Thickness Measurement of Endothelium-Descemet Membrane in Descemt Membrane Detachment Patients Using High-Definition Optical Coherence Tomography
Abstract
:1. Introduction
2. Subjects
3. Image Acquisition and Processing
4. Statistical Analysis
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melles, G.R.; San Ong, T.; Ververs, B.; van der Wees, J. Descemet membrane endothelial keratoplasty (DMEK). Cornea 2006, 25, 987–990. [Google Scholar] [PubMed]
- Godinho, J.V.; Mian, S. Update on Descemet membrane endothelial keratoplasty. Curr. Opin. Ophthalmol. 2019, 30, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Gupta, P.; Lass, J.; Price, F.W. EK (DLEK, DSEK, DMEK): New Frontier in Cornea Surgery. Annu. Rev. Vis. Sci. 2017, 3, 69–90. [Google Scholar] [CrossRef]
- Price, M.O.; Price, F.W., Jr. Descemet’s membrane endothelial keratoplasty surgery: Update on the evidence and hurdles to acceptance. Curr. Opin. Ophthalmol. 2013, 24, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Holz, H.A.; Meyer, J.J.; Espandar, L.; Tabin, G.C.; Mifflin, M.D.; Moshirfar, M. Corneal profile analysis after Descemet stripping endothelial keratoplasty and its relationship to postoperative hyperopic shift. J. Cataract. Refract. Surg. 2008, 34, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Price, F.W., Jr.; Feng, M.T.; Price, M.O. Evolution of Endothelial Keratoplasty: Where Are We Headed? Cornea 2015, 34 (Suppl. 