Safety of Obtaining an Extra Biobank Kidney Biopsy Core
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Selection
2.2. Clinical Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Population
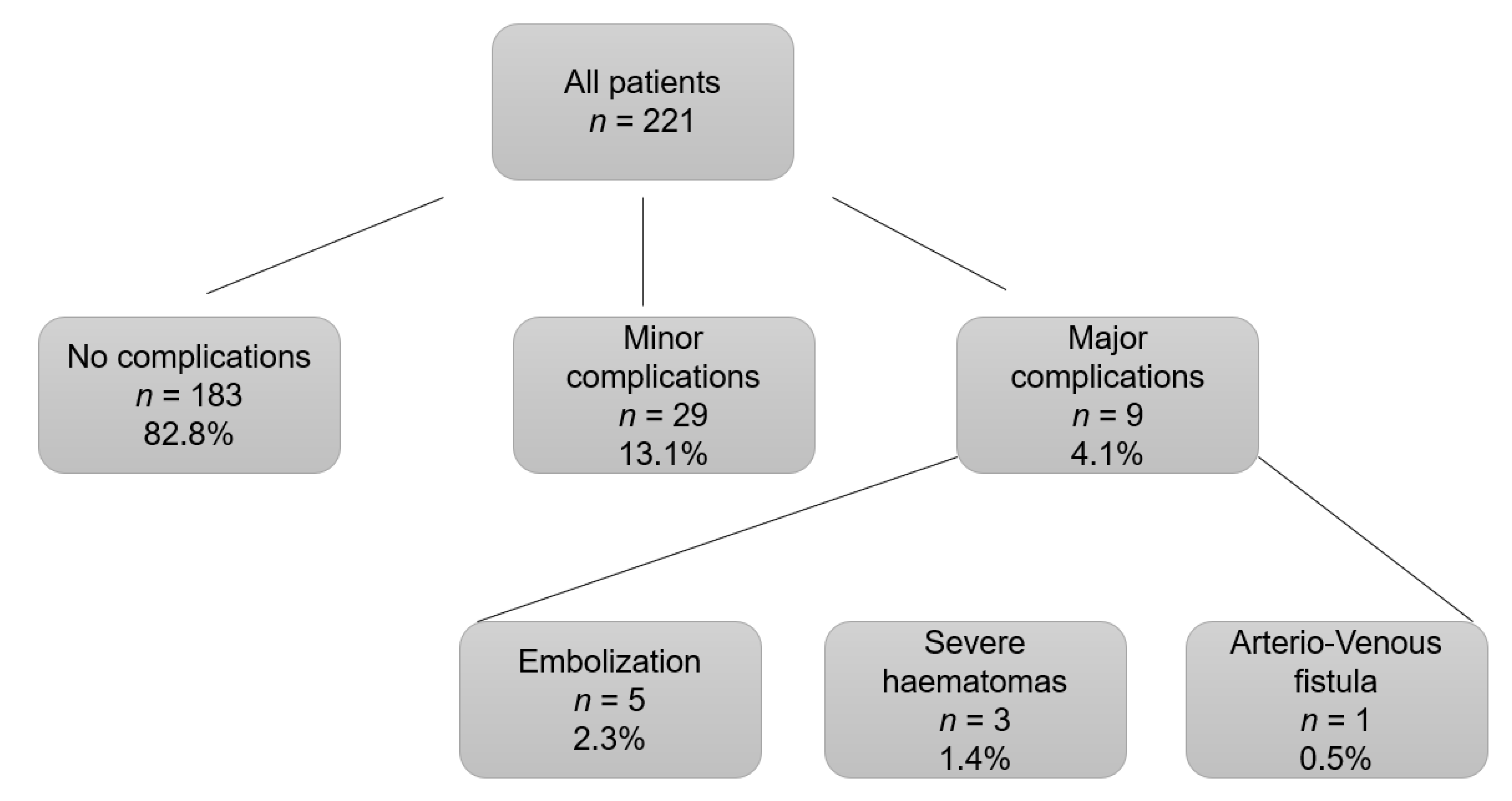
3.2. Post-Kidney Biopsy Complications
3.3. Risk Factors for Complications Associated with Kidney Biopsy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luciano, R.L.; Moeckel, G.W. Update on the Native Kidney Biopsy: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Palsson, R.; Short, S.A.P.; Kibbelaar, Z.A.; Amodu, A.; Stillman, I.E.; Rennke, H.G.; McMahon, G.M.; Waikar, S.S. Bleeding Complications After Percutaneous Native Kidney Biopsy: Results From the Boston Kidney Biopsy Cohort. Kidney Int. Rep. 2020, 5, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Ashley Jefferson, J. How safe is a native kidney biopsy? Clin. J. Am. Soc. Nephrol. 2020, 15, 1541–1542. [Google Scholar] [CrossRef]
- Stojimirovic, B. Aspiration biopsy of the kidney. Med. Glas. 1959, 13, 437–440. [Google Scholar] [PubMed]
- Kark, R.M.; Muehrcke, R.C. Biopsy of Kidney in Prone Position. Lancet 1954, 263, 1047–1049. [Google Scholar] [CrossRef]
- Poggio, E.D.; McClelland, R.L.; Blank, K.N.; Hansen, S.; Bansal, S.; Bomback, A.S.; Canetta, P.A.; Khairallah, P.; Kiryluk, K.; Lecker, S.H.; et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin. J. Am. Soc. Nephrol. 2020, 15, 1595–1602. [Google Scholar] [CrossRef]
- Hogan, J.J.; Mocanu, M.; Berns, J.S. The native kidney biopsy: Update and evidence for best practice. Clin. J. Am. Soc. Nephrol. 2016, 11, 354–356. [Google Scholar] [CrossRef]
- Rathod, K.R.; Popat, B.A.; Pandey, A.; Jamale, T.E.; Hase, N.K.; Deshmukh, H.L. Safety and effectiveness of transjugular renal biopsy: A single center study. Indian J. Nephrol. 2017, 27, 118–123. [Google Scholar] [CrossRef]
- Bolufer, M.; García-Carro, C.; Agraz, I.; Díez Miranda, I.; Jaramillo, J.; Arredondo, K.; Bury, R.; Ramos, N.; Azancot, M.A.; Gabaldón, A.; et al. Biopsia renal transyugular. La alternativa a la biopsia percutánea en pacientes de alto riesgo. Nefrología 2020, 40, 634–639. [Google Scholar] [CrossRef]
- Bolufer Cardona, M.; Soler Romeo, M.J.; McMahon, G.M. Transjugular Kidney Biopsy as a Safe Method to Increase the Etiological Diagnosis in Kidney Disease. Kidney Int. Rep. 2021, 6, 2535–2536. [Google Scholar] [CrossRef]
