Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Characteristics
2.2. Imaging Protocols
2.3. Quantitative Image Analysis
2.4. Laboratory Parameters
2.5. Statistical Analysis
3. Results
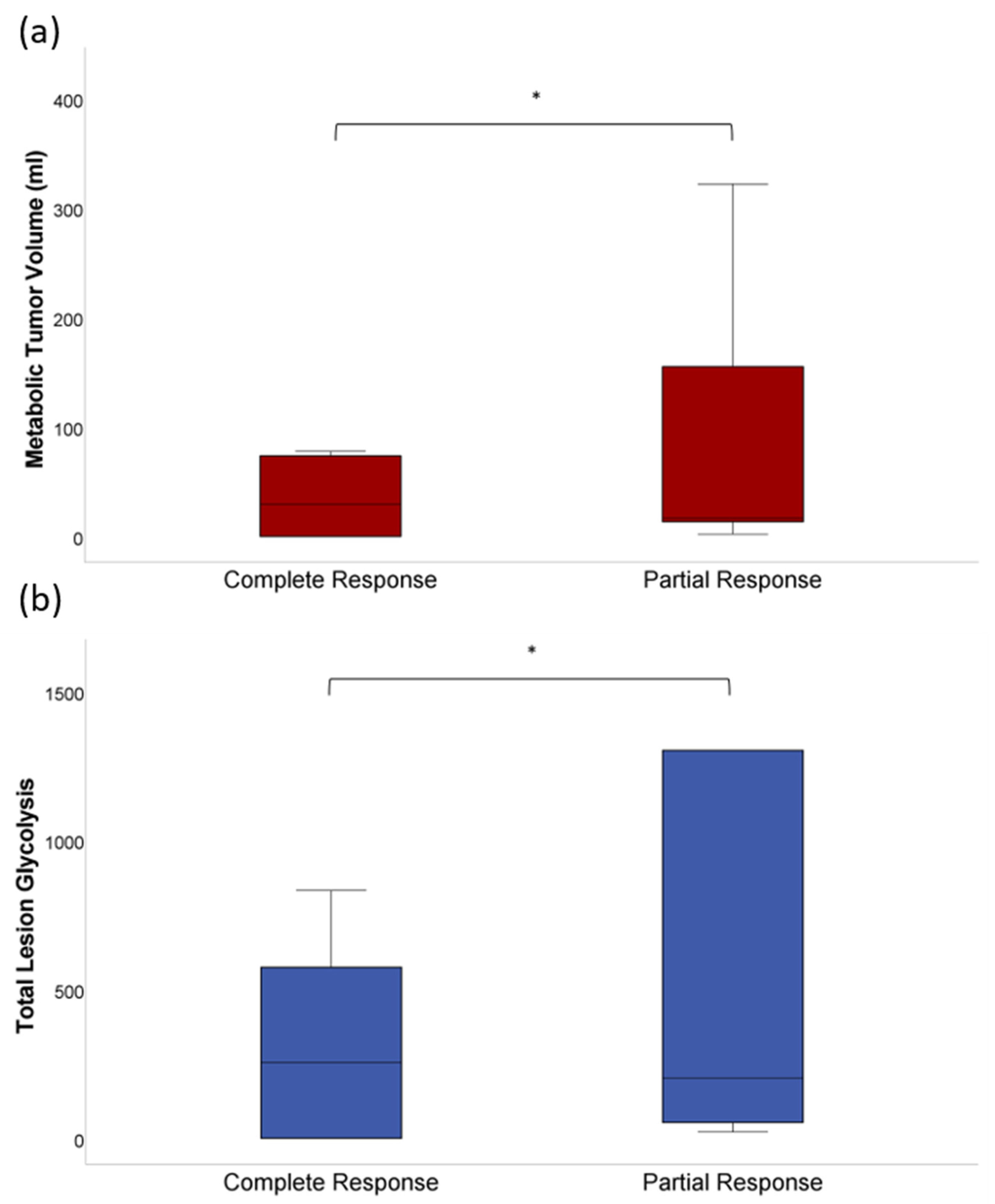
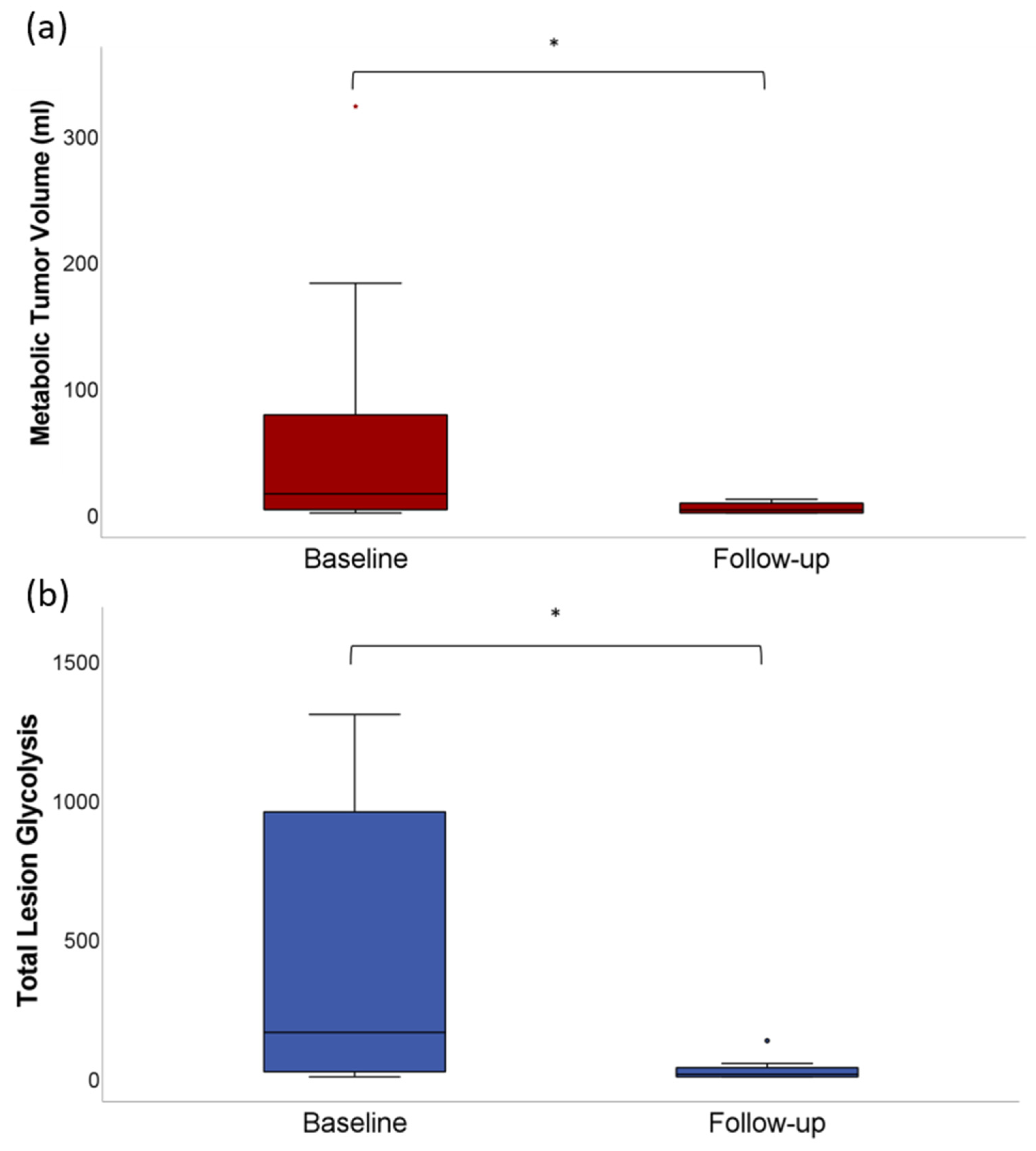
3.1. Quantitative Analysis of Tumor Volumetric Parameters
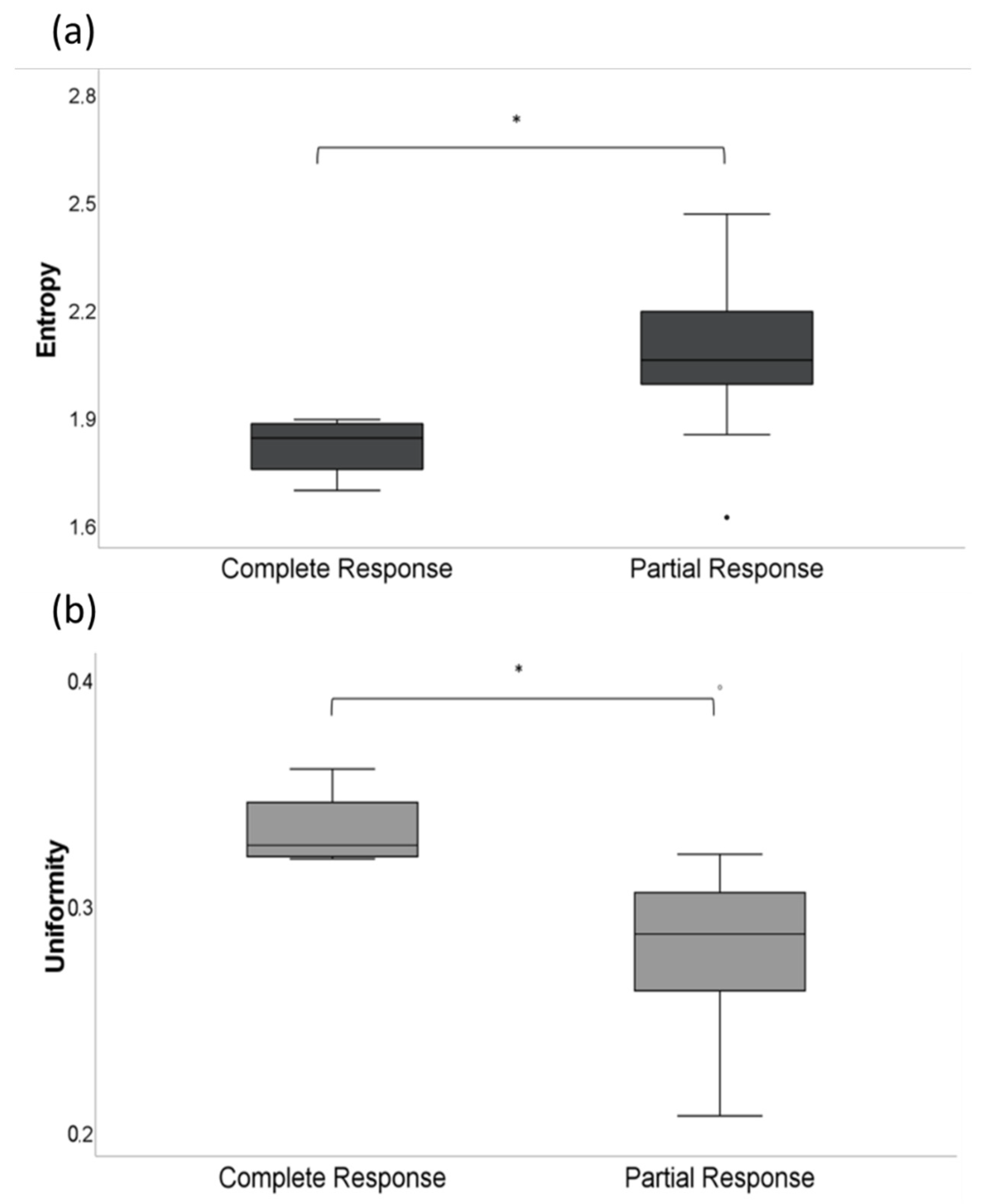
3.2. CT-Texture Analysis
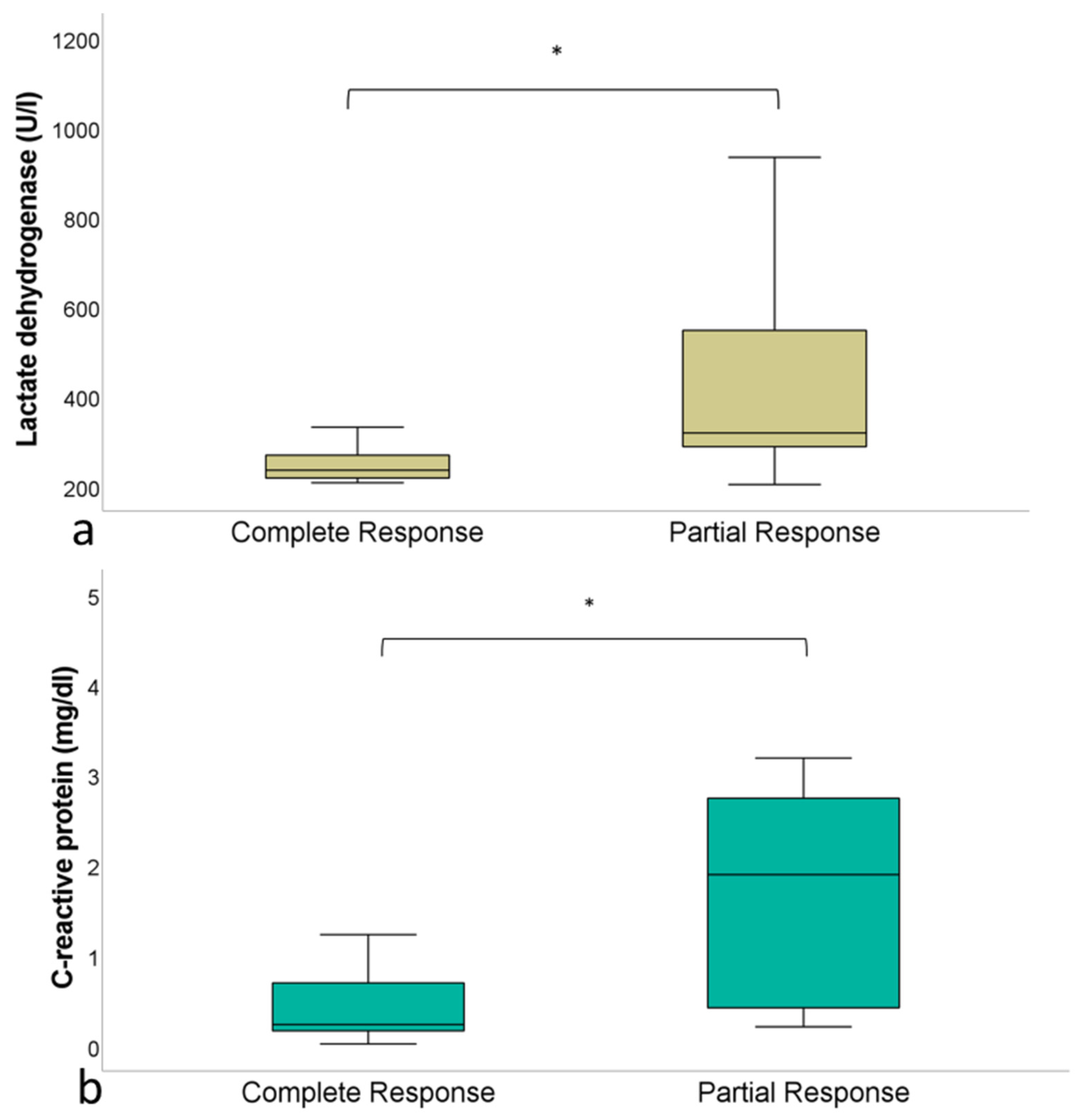
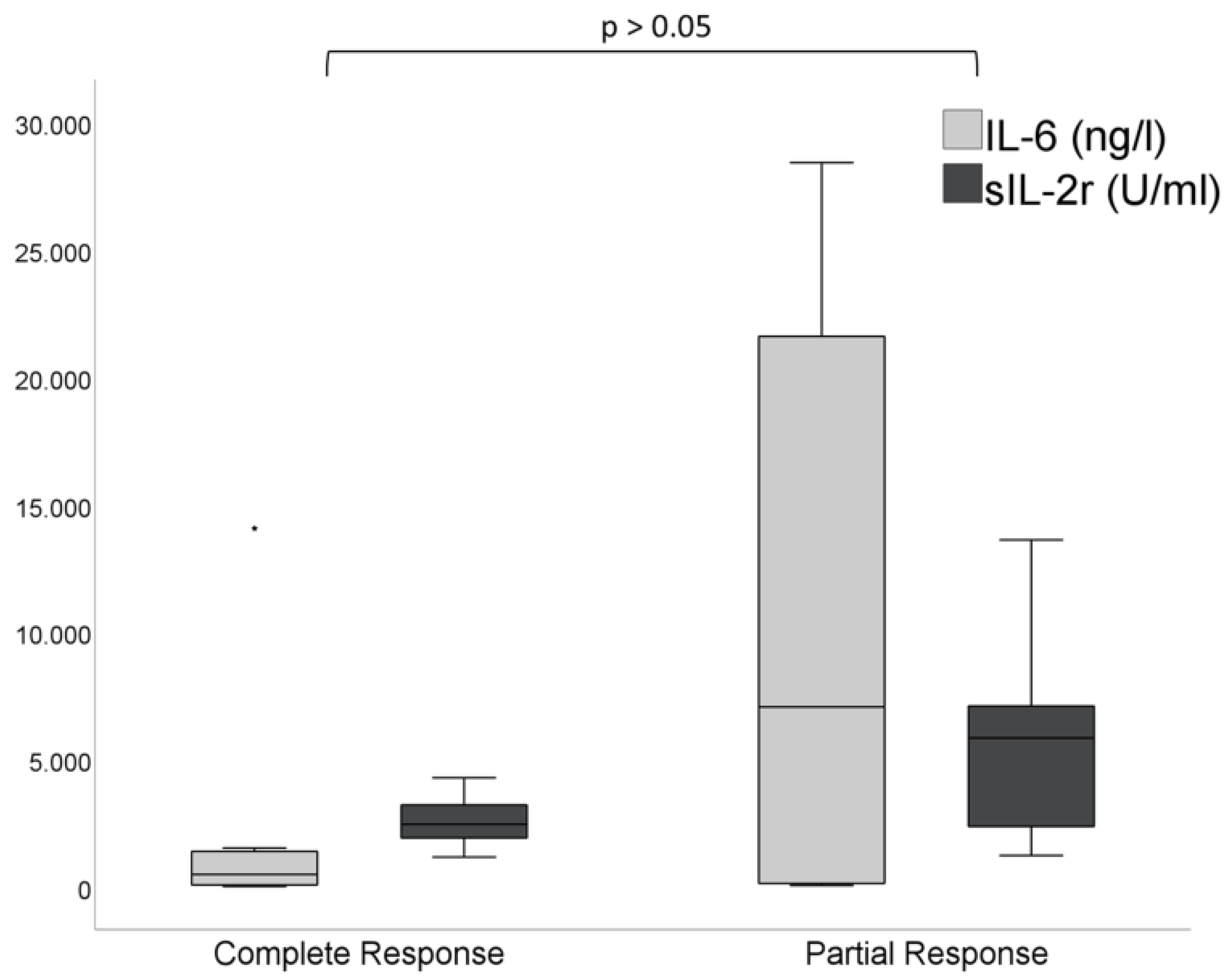
3.3. Laboratory Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | chimeric antigen receptor |
| CD19 | cluster of differentiation 19 |
| CR | complete remission |
| CRS | cytokine release syndrome |
| CTTA | CT texture analysis |
| Deauville 5PS | Deauville five-point scale |
| DLBCL | diffuse large B-cell lymphoma |
| FDG | 18F- Fluorodeoxyglucose |
| ICANS | immune effector cell-associated neurotoxicity |
| IL | interleukin |
| IPI | international prognostic index |
| LDH | lactate dehydrogenase |
| MBq | megabecquerel |
| MTV | metabolic tumor volume |
| PFS | progression-free survival |
