and CD68+ Tumor-Associated Macrophages in High-Risk HPV-Related Cervical Cancer. J. Cancer 2017, 8, 3868–3875. [Google Scholar] [CrossRef] [Green Version]
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V.V.; Stanley, M.; Wentzensen, N. Infiltrating T-Cell Markers in Cervical Carcinogenesis: A Systematic Review and Meta-Analysis. Br. J. Cancer 2021, 124, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Jayshree, R.S. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions—Evidence for Estrogen as an Immunomodulator. Front. Cell. Infect. Microbiol. 2021, 11, 1–25. [Google Scholar] [CrossRef]
- Wang, Y.; He, M.; Zhang, G.; Cao, K.; Yang, M.; Zhang, H.; Liu, H. The Immune Landscape during the Tumorigenesis of Cervical Cancer. Cancer Med. 2021, 10, 2380–2395. [Google Scholar] [CrossRef]
- Adurthi, S.; Krishna, S.; Mukherjee, G.; Bafna, U.D.; Devi, U.; Jayshree, R.S. Regulatory T Cells in a Spectrum of HPV-Induced Cervical Lesions: Cervicitis, Cervical Intraepithelial Neoplasia and Squamous Cell Carcinoma. Am. J. Reprod. Immunol. 2008, 60, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sahebali, S.; van den Eynden, G.; Murta, E.F.; Michelin, M.A.; Cusumano, P.; Petignat, P.; Bogers, J.J. Stromal Issues in Cervical Cancer: A Review of the Role and Function of Basement Membrane, Stroma, Immune Response and Angiogenesis in Cervical Cancer Development. Eur. J. Cancer Prev. 2010, 19, 204–215. [Google Scholar] [CrossRef]
- Mezache, L.; Paniccia, B.; Nyinawabera, A.; Nuovo, G.J. Enhanced Expression of PD L1 in Cervical Intraepithelial Neoplasia and Cervical Cancers. Mod. Pathol. 2015, 28, 1594–1602. [Google Scholar] [CrossRef]
- Yang, W.; Song, Y.; Lu, Y.L.; Sun, J.Z.; Wang, H.W. Increased Expression of Programmed Death (PD)-1 and Its Ligand PD-L1 Correlates with Impaired Cell-Mediated Immunity in High-Risk Human Papillomavirus-Related Cervical Intraepithelial Neoplasia. Immunology 2013, 139, 513–522. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, H.; Morihara, J.; Stern, J.; Yu, M.; Kiviat, N.; Hellstrom, I.; Hellstrom, K.E. Th2 Type Inflammation Promotes the Gradual Progression of HPV-Infected Cervical Cells to Cervical Carcinoma. Gynecol. Oncol. 2012, 127, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front. Immunol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The Inhibitory Cytokine IL-35 Contributes to Regulatory T-Cell Function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Powrie, F.; Carlino, J.; Leach, M.W.; Mauze, S.; Coffman, R.L. A Critical Role for Transforming Growth Factor-β but Not Interleukin 4 in the Suppression of T Helper Type 1-Mediated Colitis by CD45RBlow CD4+ T Cells. J. Exp. Med. 1996, 183, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berraondo, P.; Minute, L.; Ajona, D.; Corrales, L.; Melero, I.; Pio, R. Innate Immune Mediators in Cancer: Between Defense and Resistance. Immunol. Rev. 2016, 274, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Hagerling, C.; Casbon, A.-J.; Werb, Z. Balancing the Innate Immune System in Tumor Development. Trends Cell Biol. 2015, 25, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Palucka, K.; Banchereau, J. Dendritic Cells: A Link between Innate and Adaptive Immunity. J. Clin. Immunol. 1999, 19, 12–25. [Google Scholar] [CrossRef]
- Origoni, M.; Parma, M.; Dell’Antonio, G.; Gelardi, C.; Stefani, C.; Salvatore, S.; Candiani, M. Prognostic Significance of Immunohistochemical Phenotypes in Patients Treated for High-Grade Cervical Intraepithelial Neoplasia. BioMed Res. Int. 2013, 2013, 831907. [Google Scholar] [CrossRef]
- Johnson, T.S.; Munn, D.H. Host Indoleamine 2,3-Dioxygenase: Contribution to Systemic Acquired Tumor Tolerance. Immunol. Investig. 2012, 41, 765–797. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Shannon, B.; Yi, T.J.; Perusini, S.; Gajer, P.; Ma, B.; Humphrys, M.S.; Thomas-Pavanel, J.; Chieza, L.; Janakiram, P.; Saunders, M.; et al. Association of HPV Infection and Clearance with Cervicovaginal Immunology and the Vaginal Microbiota. Mucosal Immunol. 2017, 10, 1310–1319. [Google Scholar] [CrossRef] [Green Version]