- St Jeor, J.D.; Reisenauer, C.J.; Andrews, J.C.; Fleming, C.J.; Misra, S.; Takahashi, E.A. Transjugular Renal Biopsy Bleeding Risk and Diagnostic Yield: A Systematic Review. J. Vasc. Interv. Radiol. 2020, 31, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Pombas, B.; Rodríguez, E.; Sánchez, J.; Radosevic, A.; Gimeno, J.; Busto, M.; Barrios, C.; Sans, L.; Pascual, J.; Soler, M.J. Risk factors associated with major complications after ultrasound-guided percutaneous renal biopsy of native kidneys. Kidney Blood Press Res. 2020, 45, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Nasic, S.; Segelmark, M. Clinical parameters predicting complications in native kidney biopsies. Clin. Kidney J. 2020, 13, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Halimi, J.M.; Gatault, P.; Longuet, H.; Barbet, C.; Bisson, A.; Sautenet, B.; Herbert, J.; Buchler, M.; Grammatico-Guillon, L.; Fauchier, L. Major bleeding and risk of death after percutaneous native kidney biopsies a french nationwide cohort study. Clin. J. Am. Soc. Nephrol. 2020, 15, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. Approach to renal biopsy. Am. J. Kidney Dis. 2003, 42, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Whittier, W.L. Complications of the Percutaneous Kidney Biopsy. Adv. Chronic. Kidney Dis. 2012, 19, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tøndel, C.; Vikse, B.E.; Bostad, L.; Svarstad, E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin. J. Am. Soc. Nephrol. 2012, 7, 1591–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiro, M.; Katoh, T.W.T. Risk factors for bleeding complications in percutaneous renal biopsy. Clin. Exp. Nephrol. 2005, 9, 40–45. [Google Scholar] [CrossRef]
- Peters, B.; Andersson, Y.; Stegmayr, B.; Mölne, J.; Jensen, G.; Dahlberg, P.; Holm-Gunnarsson, I.; Ekberg, J.; Bjurström, K.; Haux, S.B.; et al. A study of clinical complications and risk factors in 1001 native and transplant kidney biopsies in Sweden. Acta Radiol. 2014, 55, 890–896. [Google Scholar] [CrossRef]
- Manno, C.; Strippoli, G.F.M.; Arnesano, L.; Bonifati, C.; Campobasso, N.; Gesualdo, L.; Schena, F.P. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004, 66, 1570–1577. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Buxo, J.A.D.J.J. Complications of percutaneous renal biopsy: An analysis of 1000 consecutive biopsies. Clin. Nephrol. 1975, 4, 223–227. [Google Scholar] [PubMed]