10), S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.E.; Laaser, K.; Cursiefen, C.; Heindl, L.M.; Schlötzer-Schrehardt, U.; Riss, S.; Bachmann, B.O. A Stepwise Approach to Donor Preparation and Insertion Increases Safety and Outcome of Descemet Membrane Endothelial Keratoplasty. Cornea 2011, 30, 580–587. [Google Scholar] [CrossRef]
- Kruse, F.E.; Schrehardt, U.S.; Tourtas, T. Optimizing outcomes with Descemet’s membrane endothelial keratoplasty. Curr. Opin. Ophthalmol. 2014, 25, 325–334. [Google Scholar] [CrossRef]
- Steven, P.; Le Blanc, C.; Velten, K.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Hüttmann, G.; Cursiefen, C. Optimizing Descemet Membrane Endothelial Keratoplasty Using Intraoperative Optical Coherence Tomography. JAMA Ophthalmol. 2013, 131, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- De Mora MR, C.; Groeneveld-van Beek, E.A.; Frank, L.E.; van der Wees, J.; Oellerich, S.; Bruinsma, M.; Melles, G.R. Association Between Graft Storage Time and Donor Age with Endothelial Cell Density and Graft Adherence After Descemet Membrane Endothelial Keratoplas-ty. JAMA Ophthalmol. 2016, 134, 91–94. [Google Scholar]