- Fausch, S.C.; da Silva, D.M.; Rudolf, M.P.; Kast, W.M. Human Papillomavirus Virus-Like Particles Do Not Activate Langerhans Cells: A Possible Immune Escape Mechanism Used by Human Papillomaviruses. J. Immunol. 2002, 169, 3242–3249. [Google Scholar] [CrossRef] [Green Version]
- Hibma, M.H. The Immune Response to Papillomavirus During Infection Persistence and Regression. Open Virol. J. 2013, 6, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Daud, I.I.; Scott, M.E.; Ma, Y.; Shiboski, S.; Farhat, S.; Moscicki, A.-B. Association between Toll-like Receptor Expression and Human Papillomavirus Type 16 Persistence. Int. J. Cancer 2011, 128, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halec, G.; Scott, M.E.; Farhat, S.; Darragh, T.M.; Moscicki, A.B. Toll-like Receptors: Important Immune Checkpoints in the Regression of Cervical Intra-Epithelial Neoplasia 2. Int. J. Cancer 2018, 143, 2884–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, F.; Vacchelli, E.; Obrist, F.; Eggermont, A.; Galon, J.; Fridman, W.H.; Cremer, I.; Tartour, E.; Zitvogel, L.; Kroemer, G.; et al. Trial Watch: Adoptive Cell Transfer for Anticancer Immunotherapy. OncoImmunology 2014, 3, e28344. [Google Scholar] [CrossRef] [Green Version]
- de Vos Van Steenwijk, P.J.; Ramwadhdoebe, T.H.; Goedemans, R.; Doorduijn, E.M.; van Ham, J.J.; Gorter, A.; van Hall, T.; Kuijjer, M.L.; van Poelgeest, M.I.E.; van der Burg, S.H.; et al. Tumor-Infiltrating CD14-Positive Myeloid Cells and CD8-Positive T-Cells Prolong Survival in Patients with Cervical Carcinoma. Int. J. Cancer 2013, 133, 2884–2894. [Google Scholar] [CrossRef]
- Ong, C.B.; Brandenberger, C.; Kiupel, M.; Kariagina, A.; Langohr, I.M. Immunohistochemical Characterization and Morphometric Analysis of Macrophages in Rat Mammary Tumors. Vet. Pathol. 2015, 52, 414–418. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-Associated Macrophages: From Basic Research to Clinical Application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology: Functions and Disorders of the Immune System. In Basic Immunology: Functions and Disorders of the Immune System; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–22. ISBN 978-0-323-54943-1. [Google Scholar]
- Øvestad, I.T.; Gudlaugsson, E.; Skaland, I.; Malpica, A.; Kruse, A.J.; Janssen, E.A.M.; Baak, J.P.A. Local Immune Response in the Microenvironment of CIN2-3 with and without Spontaneous Regression. Mod. Pathol. 2010, 23, 1231–1240. [Google Scholar] [CrossRef]
- Lord, S.J.; Rajotte, R.V.; Korbutt, G.S.; Bleackley, R.C. Granzyme B: A Natural Born Killer. Immunol. Rev. 2003, 193, 31–38. [Google Scholar] [CrossRef]
- Trimble, C.L.; Clark, R.A.; Thoburn, C.; Hanson, N.C.; Tassello, J.; Frosina, D.; Kos, F.; Teague, J.; Jiang, Y.; Barat, N.C.; et al. Human Papillomavirus 16-Associated Cervical Intraepithelial Neoplasia in Humans Excludes CD8 T Cells from Dysplastic Epithelium. J. Immunol. 2010, 185, 7107–7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortekaas, K.E.; Santegoets, S.J.; Abdulrahman, Z.; van Ham, V.J.; van der Tol, M.; Ehsan, I.; van Doorn, H.C.; Bosse, T.; van Poelgeest, M.I.E.; van der Burg, S.H. High Numbers of Activated Helper T Cells Are Associated with Better Clinical Outcome in Early Stage Vulvar Cancer, Irrespective of HPV or P53 Status. J. ImmunoTher. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulrahman, Z.; Kortekaas, K.E.; de Vos Van Steenwijk, P.J.; van der Burg, S.H.; van Poelgeest, M.I.E. The Immune Microenvironment in Vulvar (Pre)Cancer: Review of Literature and Implications for Immunotherapy. Expert Opin. Biol. Ther. 2018, 18, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Kawana, K.; Tomio, K.; Yamashita, A.; Taguchi, A.; Miura, S.; Adachi, K.; Nagamatsu, T.; Nagasaka, K.; Matsumoto, Y.; et al. The Prevalence Of Cervical Regulatory T Cells in HPV-Related Cervical Intraepithelial Neoplasia (CIN) Correlates Inversely with Spontaneous Regression of CIN. Am. J. Reprod. Immunol. 