| PR | partial remission |
| R-CHOP | Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone |
| ROI | region of interest |
| SUV | uptake value |
| TLG | total lesion glycolysis |
| VOI | volume of interest |
References
- Hosen, N. CAR T cell therapy. Immunol. Med. 2020, 44, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Valera, A.; Colomo, L.; Setoain, X.; Rodríguez, S.; Martínez-Trillos, A.; Giné, E.; Dlouhy, I.; Magnano, L.; Gaya, A.; et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann. Hematol. 2014, 94, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishton, M.; Hughes, S.M.; Richardson, F.; James, E.; Bessell, E.; Sovani, V.; Ganatra, R.; Haynes, A.P.; McMillan, A.K.; Fox, C.P. Delineating outcomes of patients with diffuse large b cell lymphoma using the national comprehensive cancer network-international prognostic index and positron emission tomography-defined remission status; a population-based analysis. Br. J. Haematol. 2015, 172, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisselbrecht, C.; Glass, B.; Mounier, N.; Gill, D.S.; Linch, D.C.; Trneny, M.; Bosly, A.; Ketterer, N.; Shpilberg, O.; Hagberg, H.; et al. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J. Clin. Oncol. 2010, 28, 4184–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Baratto, L.; Wu, F.; Minamimoto, R.; Hatami, N.; Liang, T.; Sabile, J.; Advani, R.H.; Mittra, E. Correlation of 18-fluorodeoxyglucose PET/computed tomography parameters and clinical features to predict outcome for diffuse large B-cell lymphoma. Nucl. Med. Commun. 2021, 42, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiao, X.; Jiang, C.; Liu, S.; Zhou, Z. Texture Analysis Improves the Value of Pretreatment 18F-FDG PET/CT in Predicting Interim Response of Primary Gastrointestinal Diffuse Large B-Cell Lymphoma. Contrast Media Mol. Imaging 2020, 2020, 2981585. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Fruchart, C.; Nganoa, C.; Gac, A.-C.; Lasnon, C. Baseline 18F-FDG PET radiomic features as predictors of 2-year event-free survival in diffuse large B cell lymphomas treated with immunochemotherapy. Eur. Radiol. 2020, 30, 4623–4632. [Google Scholar] [CrossRef]