- Bizheva, K.; Haines, L.; Mason, E.; MacLellan, B.; Tan, B.; Hileeto, D.; Sorbara, L. In Vivo Imaging and Morphometry of the Human Pre-Descemet’s Layer and Endothelium with Ultrahigh-Resolution Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2782–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wertheimer, C.M.; Elhardt, C.; Wartak, A.; Luft, N.; Kassumeh, S.; Dirisamer, M.; Siedlecki, J.; Vounotrypidis, E.; Priglinger, S.G.; Mayer, W.J. Corneal optical density in Fuchs endothelial dystrophy determined by anterior segment optical coherence tomography. Eur. J. Ophthalmol. 2020, 31, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Keidel, L.; Elhardt, C.; Hohenfellner, K.; Priglinger, S.; Schworm, B.; Wertheimer, C.; Priglinger, C.; Luft, N.; Pozza, S.B.D.; Bergmann, C.; et al. Establishing an objective biomarker for corneal cystinosis using a threshold-based Spectral domain optical coherence tomography imaging algorithm. Acta Ophthalmol. 2020, 99, e189–e195. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.A.; Gameiro, G.R.; Ruggeri, M.; Elsawy, A.; Sayed-Ahmed, I.; Roongpoovapatr, V.; Abdel-Mottaleb, M.; Shousha, M.A. Evaluation of endothelial/Descemet membrane complex of eye bank donor corneas using enhanced depth imaging optical coherence tomography. Clin. Ophthalmol. 2019, 13, 789–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livny, E.; Bahar, I.; Nahum, Y. “Ghost DMEK” Technique: Circular Peripheral Staining of Descemet’s Membrane Endo-thelial Keratoplasty Grafts. Cornea 2019, 38, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-E.; Devarajan, K.; Seah, X.