2013, 69, 134–141. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- van Esch, E.M.; Welters, M.J.; Jordanova, E.S.; Trimbos, J.B.M.; van der Burg, S.H.; van Poelgeest, M.I. Treatment Failure in Patients with HPV 16-Induced Vulvar Intraepithelial Neoplasia: Understanding Different Clinical Responses to Immunotherapy. Expert Rev. Vaccines 2012, 11, 821–840. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.L.; Flavell, R.A. Transforming Growth Factor-β Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Liu, K.; Huang, A.; Nie, J.; Tan, J.; Xing, S.; Qu, Y.; Jiang, K. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front. Immunol. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Prata, T.T.M.; Bonin, C.M.; Ferreira, A.M.T.; Padovani, C.T.J.; Fernandes, C.E.; Machado, A.P.; Tozetti, I.A. Local Immunosuppression Induced by High Viral Load of Human Papillomavirus: Characterization of Cellular Phenotypes Producing Interleukin-10 in Cervical Neoplastic Lesions. Immunology 2015, 146, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Peghini, B.C.; Abdalla, D.R.; Barcelos, A.C.M.; Teodoro, L.D.G.V.L.; Murta, E.F.C.; Michelin, M.A. Local Cytokine Profiles of Patients with Cervical Intraepithelial and Invasive Neoplasia. Hum. Immunol. 2012, 73, 920–926. [Google Scholar] [CrossRef]
- Li, S.; Gowans, E.J.; Chougnet, C.; Plebanski, M.; Dittmer, U. Natural Regulatory T Cells and Persistent Viral Infection. J. Virol. 2008, 82, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglennon, G.A.; McIntosh, P.; Doorbar, J. Persistence of Viral DNA in the Epithelial Basal Layer Suggests a Model for Papillomavirus Latency Following Immune Regression. Virology 2011, 414, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravitt, P.E. The Known Unknowns of HPV Natural History. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef] [Green Version]
- Trimble, C.L.; Piantadosi, S.; Gravitt, P.; Ronnett, B.; Pizer, E.; Elko, A.; Wilgus, B.; Yutzy, W.; Daniel, R.; Shah, K.; et al. Spontaneous Regression of High-Grade Cervical Dysplasia: Effects of Human Papillomavirus Type and HLA Phenotype Human Cancer Biology. Clin Cancer Res. 2005, 11, 4717–4723. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, M.A.L.; Nikolopoulos, M.; Garner, J.E.; Adib, T.R.; Mukhopadhyay, D.; Rains, J.S.; Harper, C.A.; Wuntakal, R. Conservative Management of Cervical Intraepithelial Neoplasia Grade 2 (CIN2) in Women under 30 Years of Age: A Cohort Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 267–273. [Google Scholar] [CrossRef]
- Lee, M.H.; Finlayson, S.J.; Gukova, K.; Hanley, G.; Miller, D.; Sadownik, L.A. Outcomes of Conservative Management of High Grade Squamous Intraepithelial Lesions in Young Women. J. Low. Genit. Tract Dis. 2018, 22, 212–218. [Google Scholar] [CrossRef]
- Koeneman, M.M.; van Lint, F.H.M.; van Kuijk, S.M.J.; Smits, L.J.M.; Kooreman, L.F.S.; Kruitwagen, R.F.P.M.; Kruse, A.J. A Prediction Model for Spontaneous Regression of Cervical Intraepithelial Neoplasia Grade 2, Based on Simple Clinical Parameters. Hum. Pathol. 2017, 59, 62–69. [Google Scholar] [CrossRef]
- Loopik, D.L.; Doucette, S.; Bekkers, R.L.M.; Bentley, J.R. Regression and Progression Predictors of CIN2 in Women Younger than 25 Years. J. Low. Genit. Tract Dis. 2016, 20, 213–217. [Google Scholar] [CrossRef]
- Kingnate, C.; Supoken, A.; Kleebkaow, P.; Chumworathayi, B.; Luanratanakorn, S.; Kietpeerakool, C. Is Age an Independent Predictor of High-Grade Histopathology in Women Referred for Colposcopy after Abnormal Cervical Cytology? Asian Pac. J. Cancer Prev. 2015, 16, 7231–7235. [Google Scholar] [CrossRef] [Green Version]