- Christensen, J.; Lindequist, S.; Knudsen, D.U.; Pedersen, R.S. Ultrasound-guided renal biopsy with biopsy gun technique-efficacy and complications. Acta Radiol. 1995, 36, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Shidham, G.B.; Siddiqi, N.; Beres, J.A.; Logan, B.; Nagaraja, H.N.; Shidham, S.G.; Piering, W.F. Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology 2005, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Corapi, K.M.; Chen, J.L.T.; Balk, E.M.; Gordon, C.E. Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am. J. Kidney Dis. 2012, 60, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Massak, M.; Czaplicki, M.; Young, E.; Sharma, S.; Chang, A.; Anand, P. Use a smartphone camera at the bedside to assess adequacy of kidney biopsies. J. Am. Soc. Nephrol. 2021, 32, 3024–3026. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Bonifati, C.; Torres, D.D.; Campobasso, N.; Schena, F.P. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: A randomized controlled trial. Am. J. Kidney Dis. 2011, 57, 850–855. [Google Scholar] [CrossRef]
- Peters, B.; Hadimeri, H.; Mölne, J.; Nasic, S.; Jensen, G.; Stegmayr, B. Desmopressin (Octostim®) before a native kidney biopsy can reduce the risk for biopsy complications in patients with impaired renal function: A pilot study. Nephrology 2018, 23, 366–370. [Google Scholar] [CrossRef]
- Hogan, J.J.; Owen, J.G.; Blady, S.J.; Almaani, S.; Avasare, R.S.; Bansal, S.; Lenz, O.; Luciano, R.L.; Parikh, S.V.; Ross, M.J.; et al. The Feasibility and Safety of Obtaining Research Kidney Biopsy Cores in Patients with Diabetes. Clin. J. Am. Soc. Nephrol. 2020, 15, 1024–1026. [Google Scholar] [CrossRef]
- Leclerc, S.; Nadeau-Fredette, A.C.; Elftouh, N.; Lafrance, J.P.; Pichette, V.; Laurin, L.P. Use of Desmopressin Prior to Kidney Biopsy in Patients With High Bleeding Risk. Kidney Int. Rep. 2020, 5, 1180–1187. [Google Scholar] [CrossRef]
- Sousanieh, G.; Whittier, W.L.; Rodby, R.A.; Peev, V.; Korbet, S.M. Percutaneous Renal Biopsy Using an 18-Gauge Automated Needle Is Not Optimal. Am. J. Nephrol. 2021, 51, 982–987. [Google Scholar] [CrossRef]
- Waldo, B.; Korbet, S.M.; Freimanis, M.G.; Lewis, E.J. The value of post-biopsy ultrasound in predicting complications after percutaneous renal biopsy of native kidneys. Nephrol. Dial. Transplant. 2009, 24, 2433–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mier, M.V.P.R.; Espinosa-Hernández, M.; Rodelo-Haad, C.; Motta EE de Gómez-Carrasco, J.; Ortega, R.; Aljama, P. Estudio prospectivo de las complicaciones asociadas a la biopsia percutánea en riñón nativo: Experiencia en un centro. Nefrologia 2014, 34, 383–387. [Google Scholar] [CrossRef]