-Y.; Lin, S.-J.; Peh, G.S.L.; Cajucom-Uy, H.Y.; Ang, M.H.N.; Tan, D.T.H. Lamellar Dissection Technique for Descemet Membrane Endothelial Keratoplasty Graft Preparation. Cornea 2020, 39, 23–29. [Google Scholar] [CrossRef]
- Xu, L.; Cao, W.F.; Wang, Y.X.; Chen, C.X.; Jonas, J.B. Anterior Chamber Depth and Chamber Angle and Their Associations with Ocular and General Parameters: The Beijing Eye Study. Am. J. Ophthalmol. 2008, 145, 929–936. [Google Scholar] [CrossRef]
- Seitz, B.; Daas, L.; Flockerzi, E.; Suffo, S. Descemet membrane endothelial keratoplasty DMEK-Donor and recipient step by step. Ophthalmologe 2020, 117, 811–828. [Google Scholar] [CrossRef]
- Murphy, C.; Alvarado, J.; Juster, R. Prenatal and postnatal growth of the human Descemet’s membrane. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1402–1415. [Google Scholar]
- Johnson, D.H.; Bourne, W.M.; Campbell, R.J. The Ultrastructure of Descemet’s Membrane. Arch. Ophthalmol. 1982, 100, 1942–1947. [Google Scholar] [CrossRef]
- Abou Shousha, M.; Perez, V.L.; Wang, J.; Ide, T.; Jiao, S.; Chen, Q.; Chang, V.; Buchser, N.; Dubovy, S.R.; Yoo, S.H.; et al. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology 2010, 117, 1220–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human corneal anatomy redefined: A novel pre-Descemet’s layer (Dua’s layer). Ophthalmology 2013, 120, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Sinha, R.; D’Souza, S.; Potgieter, F.; Ross, A.; Kenawy, M.; Scott, I.; Said, D.G. “Descemet Membrane Detachment”: A Novel Concept in Diagnosis and Classi-fication. Am. J. Ophthalmol. 2020, 218, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, Y.; Uji, A.; Yoshimura, N. Swept-Source Optical Coherence Tomography Detecting Intraoperative Acute Descemet’s Fold Formation. Case Rep. Ophthalmol. 2016, 7, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.E. Corneal edema: Cause and treatment. Surv. Ophthalmol. 1975, 20, 190–204. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Rodrigues, M.M.; Krachmer, J.H.; Fujikawa, L.S. Immunohistochemical characterization of extracellular matrix in the developing human cornea. Curr. Eye Res. 1986, 5, 105–117. [Google Scholar] [CrossRef]
- Kohno, T.; Sorgente, N.; Ishibashi, T.; Goodnight, R.; Ryan, S.J. Immunofluorescent studies of fibronectin and laminin in the human eye. Investig. Ophthalmol. Vis. Sci. 1987, 28, 506–514. [Google Scholar]
- Levy, S.G.; McCartney, A.C.; Moss, J. The distribution of fibronectin and P component in Descemet’s membrane: An immunoelectron microscopic study. Curr. Eye Res. 1995, 14, 865–870. [Google Scholar] [CrossRef]
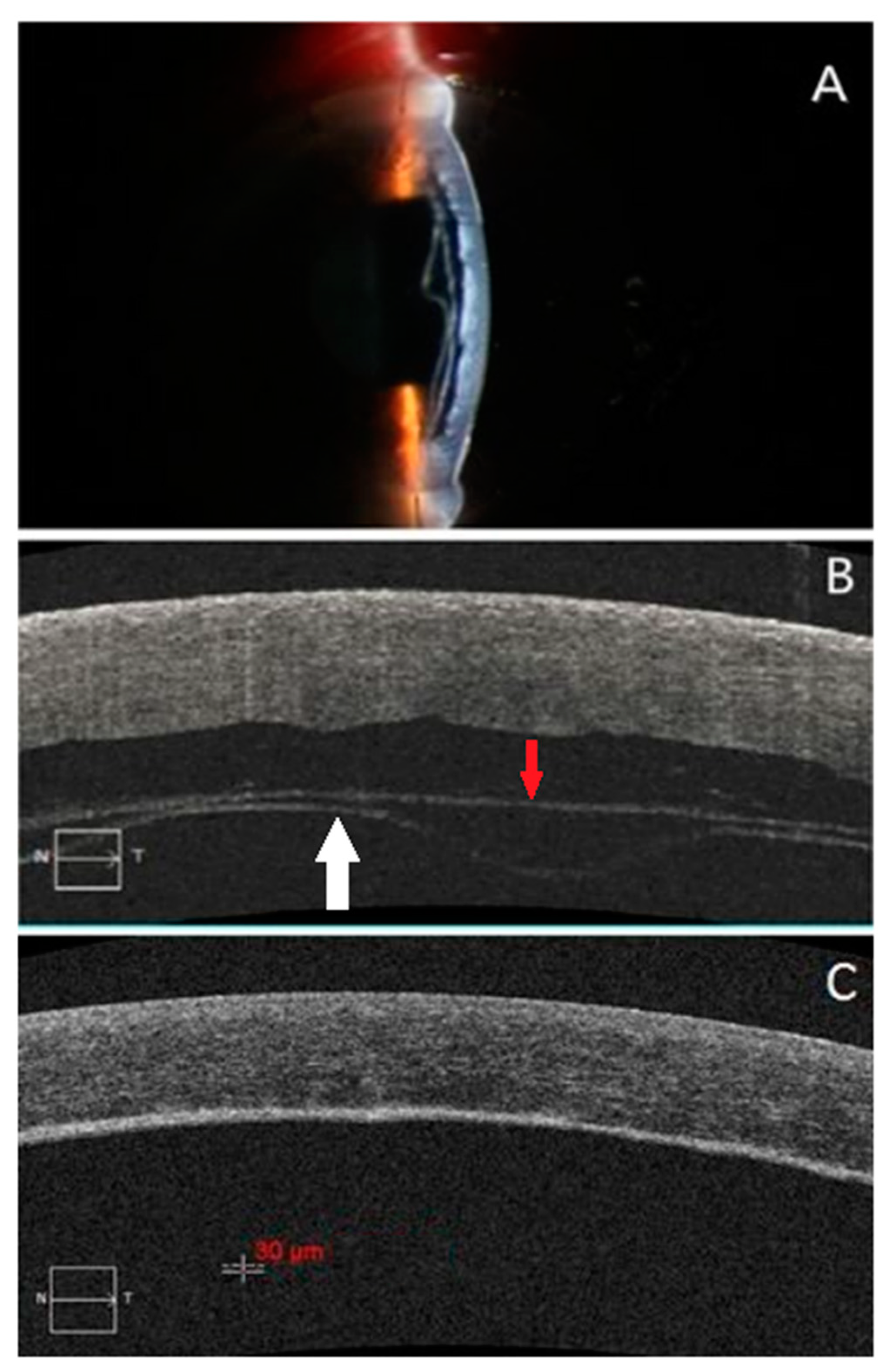