- Moscicki, A.B.; Ma, Y.; Wibbelsman, C.; Darragh, T.M.; Powers, A.; Farhat, S.; Shiboski, S. Rate of and Risks for Regression of Cervical Intraepithelial Neoplasia 2 in Adolescents and Young Women. Obstet. Gynecol. 2010, 116, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, T.; Kay, M.M.B. Age Influence on the Immune System. Adv. Immunol. 1980, 29, 287–330. [Google Scholar] [CrossRef] [PubMed]
- Giannella, L.; Giorgi Rossi, P.; Delli Carpini, G.; di Giuseppe, J.; Bogani, G.; Gardella, B.; Monti, E.; Liverani, C.A.; Ghelardi, A.; Insinga, S.; et al. Age-Related Distribution of Uncommon HPV Genotypes in Cervical Intraepithelial Neoplasia Grade 3. Gynecol. Oncol. 2021, 161, 741–747. [Google Scholar] [CrossRef]
- Gadducci, A.; Barsotti, C.; Cosio, S.; Domenici, L.; Genazzani, A.R. Smoking Habit, Immune Suppression, Oral Contraceptive Use, and Hormone Replacement Therapy Use and Cervical Carcinogenesis: A Review of the Literature. Gynecol. Endocrinol. 2011, 27, 597–604. [Google Scholar] [CrossRef]
- de Mello Silva, M.V.; Coutinho, I.C.; Heráclio, S.D.A.; Fittipaldi, H.M.; Katz, L. Factors Associated with the Persistence/Recurrence of CIN2/3 in Women Submitted to Loop Electrosurgical Excision Procedure in a Teaching Hospital in Northeastern Brazil: A Case-Control Study. J. Low. Genit. Tract Dis. 2014, 18, 286–290. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oki, A.; Furuta, R.; Maeda, H.; Yasugi, T.; Takatsuka, N.; Hirai, Y.; Mitsuhashi, A.; Fujii, T.; Iwasaka, T.; et al. Tobacco Smoking and Regression of Low-Grade Cervical Abnormalities. Cancer Sci. 2010, 101, 2065–2073. [Google Scholar] [CrossRef]
- Poppe, W.A.J.; Ide, P.S.; Drijkoningen, M.P.G.; Lauweryns, J.M.; van Assche, A. Tobacco Smoking Impairs the Local Immunosurveillance in the Uterine Cervix. Gynecol. Obstet. Investig. 1995, 39, 34–38. [Google Scholar] [CrossRef]
- Barton, S.E.; Jenkins, D.; Cuzick, J.; Maddox, P.H.; Edwards, R.; Singer, A. Effect of Cigarette Smoking on Cervical Epithelial Immunity: A Mechanism for Neoplastic Change? Lancet 1988, 332, 652–654. [Google Scholar] [CrossRef]
- Lam, J.U.H.; Rebolj, M.; Dugué, P.A.; Bonde, J.; von Euler-Chelpin, M.; Lynge, E. Condom Use in Prevention of Human Papillomavirus Infections and Cervical Neoplasia: Systematic Review of Longitudinal Studies. J. Med. Screen. 2014, 21, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Munk, A.C.; Gudlaugsson, E.; Ovestad, I.T.; Lovslett, K.; Fiane, B.; Hidle, B.V.D.; Kruse, A.J.; Skaland, I.; Janssen, E.A.M.; Baak, J.P.A. Interaction of Epithelial Biomarkers, Local Immune Response and Condom Use in Cervical Intraepithelial Neoplasia 2–3 Regression. Gynecol. Oncol. 2012, 127, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, C.J.A.; Bleeker, M.C.G.; van den Brule, A.J.C.; Voorhorst, F.J.; Snijders, P.J.F.; Berkhof, J.; Westenend, P.J.; Meijer, C.J.L.M. Condom Use Promotes Regression of Cervical Intraepithelial Neoplasia and Clearance of Human Papillomavirus: A Randomized Clinical Trial. Int. J. Cancer 2003, 107, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Munk, A.C.; Gudlaugsson, E.; Malpica, A.; Fiane, B.; Løvslett, K.I.; Kruse, A.J.; Øvestad, I.T.; Voorhorst, F.; Janssen, E.A.M.; Baak, J.P.A. Consistent Condom Use Increases the Regression Rate of Cervical Intraepithelial Neoplasia 2–3. PLoS ONE 2012, 7, e45114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeneman, M.M.; Hendriks, N.; Kooreman, L.F.S.; Winkens, B.; Kruitwagen, R.F.P.M.; Kruse, A.J. Prognostic Factors for Spontaneous Regression of High-Risk Human Papillomavirus-Positive Cervical Intra-Epithelial Neoplasia Grade 2. Int. J. Gynecol. Cancer 2019, 29, 1003–1009. [Google Scholar] [CrossRef]