- Li, Q.; Lin, X.; Zhang, X.; Samir, A.E.; Arellano, R.S. Imaging-Related Risk Factors for Bleeding Complications of US-Guided Native Renal Biopsy: A Propensity Score Matching Analysis. J. Vasc. Interv. Radiol. 2019, 30, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Lees, J.S.; McQuarrie, E.P.; Mordi, N.; Geddes, C.C.; Fox, J.G.; Mackinnon, B. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin. Kidney J. 2017, 10, 573–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, A.T.; Valdez-Ortiz, R.; González-Parra, C.; Espinoza-Dávila, E.; Morales-Buenrostro, L.E.; Correa-Rotter, R. Percutaneous renal biopsy of native kidneys: Efficiency, safety and risk factors associated with major complications. Arch Med Sci. 2011, 7, 823–831. [Google Scholar] [CrossRef]
- Bonani, M.; Seeger, H.; Weber, N.; Lorenzen, J.M.; Wüthrich, R.P.; Kistler, A.D. Safety of kidney biopsy when performed as an outpatient procedure. Kidney Blood Press Res. 2021, 46, 310–322. [Google Scholar] [CrossRef]
- Whittier, W.L.; Sayeed, K.; Korbet, S.M. Clinical factors influencing the decision to transfuse after percutaneous native kidney biopsy. Clin. Kidney J. 2016, 9, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Townsend, R.R.; Guarnieri, P.; Argyropoulos, C.; Blady, S.; Boustany-Kari, C.M.; Devalaraja-Narashimha, K.; Morton, L.; Mottl, A.K.; Patel, U.; Palmer, M.; et al. Rationale and design of the Transformative Research in Diabetic Nephropathy (TRIDENT) Study. Kidney Int. 2020, 97, 10–13. [Google Scholar] [CrossRef]

| Major Complications | Minor Complications |
|---|---|
| Need of transfusion | Pain |
| Embolization | Hematuria |
| Nephrectomy | Hematoma without blood transfusion |
| Death |
| Characteristics | All Patients |
|---|---|
| Patients (n) | 221 |
| Age (years) | 56.6 (±16.8) |
| Sex (Women/Men) (n, %) | 91 (41.2%)/130 (58.8%) |
| Hypertension (>140/90 mmHg) (n, %) | 128 (57.9%) |
| Diabetes Mellitus (n, %) | 52 (23.5%) |
| Weight (kg) | 75.8 (±24.6) |
| BMI (kg/m2) | 27.6 (±5.01) |
| Systolic blood pressure (mmHg) | 136 (±24.6) |
| Diastolic blood pressure (mmHg) | 76 (±15) |
| Creatinine (mg/dL) | 2.24 (±1.94) |
| Proteinuria (g/24 h) | 1.56 (0.506–3.59) |
| Urinary albumin/creatinine ratio (mg/g) | 613 (100.3–2300) |
| Urinary protein/creatinine ratio (mg/g) | 1153.8 (482.8–3156) |
| Platelets (n) | 250.995 (±89.742) |
| INR | 0.99 (±0.1) |
| PT (seconds) | 11.86 (±1.2) |
| Hb pre (g/dL) | 12.03 (±2.3) |
| Hb post (g/dL) | 11.3 (±2.3) |
| Microhematuria (n, %) | 100 (45.2%) |
| Kidney size (cm) | 11.04 (±1.2) |
| Cortical size (cm) | 1.67 (±0.64) |
| Technique (US-guided/transjugular) (n, %) | 213 (96.4%)/8 (3.6%) |
| Number of renal cores (n, %): | |
| 3 | 152 (68.8%) |
| 2 | 62 (28%) |
| 1 | 7 (3.2%) |
| Anticoagulants (n, %): | |