- Shagera, Q.A.; Cheon, G.J.; Koh, Y.; Yoo, M.Y.; Kang, K.W.; Lee, D.S.; Kim, E.E.; Yoon, S.-S.; Chung, J.-K. Prognostic value of metabolic tumour volume on baseline 18F-FDG PET/CT in addition to NCCN-IPI in patients with diffuse large B-cell lymphoma: Further stratification of the group with a high-risk NCCN-IPI. Eur. J. Pediatr. 2019, 46, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, stag-ing, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Zhai, W.; Cheng, S.; Zhang, H.; Xie, Y.; He, W. Predictive value of F-18 FDG PET/CT quantization parameters for progression-free survival in patients with diffuse large B-cell lymphoma. Hematology 2015, 21, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Chen, Y.; Huang, H.; Zhou, X.; Liu, J.; Huang, G. Prognostic value of total lesion glycolysis of baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B-cell lymphoma. Oncotarget 2016, 7, 83544–83553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Wu, K.; Liu, Y.; Jiang, Q.; Xie, Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: A meta-analysis with 702 participants. Med. Oncol. 2014, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sasanelli, M.; Meignan, M.; Haioun, C.; Berriolo-Riedinger, A.; Casasnovas, R.-O.; Biggi, A.; Gallamini, A.; Siegel, B.A.; Cashen, A.F.; Véra, P.; et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur. J. Pediatr. 2014, 41, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Meignan, M.; Cottereau, A.-S.; Versari, A.; Chartier, L.; Dupuis, J.; Boussetta, S.; Grassi, I.; Casasnovas, R.-O.; Haioun, C.; Tilly, H.; et al. Baseline Metabolic Tumor Volume Predicts Outcome in High–Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J. Clin. Oncol. 2016, 34, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Talbot, M.; Fruchart, C.; Damaj, G.; Lasnon, C. Diagnostic and prognostic value of baseline FDG PET/CT skeletal textural features in diffuse large B cell lymphoma. Eur. J. Pediatr. 