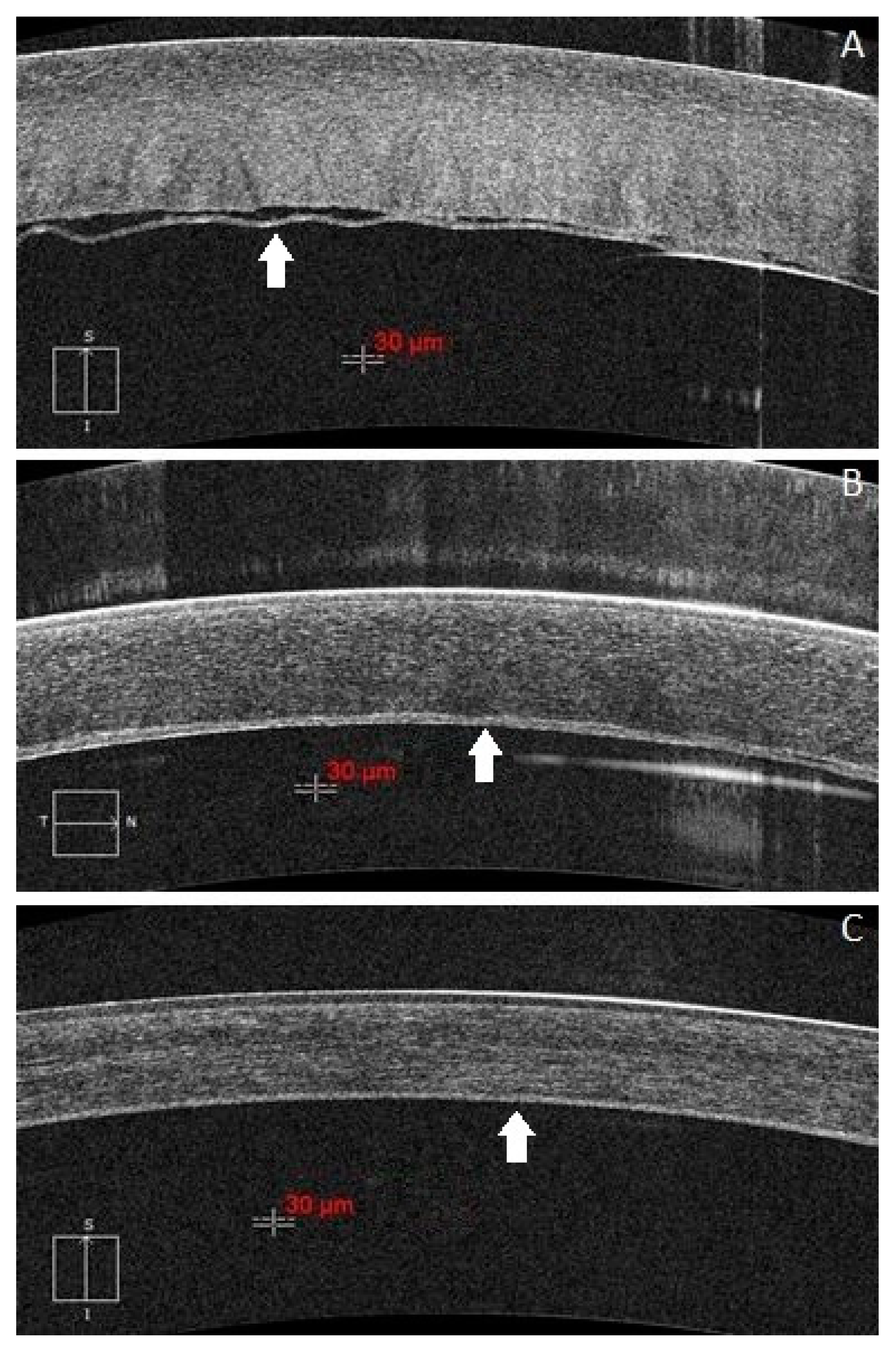
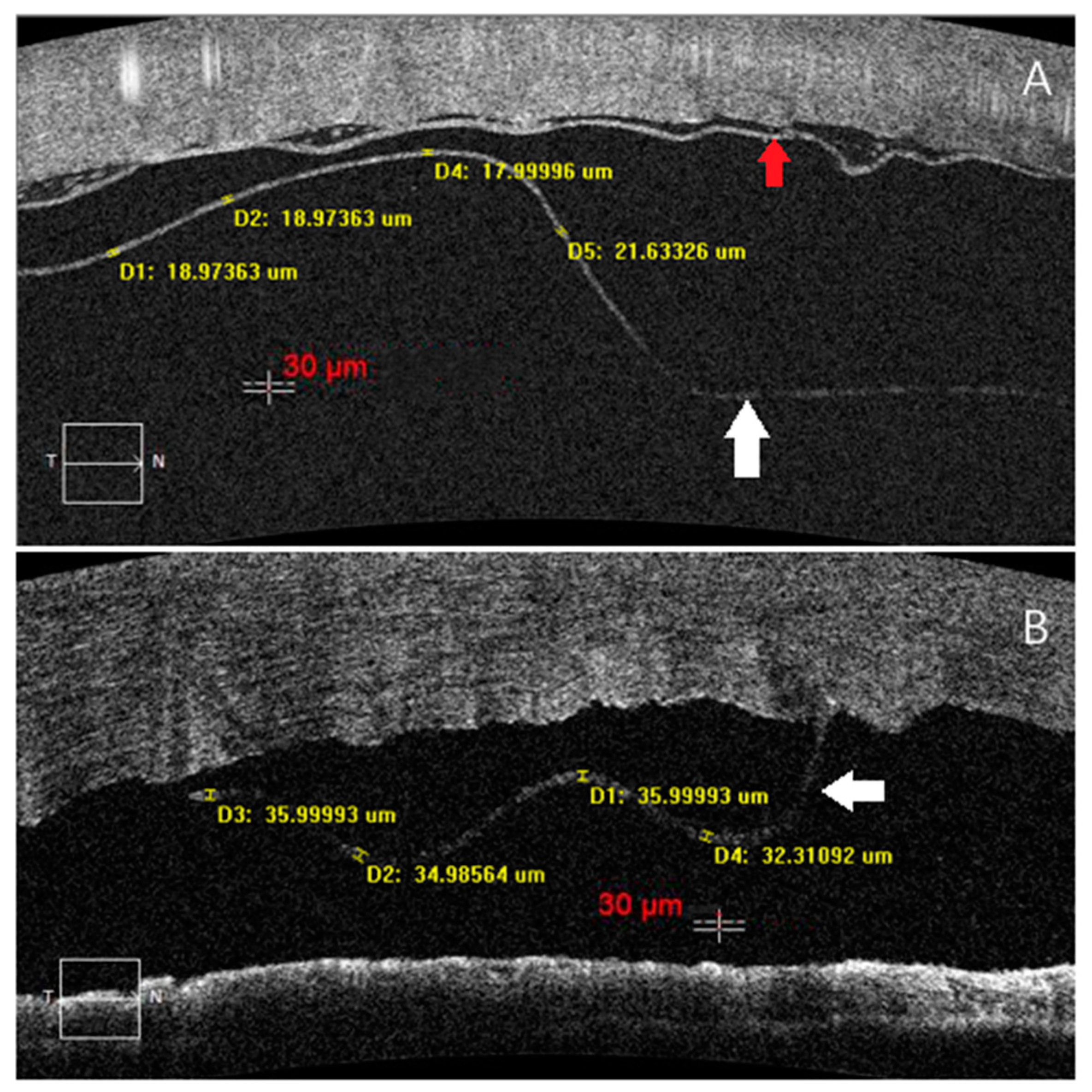
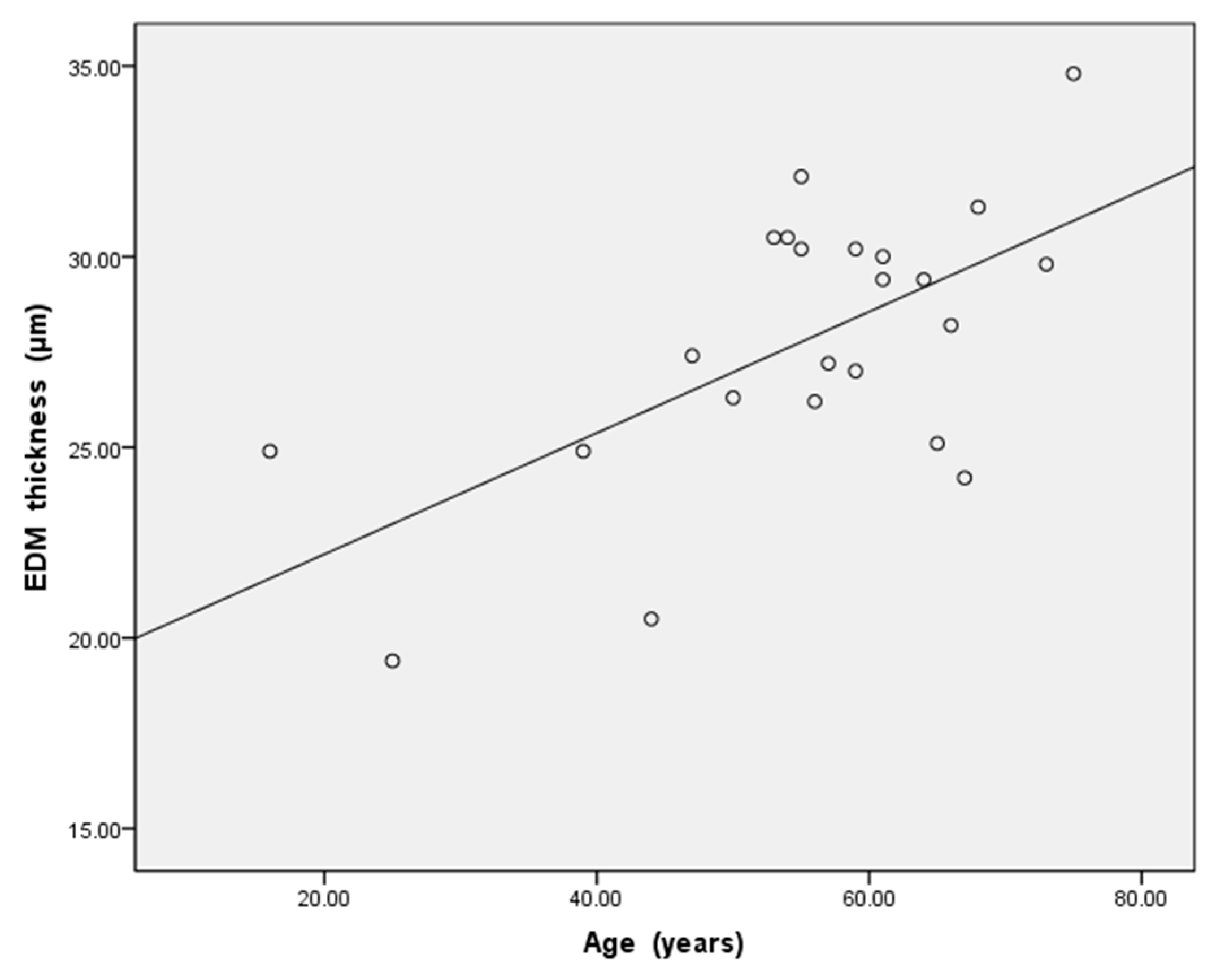
| ≤50-Year-Old n = 6 | >50-Year-Old n = 17 | p Value | ||
|---|---|---|---|---|
| Age (years) | 36.8 ± 13.5 | 61.6 ± 6.6 | 0.015 | |
| the EDM thickness on the first post-operative day (μm) | 23.9 ± 3.2 | 29.2 ± 2.6 | 0.001 | |
| the DMD cause | dDALK | 1 | 1 | |
| pdDALK | 5 | 7 | ||
| PKP | 0 | 2 | ||
| DMEK | 0 | 4 | ||
| phaco | 0 | 3 | ||
| No. | Age (year) | The EDM Thickness on the First Examination (μm) | The EDM Thickness on the Second Examination (μm) | The Interval between Two Examinations (day) | The DMD Cause |
|---|---|---|---|---|---|
| 1 | 16 | 24.9 | 25.1 | 1 | dDALK |
| 2 | 25 | 19.4 | 19.4 | 4 | pdDALK |
| 7 | 53 ! | 30.5 | 30.9 | 5 | PKP |
| 8 | 54 | 30.5 | 30.7 | 1 | pdDALK |
| 15 | 61 | 30.0 | 30.3 | 1 | dDALK |
| 17 | 64 ! | 29.4 | 29.5 | 1 | DMEK |
| 18 | 65 ! | 25.1 | 24.4 | 3 | DMEK |
| 22 | 73 | 29.8 | 30.5 | 1 | phcao |
| No. | Age (year) | The EDM Thickness with DM Detachment (μm) | The EDM Thickness with DM Reattachment (μm) | The DMD Cause |
|---|---|---|---|---|
| 1 | 16 | 24.9 | 24.2 | dDALK |
| 7 | 53 ! | 30.5 | 27 | PKP |
| 11 | 56 ! | 26.2 | 24.2 | DMEK |
| 15 | 61 | 30.0 | 24.4 | dDALK |
| 17 | 64 ! | 29.4 | 24.2 | DMEK |
| 18 | 65 ! | 25.1 | 18.3 | DMEK |
| 20 | 67 | 24.2 | 21.4 | phaco |