| Acenocoumarol | 7 (3.2%) |
| Heparin | 3 (1.4%) |
| Others | 5 (2.3%) |
| Antiplatelets (n, %): | |
| ASA 100 mg | 30 (13.6%) |
| ASA 300 mg | 1 (0.5%) |
| Clopidogrel | 5 (2.3%) |
| Characteristics | Number of Kidney Cores | p | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Patients (n, %) | 7 (3.2%) | 62 (28.1%) | 151 (68.8%) | - |
| Age (years) | 45.4 (±17.9) | 56 (±17.2) | 57.4 (±16.5) | 0.171 |
| Sex (Women/Men) (n, %) | 2 (28.6%)/ 5 (71.4%) | 29 (46.8%)/ 33 (53.2%) | 60 (39.5%)/ 92 (60.5%) | 0.486 |
| Hypertension (n, %) | 5 (71.4%) | 37 (59.7%) | 86 (56.6%) | 0.699 |
| Diabetes Mellitus (n, %) | 0 (0%) | 15 (24.2%) | 37 (24.3%) | 0.329 |
| Weight (kg) | 68.3 (±19.2) | 74.8 (±15.3) | 76.6 (±15.3) | 0.321 |
| BMI (kg/m2) | 25.4 (±2.8) | 27.1 (±5) | 27.9 (±5.1) | 0.298 |
| Systolic blood pressure (mmHg) | 151.3 (±25.9) | 134.3 (±28.2) | 135.4 (±22.8) | 0.22 |
| Diastolic blood pressure (mmHg) | 87.7 (±19.2) | 76.6 (±15.5) | 75.2 (±15) | 0.101 |
| Creatinine (mg/dL) | 4.5 (±3.3) | 2.5 (±2.3) | 2 (±1.6) | 0.002 |
| Proteinuria (g/24 h) | 2.8 [2.3–7.8] | 3 [2–4] | 2.8 [2.2–3.4] | 0.948 |
| Urinary albumin/creatinine ratio (mg/g) | 387.4 [51.7–826.5] | 1577.2 [950–2204.4] | 1616 [1243–1989.1] | 0.403 |
| Urinary protein/creatinine ratio (mg/g) | 131.1 [57.2–2005.1] | 2730.8 [1757.9–3703.6] | 2740.6 [2080.6–3400.7] | 0.619 |
| Platelets (n) | 260,428 (±58,266) | 238,725 (±103,365) | 255,565 (±84,828) | 0.444 |
| INR | 0.99 (±0.1) | 0.99 (±0.1) | 0.98 (±0.1) | 0.807 |
| PT (seconds) | 11.9 (±1.2) | 11.9 (±1.1) | 11.8 (±1.2) | 0.843 |
| Hb pre (g/dL) | 12.5 (±2.6) | 11.7 (±2.4) | 12.1 (±2.3) | 0.466 |
| Hb post (g/dL) | 11.4 (±2.7) | 11.1 (±2.6) | 11.4 (±2.1) | 0.784 |
| Microhematuria (n. %) | 4 (57.1%) | 26 (41.9%) | 70 (46.4%) | 0.689 |
| Kidney size (cm) | 10.7 (±1.2) | 11.2 (±1.3) | 11 (±1.2) | 0.449 |
| Cortical size (cm) | 1.7 (±0.2) | 1.7 (±0.3) | 1.7 (±0.3) | 0.985 |
| Left kidney/Right kidney (n, %) | 3 (42.9%)/ 4 (57.1%) | 19 (31.1%)/ 42 (68.9%) | 77 (51%)/ 74 (49%) | 0.031 |
| Technique (US-guided/ Transjugular) (n, %) | 7 (100%)/ 0 | 58 (93.5%)/ 4 (6.5%) | 148 (97.4%)/ 4 (2.6%) | 0.348 |
| Transfusion (n, %) | 2 (28.6%) | 9 (14.5%) | 14 (9.2%) | 0.184 |
| Anticoagulants (n. %): | ||||
| Acenocoumarol | 0 | 3 (4.8%) | 4 (2.6%) | 0.701 |
| Heparin | 0 | 1 (1.6%) | 2 (1.3%) | |
| Others | 0 | 3 (4.8%) | 2 (1.3%) | |
| Antiplatelets (n. %): | ||||
| ASA 100 mg | 0 | 6 (9.7%) | 24 (15.8%) | |
| ASA 300 mg | 0 | 0 | 1 (0.7%) | 0.743 |
| Clopidogrel | 0 | 2 (3.2%) | 3 (2%) | |