2017, 45, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Mannil, M.; von Spiczak, J.; Manka, R.; Alkadhi, H. Texture Analysis and Machine Learning for Detecting Myocardial Infarction in Noncontrast Low-Dose Computed Tomography: Unveiling the Invisible. Investig. Radiol. 2018, 53, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Neisius, U.; El-Rewaidy, H.; Nakamori, S.; Rodriguez, J.; Manning, W.J.; Nezafat, R. Radiomic Analysis of Myocardial Native T1 Imaging Discriminates Between Hypertensive Heart Disease and Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; O’Doherty, M.J.; Marsden, P.K. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Shiradkar, R.; Liu, Z. Integrating pathomics with radiomics and genomics for cancer prognosis: A brief review. Chin. J. Cancer Res. (Chung-Kuo Yen Cheng Yen Chiu) 2021, 33, 563–573. [Google Scholar] [CrossRef] [PubMed]
| I | II | III | IV | |
|---|---|---|---|---|
| Ann-Arbor stage | 2 | 3 | 5 | 11 |
| International Prognostic Index Score | 3 | 0 | 9 | 9 |
| Cytokine Release Syndrome Grade | 10 | 7 | 2 | 1 |
| 1st Order Feature | Definition |
|---|---|
| Entropy |
|
| Mean |
|
| Skewness |
|
| Uniformity |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinert, C.P.; Perl, R.M.; Faul, C.; Lengerke, C.; Nikolaou, K.; Dittmann, H.; Bethge, W.A.; Horger, M. Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy. J. Clin. Med. 2022, 11, 1522. https://doi.org/10.3390/jcm11061522
Reinert CP, Perl RM, Faul C, Lengerke C, Nikolaou K, Dittmann H, Bethge WA, Horger M. Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy. Journal of Clinical Medicine. 2022; 11(6):1522. https://doi.org/10.3390/jcm11061522
Chicago/Turabian StyleReinert, Christian Philipp, Regine Mariette Perl, Christoph Faul, Claudia Lengerke, Konstantin Nikolaou, Helmut Dittmann, Wolfgang A. Bethge, and Marius Horger. 2022. "Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy" Journal of Clinical Medicine 11, no. 6: 1522. https://doi.org/10.3390/jcm11061522
APA StyleReinert, C. P., Perl, R. M., Faul, C., Lengerke, C., Nikolaou, K., Dittmann, H., Bethge, W. A., & Horger, M. (2022). Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy. Journal of Clinical Medicine, 11(6), 1522. https://doi.org/10.3390/jcm11061522