| 22 | 73 | 29.8 | 24.5 | phaco |
| 23 | 75 ! | 34.8 | 22.7 | PKP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Xu, L.; Su, G.; Luo, B.; Gao, J.; Tan, Y.; Zhang, H.; Li, G. Thickness Measurement of Endothelium-Descemet Membrane in Descemt Membrane Detachment Patients Using High-Definition Optical Coherence Tomography. J. Clin. Med. 2022, 11, 1534. https://doi.org/10.3390/jcm11061534
Wang W, Xu L, Su G, Luo B, Gao J, Tan Y, Zhang H, Li G. Thickness Measurement of Endothelium-Descemet Membrane in Descemt Membrane Detachment Patients Using High-Definition Optical Coherence Tomography. Journal of Clinical Medicine. 2022; 11(6):1534. https://doi.org/10.3390/jcm11061534
Chicago/Turabian StyleWang, Wei, Lingjuan Xu, Guanyu Su, Ban Luo, Jing Gao, Yongyao Tan, Hong Zhang, and Guigang Li. 2022. "Thickness Measurement of Endothelium-Descemet Membrane in Descemt Membrane Detachment Patients Using High-Definition Optical Coherence Tomography" Journal of Clinical Medicine 11, no. 6: 1534. https://doi.org/10.3390/jcm11061534
APA StyleWang, W., Xu, L., Su, G., Luo, B., Gao, J., Tan, Y., Zhang, H., & Li, G. (2022). Thickness Measurement of Endothelium-Descemet Membrane in Descemt Membrane Detachment Patients Using High-Definition Optical Coherence Tomography. Journal of Clinical Medicine, 11(6), 1534. https://doi.org/10.3390/jcm11061534