| Characteristics | Without Complications | With Complications | p |
|---|---|---|---|
| Patients (n, %) | 183 (82.8%) | 38 (17.2%) | - |
| Age (years) | 57.7 (±16.3) | 51.4 (±18.2) | 0.034 |
| Sex (Women/Men) (n, %) | 73 (39.9%)/ 110 (40.1%) | 18 (47.4%)/ 20 (52.6%) | 0.469 |
| Hypertension (n, %) | 108 (59%) | 20 (52.6%) | 0.476 |
| Diabetes Mellitus (n, %) | 10 (26.3%) | 42 (23%) | 0.676 |
| Weight (kg) | 76.9 (±15.4) | 70.5 (±14.5) | 0.022 |
| BMI (kg/m2) | 27.8 (±4.97) | 26.2 (±5.1) | 0.096 |
| Systolic blood pressure (mmHg) | 134.5 (±23.8) | 141 (±27.5) | 0.134 |
| Diastolic blood pressure (mmHg) | 75.5 (±15.6) | 78.5 (±14.2) | 0.262 |
| Creatinine (mg/dL) | 2.26 (±1.9) | 2.15 (±1.97) | 0.751 |
| Proteinuria (g/24 h) | 2.85 (2.27–3.43] | 3.01 (1.88–4.15] | 0.809 |
| Urinary albumin/creatinine ratio (mg/g) | 1556.57 (1214.84–1898.31] | 1622.96 (856.65–2389.27] | 0.875 |
| Urinary protein/creatinine ratio (mg/g) | 2653.11 (2065.71–3240.52] | 2907.7 (1613.22–4202.19] | 0.725 |
| Platelets (n) | 245,295 (±84,011) | 278,447 (±110,613) | 0.038 |
| INR | 0.99 (±0.1) | 0.96 (0.09) | 0.07 |
| PT (seconds) | 11.92 (±1.22) | 11.54 (±1.08) | 0.05 |
| Hb pre (g/dL) | 12.07 (±2.27) | 11.84 (±2.56) | 0.057 |
| Hb post (g/dL) | 11.4 (±2.2) | 10.8 (±2.5) | 0.14 |
| Microhematuria (n, %) | 84 (46.2%) | 16 (42.1%) | 0.722 |
| Kidney size (cm) | 11.08 (±1.25) | 10.84 (±1.09) | 0.277 |
| Cortical size (cm) | 1.67 (±0.69) | 1.64 (±0.32) | 0.807 |
| Left kidney/Right kidney (n, %) | 85 (47%)/ 96 (53%) | 24 (63.2%)/ 14 (36.8%) | 0.286 |
| Technique (US-guided/transjugular) (n, %) | 177 (96.7%)/ 6 (3.3%) | 36 (94.7%)/ 2 (5.3%) | 0.628 |
| Transfusion (n, %) | 15 (8.2%) | 10 (26.3%) | 0.003 |
| Number of renal cores | |||
| (n, %): | |||
| 3 | 130 (71%) | 22 (57.9%) | |
| 2 | 50 (27.3%) | 12 (31.6%) | 0.012 |
| 1 | 3 (1.6%) | 4 (10.5%) | |
| Anticoagulants (n, %): | |||
| Acenocoumarol | 7 (3.8%) | 0 | |
| Heparin | 3 (1.6%) | 0 | 0.342 |
| Others | 5 (2.7%) | 0 | |
| Antiplatelets (n, %): | |||
| AAS 100 mg | 24 (13.1%) | 6 (15.8%) | |
| AAS 300 mg | 1 (0.5%) | 0 | 0.935 |
| Clopidogrel | 4 (2.2%) | 1 (2.6%) |
| Characteristics | Without Complications | Minor Complications | Major Complications | p |
|---|---|---|---|---|
| Patients (n, %) | 183 (82.8%) | 29 (13.1%) | 9 (4.1%) | - |
| Age (years) | 57.7 (±16.3) | 52.9 (±17.8) | 46.6 (±20) | 0.065 |
| Sex (Women/Men) (n, %) | 73 (39.9%)/ 110 (40.1%) | 13 (44.8%)/ 16 (55.2%) | 5 (55.6%)/ 4 (44.4%) | 0.591 |
| Hypertension (n, %) | 108 (59%) | 17 (58.6%) | 3 (33.3%) | 0.312 |
| Diabetes Mellitus (n, %) | 10 (26.3%) | 9 (31%) | 1 (11.1%) | 0.425 |