- Jensen, K.E.; Schmiedel, S.; Norrild, B.; Frederiksen, K.; Iftner, T.; Kjaer, S.K. Parity as a Cofactor for High-Grade Cervical Disease among Women with Persistent Human Papillomavirus Infection: A 13-Year Follow-Up. Br. J. Cancer 2013, 108, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, N.; Franceschi, S.; Bosetti, C.; Moreno, V.; Herrero, R.; Smith, J.S.; Shah, K.V.; Meijer, C.J.; Bosch, F.X. Role of Parity and Human Papillomavirus in Cervical Cancer: The IARC Multicentric Case-Control Study. Lancet 2002, 359, 1093–1101. [Google Scholar] [CrossRef]
- Castle, P.E.; Schiffman, M.; Wheeler, C.M.; Solomon, D. Evidence for Frequent Regression of Cervical Intraepithelial Neoplasia–Grade 2. Obstet. Gynecol. 2009, 113, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Kulmala, S.M.A.; Syrjänen, S.M.; Gyllensten, U.B.; Shabalova, I.P.; Petrovichev, N.; Tosi, P.; Syrjänen, K.J.; Johansson, B.C. Early Integration of High Copy HPV16 Detectable in Women with Normal and Low Grade Cervical Cytology and Histology. J. Clin. Pathol. 2006, 59, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.W.; Chao, S.L.; Lee, B.H. Integration of Human Papillomavirus Type-16 and Type-18 Is a Very Early Event in Cervical Carcinogenesis. J. Clin. Pathol. 2008, 61, 627–631. [Google Scholar] [CrossRef]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2021, 88, 187–197. [Google Scholar] [CrossRef]
- Singer, M.; Borg, M.; Ouburg, S.; Morré, S.A. The Relation of the Vaginal Microbiota to Early Pregnancy Development during in Vitro Fertilization Treatment—A Meta-Analysis. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.-B. Does the Vaginal Microbiota Play a Role in the Development of Cervical Cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliana, N.C.A.; Suiters, M.J.M.; Al-Nasiry, S.; Morré, S.A.; Peters, R.P.H.; Ambrosino, E. The Association Between Vaginal Microbiota Dysbiosis, Bacterial Vaginosis, and Aerobic Vaginitis, and Adverse Pregnancy Outcomes of Women Living in Sub-Saharan Africa: A Systematic Review. Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The Microbiome and Gynaecological Cancer Development, Prevention and Therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; MacIntyre, D.A.; Paraskevaidi, M.; Moscicki, A.-B.; Mahajan, V.; Smith, A.; Lee, Y.S.; Lyons, D.; Paraskevaidis, E.; Marchesi, J.R.; et al. The Vaginal Microbiota and Innate Immunity after Local Excisional Treatment for Cervical Intraepithelial Neoplasia. Genome Med. 2021, 13, 176. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Bacterial Vaginosis and Health-Associated Bacteria Modulate the Immunometabolic Landscape in 3D Model of Human Cervix. Npj Biofilms Microbiomes 2021, 7, 88. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [Green Version]
- Abdulrahman, Z.; de Miranda, N.F.C.C.; Hellebrekers, B.W.J.; de Vos van Steenwijk, P.J.; van Esch, E.M.G.; van der Burg, S.H.; van Poelgeest, M.I.E. A Pre-Existing Coordinated Inflammatory Microenvironment is Associated with Complete Response of Vulvar High-Grade Squamous Intraepithelial Lesions to Different Forms of Immunotherapy. Int. J. Cancer 2020, 147, 2914–2923. [Google Scholar] [CrossRef]
- Abdulrahman, Z.; de Miranda, N.; van Esch, E.M.G.; de Vos Van Steenwijk, P.J.; Nijman, H.W.; Welters, J.P.M.; van Poelgeest, M.I.E.; van der Burg, S.H. Pre-Existing Inflammatory Immune Microenvironment Predicts the Clinical Response of Vulvar High-Grade Squamous Intraepithelial Lesions to Therapeutic HPV16 Vaccination. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlou, A.; van Seters, M.; Kleinjan, A.; Heijmans-Antonissen, C.; Santegoets, L.A.M.; Beckmann, I.; van Beurden, M.; Helmerhorst, T.J.M.; Blok, L.J. Imiquimod-Induced Clearance of HPV Is Associated with Normalization of Immune Cell Counts in Usual Type Vulvar Intraepithelial Neoplasia. Int. J. Cancer 2010, 127, 2831–2840. [Google Scholar] [CrossRef] [PubMed]
- Sauder, D.N. Immunomodulatory and Pharmacologic Properties of Imiquimod. J. Am. Acad. Dermatol. 2000, 43, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Gerster, J.F.; Owens, M.L.; Slade, H.B.; Tomai, M.A. Review Article Imiquimod Applied Topically: A Novel Immune Response Modifier and New Class of Drug. Int. J. Immunopharmacol. 1999, 21, 1–14. [Google Scholar] [CrossRef]
- de Witte, C.J.; van de Sande, A.J.M.; van Beekhuizen, H.J.; Koeneman, M.M.; Kruse, A.J.; Gerestein, C.G. Imiquimod in Cervical, Vaginal and Vulvar Intraepithelial Neoplasia: A Review. Gynecol. Oncol. 2015, 139, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Schön, M.P.; Schön, M. Imiquimod: Mode of Action. Br. J. Dermatol. 2007, 157, 8–13. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Gerard, C.L.; Delyon, J.; Wicky, A.; Homicsko, K.; Cuendet, M.A.; Michielin, O. Turning Tumors from Cold to Inflamed to Improve Immunotherapy Response. Cancer Treat. Rev. 2021, 101, 102227. [Google Scholar] [CrossRef]
- Chen, F.P. Efficacy of Imiquimod 5% Cream for Persistent Human Papillomavirus in Genital Intraepithelial Neoplasm. Taiwan. J. Obstet. Gynecol. 2013, 52, 475–478. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.T.; Qiu, J.T.; Wang, C.J.; Chang, S.D.; Tang, Y.H.; Wu, P.J.; Jung, S.M.; Huang, C.C.; Chou, H.H.; Jao, M.S.; et al. Topical Imiquimod Treatment for Human Papillomavirus Infection in Patients with and without Cervical/Vaginal Intraepithelial Neoplasia. Taiwan. J. Obstet. Gynecol. 2012, 51, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Pachman, D.R.; Barton, D.L.; Clayton, A.C.; McGovern, R.M.; Jefferies, J.A.; Novotny, P.J.; Sloan, J.A.; Loprinzi, C.L.; Gostout, B.S. Randomized Clinical Trial of Imiquimod: An Adjunct to Treating Cervical Dysplasia. Am. J. Obstet. Gynecol. 2012, 206, 42.e1–42.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Burg, S.H.; Arens, R.; Ossendorp, F.; van Hall, T.; Melief, C.J.M. Vaccines for Established Cancer: Overcoming the Challenges Posed by Immune Evasion. Nat. Rev. Cancer 2016, 16, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; Kenter, G.G.; Piersma, S.J.; Vloon, A.P.G.; Löwik, M.J.G.; Berends-van Der Meer, D.M.A.; Drijfhout, J.W.; Valentijn, A.R.P.M.; Wafelman, A.R.; Oostendorp, J.; et al. Induction of Tumor-Specific CD4+ and CD8+ T-Cell Immunity in Cervical Cancer Patients by a Human Papillomavirus Type 16 E6 and E7 Long Peptides Vaccine. Clin. Cancer Res. 2008, 14, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazer, I.H.; Quinn, M.; Nicklin, J.L.; Tan, J.; Perrin, L.C.; Ng, P.; O’Connor, V.M.; White, O.; Wendt, N.; Martin, J.; et al. Phase 1 Study of HPV16-Specific Immunotherapy with E6E7 Fusion Protein and ISCOMATRIXTM Adjuvant in Women with Cervical Intraepithelial Neoplasia. Vaccine 2004, 23, 172–181. [Google Scholar] [CrossRef]
- de Vos Van Steenwijk, P.J.; Ramwadhdoebe, T.H.; Löwik, M.J.G.; van der Minne, C.E.; Berends-Van Der Meer, D.M.A.; Fathers, L.M.; Valentijn, A.R.P.M.; Oostendorp, J.; Fleuren, G.J.; Hellebrekers, B.W.J.; et al. A Placebo-Controlled Randomized HPV16 Synthetic Long-Peptide Vaccination Study in Women with High-Grade Cervical Squamous Intraepithelial Lesions. Cancer Immunol. Immunother. 2012, 61, 1485–1492. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, A.M.; Nieland, J.D.; Jochmus, I.; Baur, S.; Friese, K.; Gabelsberger, J.; Gieseking, F.; Gissmann, L.; Glasschröder, B.; Grubert, T.; et al. Vaccination Trial with HPV16 L1E7 Chimeric Virus-like Particles in Women Suffering from High Grade Cervical Intraepithelial Neoplasia (CIN 2/3). Int. J. Cancer 2007, 121, 2794–2800. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, L.; Teague, J.E.; Morrow, M.P.; Jotova, I.; Wu, T.C.; Wang, C.; Desmarais, C.; Boyer, J.D.; Tycko, B.; Robins, H.S.; et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Sci. Transl. Med. 2014, 6, 221ra13. [Google Scholar] [CrossRef] [Green Version]
- van Pachterbeke, C.; Bucella, D.; Rozenberg, S.; Manigart, Y.; Gilles, C.; Larsimont, D.; vanden Houte, K.; Reynders, M.; Snoeck, R.; Bossens, M. Topical Treatment of CIN 2+ by Cidofovir: Results of a Phase II, Double-Blind, Prospective, Placebo-Controlled Study. Gynecol. Oncol. 2009, 115, 69–74. [Google Scholar] [CrossRef]