| Weight (kg) | 76.9 (±15.4) | 71.04 (±14.4) | 68.97 (±15.3) | 0.069 |
| BMI (kg/m2) | 27.8 (±4.97) | 26.93 (±5.36) | 24.13 (±3.73) | 0.097 |
| Systolic blood pressure (mmHg) | 134.5 (±23.8) | 144.24 (±24.5) | 130.67 (±35.4) | 0.114 |
| Diastolic blood pressure (mmHg) | 75.5 (±15.6) | 79.24 (±13.9) | 76.22 (±16) | 0.468 |
| Creatinine (mg/dL) | 2.26 (±1.9) | 1.93 (±1.65) | 2.86 (±2.76) | 0.432 |
| Proteinuria (g/24 h) | 2.85 (2.27–3.43) | 2.85 (1.57–4.2) | 3.47 (0.55–6.39) | 0.896 |
| Urinary albumin/creatinine ratio (mg/g) | 1556.57 (1214.84–1898.31) | 1412.1 (789.6–2034.6) | 2255.6 (671.1–5182.3) | 0.632 |
| Urinary protein/creatinine ratio (mg/g) | 2653.11 (2065.71–3240.52) | 2536.5 (1332–3741.02) | 4114.2 (536.9–8765.2) | 0.562 |
| Platelets (n) | 245,295 (±84,011) | 270,517 (±80,529) | 304,000 (±181,464) | 0.072 |
| INR | 0.99 (±0.1) | 0.94 (±0.07) | 1.02 (±0.11) | 0.028 |
| PT (seconds) | 11.92 (±1.22) | 11.33 (±0.94) | 12.2 (±1.29) | 0.032 |
| Hb pre (g/dL) | 12.07 (±2.27) | 12.26 (±2.67) | 10.49 (±1.64) | 0.115 |
| Hb post (g/dL) | 11.4 (±2.2) | 11.28 (±2.45) | 9.24 (±2.13) | 0.02 |
| Microhematuria (n, %) | 84 (46.2%) | 12 (41.4%) | 4 (44.4%) | 0.89 |
| Kidney size (cm) | 11.08 (±1.25) | 10.93 (±1.1) | 10.57 (±1.1) | 0.413 |
| Cortical size (cm) | 1.67 (±0.69) | 1.6 (±0.32) | 1.75 (±0.33) | 0.829 |
| Left kidney/Right kidney (n, %) | 85 (47%)/ 96 (53%) | 10 (34.5%)/ 19 (65.5%) | 4 (44.4%)/ 5 (55.6%) | 0.455 |
| Technique (US-guided/ Transjugular) (n, %) | 177 (96.7%)/ 6 (3.3%) | 29 (100%)/ 0 | 7 (77.8%)/ 2 (22.2%) | 0.006 |
| Transfusion (n, %) | 15 (8.2%) | 5 (17.2%) | 5 (55.6%) | <0.001 |
| Number of renal cores (n, %): | ||||
| 3 | 130 (71%) | 17 (58.6%) | 5 (55.6%) | |
| 2 | 50 (27.3%) | 9 (31%) | 3 (33.3%) | 0.064 |
| 1 | 3 (1.6%) | 3 (10.3%) | 1 (11.1%) | |
| Anticoagulants (n. %): | ||||
| Acenocoumarol | 7 (3.8%) | 0 | 0 | |
| Heparin | 3 (1.6%) | 0 | 0 | 0.765 |
| Others | 5 (2.7%) | 0 | 0 | |
| Antiplatelets (n. %): | ||||
| ASA 100 mg | 24 (13.1%) | 6 (20.7%) | 0 | |
| ASA 300 mg | 1 (0.5%) | 0 | 0 | 0.378 |
| Clopidogrel | 4 (2.2%) | 0 | 1 (11.1%) |
| Variable | OR | CI (95%) | Lateral Significance (p) |
|---|---|---|---|
| PT (seconds) * | 1.36 | 0.94–1.97 | 0.1 |
| Renal cores (1 core vs. 2/3 cores) | 7.09 | 1.34–37.04 | 0.021 |
| Age (years) * | 1.01 | 0.99–1.04 | 0.299 |
| Platelets (n) * | 1 | 1–1 | 0.130 |
| Weight (kg) * | 1.02 | 0.99–1.04 | 0.245 |
| Hb pre (g/dL) * | 1.13 | 0.94–1.36 | 0.2 |
| Study | Country | Year | Type of Study | Number of Patients | Type of Needles | Number of Kidney Cores | Complications Regarding Number of Kidney Cores |
|---|---|---|---|---|---|---|---|
| Singh S, et al. [25] | United States | 2021 | Prospective | 20 | 18G (95%) 16G (5%) | From 2 to 6 cores (2.9) | No data |
| Manno et al. [26] | Italy | 2011 | Randomized Clinical Trial | 162 | 16G | 2 cores | No data |
| Peters et al. [27] | Sweden | 2018 | Prospective | 576 | 16G or 18G | From 2 to 3 cores (Unknown) | No differences |
| Pombas et al. [12] | Spain | 2020 | Retrospective | 661 | 16G | 2 cores | No data |
| Hogan et al. [28] | United States | 2020 | Prospective | 160 | 16G (54%) 18G (44%) | From 1 to 4 cores (Unknown) | No differences |
| Leclerc et al. [29] | Canada | 2020 | Retrospective | 413 | 16G (98%) 18G (2%) | 2 cores (86%) 1 core (14%) | No data |
| Sousanieh et al. [30] | United States | 2020 | Prospective | 592 | 14G (57%) 16G (43%) | From 1 to 3 cores (2.2–2.3) | No data |
| Waldo et al. [31] | United States | 2009 | Prospective | 162 | 14G | No data | No data |
| Pendon-Ruiz de Mier et al. [32] | Spain | 2014 | Prospective | 241 | 16G | From 1 to 2 cores (unknown) | No differences |
| Li et al. [33] | China | 2018 | Retrospective | 551 | 16G or 18G (unknown) | From 1 to 4 cores (unknown) | No differences |
| Lees et al. [34] | UK | 2017 | Retrospective | 2563 | 16G | From 1 to 3 cores (unknown) | No data |
| Torres et al. [35] | Mexico | 2011 | Retrosepctive | 623 | 16G | No data | No data |
| Current study | Spain | 2021 | Retrospective | 221 | 16G | 1 core (3.2%) 2 cores (28.1%) 3 cores (68.8%) | More complications in patients with one kidney core obtained. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermejo, S.; García-Carro, C.; Mast, R.; Vergara, A.; Agraz, I.; León, J.C.; Bolufer, M.; Gabaldon, M.-A.; Serón, D.; Bestard, O.; et al. Safety of Obtaining an Extra Biobank Kidney Biopsy Core. J. Clin. Med. 2022, 11, 1459. https://doi.org/10.3390/jcm11051459
Bermejo S, García-Carro C, Mast R, Vergara A, Agraz I, León JC, Bolufer M, Gabaldon M-A, Serón D, Bestard O, et al. Safety of Obtaining an Extra Biobank Kidney Biopsy Core. Journal of Clinical Medicine. 2022; 11(5):1459. https://doi.org/10.3390/jcm11051459
Chicago/Turabian StyleBermejo, Sheila, Clara García-Carro, Richard Mast, Ander Vergara, Irene Agraz, Juan Carlos León, Monica Bolufer, Maria-Alejandra Gabaldon, Daniel Serón, Oriol Bestard, and et al. 2022. "Safety of Obtaining an Extra Biobank Kidney Biopsy Core" Journal of Clinical Medicine 11, no. 5: 1459. https://doi.org/10.3390/jcm11051459