- Hurt, C.N.; Jones, S.E.F.; Madden, T.A.; Fiander, A.; Nordin, A.J.; Naik, R.; Powell, N.; Carucci, M.; Tristram, A. Recurrence of Vulval Intraepithelial Neoplasia Following Treatment with Cidofovir or Imiquimod: Results from a Multicentre, Randomised, Phase II Trial (RT3VIN). BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1171–1177. [Google Scholar] [CrossRef]
- Tristram, A.; Hurt, C.N.; Madden, T.; Powell, N.; Man, S.; Hibbitts, S.; Dutton, P.; Jones, S.; Nordin, A.J.; Naik, R.; et al. Activity, Safety, and Feasibility of Cidofovir and Imiquimod for Treatment of Vulval Intraepithelial Neoplasia (RT3VIN): A Multicentre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2014, 15, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Bossens, M.; Jost, M.; van Pachterbeke, C.; de Maertelaer, V.; Simon, P.; Frankenne, F.; Hubert, P.; Evrard, B.; Snoeck, R. Safety and Tolerance of Cidofovir as a 2% Gel for Local Application in High-Grade Cervical Intraepithelial Neoplasia: A Phase 1 Investigation. Int. J. Clin. Pharmacol. Ther. 2018, 56, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, R.; Noel, J.C.; Muller, C.; de Clercq, E.; Bossens, M. Cidofovir, a New Approach for the Treatment of Cervix Intraepithelial Neoplasia Grade III (CIN III). J. Med. Virol. 2000, 60, 205–209. [Google Scholar] [CrossRef]
- Desravines, N.; Miele, K.; Carlson, R.; Chibwesha, C.; Rahangdale, L. Topical Therapies for the Treatment of Cervical Intraepithelial Neoplasia (CIN) 2–3: A Narrative Review. Gynecol. Oncol. Rep. 2020, 33, 100608. [Google Scholar] [CrossRef]
- Mutombo, A.B.; Simoens, C.; Tozin, R.; Bogers, J.; van Geertruyden, J.P.; Jacquemyn, Y. Efficacy of Commercially Available Biological Agents for the Topical Treatment of Cervical Intraepithelial Neoplasia: A Systematic Review. Syst. Rev. 2019, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Glaspy, J.A. Pembrolizumab for the Treatment of Cervical Intraepithelial Neoplasia (NCT04712851). Available online: https://clinicaltrials.gov/ct2/show/NCT04712851 (accessed on 22 February 2022).
- Hoyt, C.C. Multiplex Immunofluorescence and Multispectral Imaging: Forming the Basis of a Clinical Test Platform for Immuno-Oncology. Front. Mol. Biosci. 2021, 8, 674747. [Google Scholar] [CrossRef]
- da Silva, M.; de Albuquerque, B.; Allyrio, T.; de Almeida, V.; Cobucci, R.; Bezerra, F.; Andrade, V.; Lanza, D.; de Azevedo, J.; de Araújo, J.; et al. The Role of HPV-induced Epigenetic Changes in Cervical Carcinogenesis (Review). Biomed. Rep. 2021, 15, 60. [Google Scholar] [CrossRef]
- Kremer, W.; Steenbergen, R.; Heideman, D.; Kenter, G.; Meijer, C. The Use of Host Cell DNA Methylation Analysis in the Detection and Management of Women with Advanced Cervical Intraepithelial Neoplasia: A Review. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Kortekaas, K.E.; Santegoets, S.J.; Tas, L.; Ehsan, I.; Charoentong, P.; van Doorn, H.C.; van Poelgeest, M.I.E.; Mustafa, D.A.M.; van der Burg, S.H. Primary Vulvar Squamous Cell Carcinomas with High T Cell Infiltration and Active Immune Signaling Are Potential Candidates for Neoadjuvant PD-1/PD-L1 Immunotherapy. J. ImmunoTher. Cancer 2021, 9, e003671. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical Intraepithelial Neoplasia Disease Progression Is Associated with Increased Vaginal Microbiome Diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [Green Version]
| Country or Continent (Organization) [References] | cLSIL | cHSIL | Current Mode of Treatment |
|---|---|---|---|
| Australia (Cancer Council Australia) [28] | Observation is preferred, repeat HPV test after 1 year. | CIN 2:
| LLETZ, cold knife conization or hysterectomy. |
| Europe (European Federation of Colposcopy) [29,30] | Observation is preferred, repeat cytology after 1 year. | Treatment is recommended. | LLETZ, cold knife conization or laser excision. |
| Great Britain (The British Society for Colposcopy and Cervical Pathology) [31] | Observation is preferred, repeat cytology after 1 year. | Treatment is recommended. | LLETZ, cold knife conization or laser excision. |
| United States of America (American Society for Colposcopy and Cervical Pathology) [32] | Observation is preferred, repeat cytology after 1 year. | CIN 2:
| LLETZ, cold knife conization, laser excision, cryotherapy, laser ablation or thermoablation. |
| cLSIL Immune Microenvironment | cHSIL Immune Microenvironment | References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Stroma | Epithelium | Overall | Stroma | Epithelium | ||||
| Cellular | Pro- inflammatory | iDC | Not reported | Not reported | Not reported | ↑ | Not reported | Not reported | [45] |
| mDC | = | = | = | ↓ | Not reported | ↓ | [46,47,48,49,50] | ||
| LC | = | = | = | ↓ | Not reported | ↓ | [46,47,48,49,50] | ||
| M1 | = | = | = | ↑ | ↑ | Not reported | [45,51,52,53] | ||
| CD4+ | ↓ | ↓ | ↓ | ↓↓ | ↓↓ | ↓↓ | [37,39,54,55,56] | ||
| CD8+ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓↓ | [39,54,55,56] | ||
| Anti- inflammatory | M2 | ↑ | ↑ | ↑ | ↑↑ | ↑↑ | ↑↑ | [53] | |
| Treg | ↑ | ↑ | = | ↑↑ | ↑↑ | = | [37,45,54,56,57,58] | ||
| PD-1+ T cells | ↑ | Not reported | Not reported | ↑ | Not reported | Not reported | [37,45,54,56,57,58] | ||
| PD-L1+ cells | ↑ | ↑ | ↑ | ↑↑ | Not reported | Not reported | [59,60] | ||
| IDO-1+ cells | = | Not reported | Not reported = | ↑ | ↑ | ↑ | [45,56,61] | ||
| Cytokines | TGF-β | ↑ | Unknown | Unknown | ↑ | Unknown | Unknown | [55,62,63,64,65] | |
| IL-10 | = | Unknown | Unknown | ↑ | Unknown | Unknown | [55,62,63,64,65] | ||
| IL-35 | Not reported | Unknown | Unknown | ↑ | Unknown | Unknown | [55,63,64,65] | ||
| First Author, Year [References] | Treatment | Study Design | Sample Size of Imiquimod Users | Primary Endpoint | Main Clinical Outcome (n) |
|---|---|---|---|---|---|
| Hendriks, 2022 [27] | Imiquimod vs. LLETZ | PS | 61 | Clinical regression | Regression: 60% hrHPV clearance: 69% |
| Fonseca, 2021 [23] | Imiquimod vs. LLETZ | RCT | 49 | Clinical regression | Regression: 60.5% (23) Persistence: 39.5% (15) Progression: 0% (0) |
| Cokan, 2021 [26] | Imiquimod vs. LLETZ | RCT | 52 | Clinical regression | Regression: 62.8% (27) |
| Chen, 2013 [144] | Imiquimod after surgical treatment | RS | 76 | HPV clearance | HPV clearance: 76.3% (58) |
| Grimm, 2012 [24] | Imiquimod vs. placebo | RCT | 30 | Clinical regression | Regression: 73% (22) Remission: 47% (14) |
| Lin, 2012 [145] | Imiquimod | PS | 1. 26 | 1. HPV clearance in women with persistent HPV-infection, but normal Pap-smear | 1. 69.2% (18) |
| 2. 72 | 2. Clinical regression and HPV clearance | 2. 51.4% (37) | |||
| Pachman, 2011 [146] | Imiquimod vs. excision or ablation | RCT | 28 | Recurrence within 2 years | Recurrence: 14% (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntinga, C.L.P.; de Vos van Steenwijk, P.J.; Bekkers, R.L.M.; van Esch, E.M.G. Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy. J. Clin. Med. 2022, 11, 1432. https://doi.org/10.3390/jcm11051432
Muntinga CLP, de Vos van Steenwijk PJ, Bekkers RLM, van Esch EMG. Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy. Journal of Clinical Medicine. 2022; 11(5):1432. https://doi.org/10.3390/jcm11051432
Chicago/Turabian StyleMuntinga, Caroline L. P., Peggy J. de Vos van Steenwijk, Ruud L. M. Bekkers, and Edith M. G. van Esch. 2022. "Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy" Journal of Clinical Medicine 11, no. 5: 1432. https://doi.org/10.3390/jcm11051432
APA StyleMuntinga, C. L. P., de Vos van Steenwijk, P. J., Bekkers, R. L. M., & van Esch, E. M. G. (2022). Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy. Journal of Clinical Medicine, 11(5), 1432. https://doi.org/10.3390/jcm11051432







