Safety and Efficacy of Sorafenib and Lenvatinib in Patients Who Underwent Surgery or Whole-Brain Radiotherapy for Brain Metastasis of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Follow-Up Outcomes and Data Collected
2.3. Statistical Analysis
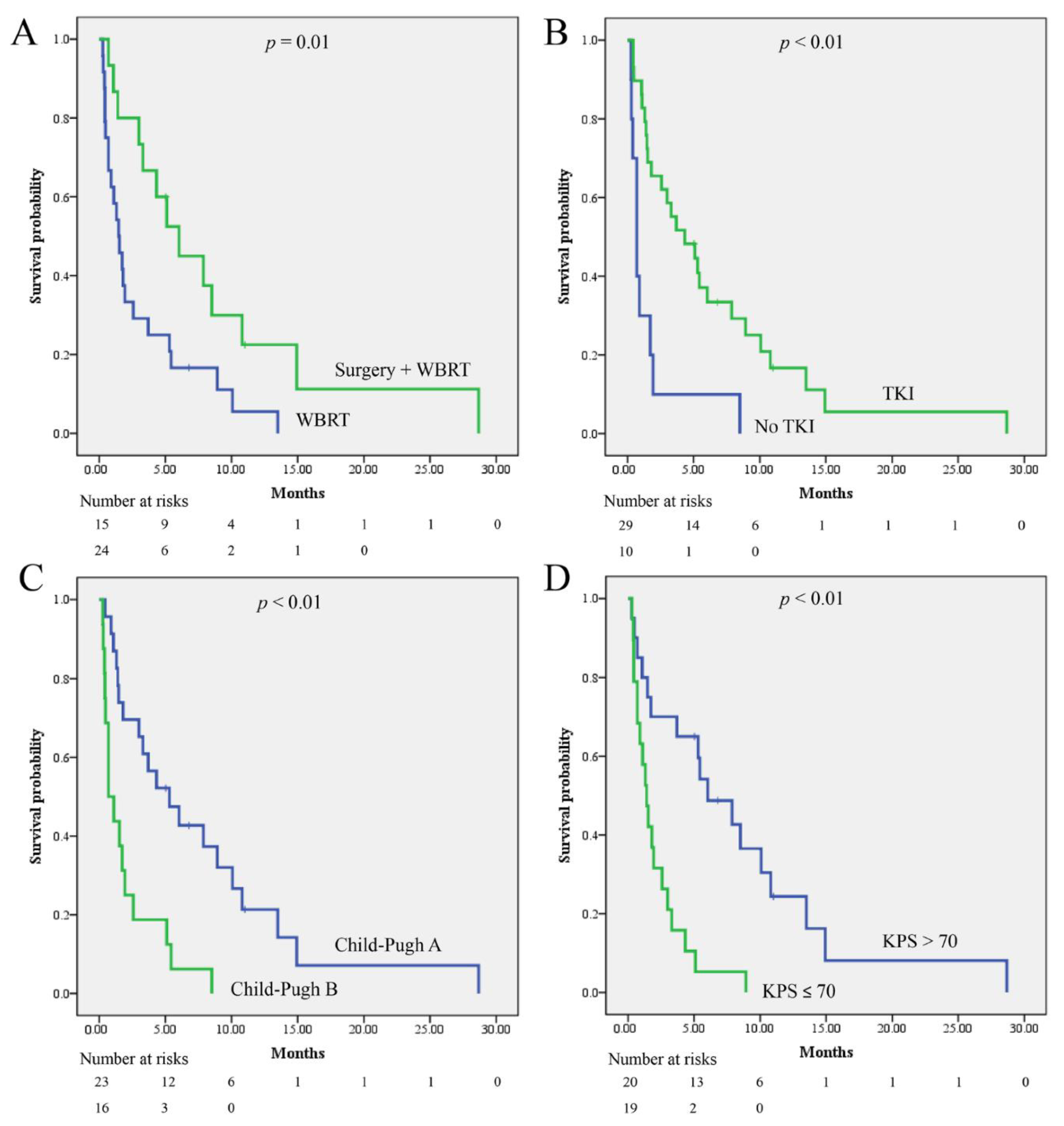
3. Results
3.1. General Demographic Characteristics
3.2. Safety of the TKIs
3.3. Survival and Prognostic Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273.e1. [Google Scholar] [CrossRef]
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M.; Chen, K.-Y.; Tseng, J.-H.; Ho, M.-C.; Lee, R.-C.; Liang, P.-C.; Liao, L.-Y.; Huang, K.-W.; et al. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2021, 120, 1051–1060. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL–EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Bai, Y.; Lim, H.Y.; Thongprasert, S.; Chao, Y.; Fan, J.; Yang, T.S.; Bhudhisawasdi, V.; Kang, W.K.; Zhou, Y.; et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013, 31, 3501–3508. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs. sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet. Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Wang, S.; Wang, A.; Lin, J.; Xie, Y.; Wu, L.; Huang, H.; Bian, J.; Yang, X.; Wan, X.; Zhao, H.; et al. Brain metastases from hepatocellular carcinoma: Recent advances and future avenues. Oncotarget 2017, 8, 25814–25829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, H.J.; Cho, B.C.; Sohn, J.H.; Shin, S.J.; Kim, S.H.; Kim, J.H.; Yoo, N.C. Brain metastases from hepatocellular carcinoma: Prognostic factors and outcome: Brain metastasis from HCC. J. Neuro-Oncol. 2009, 91, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.C.; Sung, P.S.; Song, D.S.; Kwon, J.H.; Nam, S.W.; Yoon, D.J.; Jang, J.W.; Choi, J.Y.; Yoon, S.K.; Moon, S.W.; et al. Control of intracranial disease is associated with improved survival in patients with brain metastasis from hepatocellular carcinoma. Int. J. Clin. Oncol. 2019, 24, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.B.; Ke, C.; Zhang, G.H.; Zhang, X.H.; Sai, K.; Chen, Z.P.; Mou, Y.G. Brain metastases from hepatocellular carcinoma: Clinical features and prognostic factors. BMC Cancer 2012, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Na, Y.C.; Lee, W.H.; Kim, J.H.; Chang, W.S.; Jung, H.H.; Chang, J.H.; Chang, J.W.; Park, Y.G. Treatment Options of Metastatic Brain Tumors from Hepatocellular Carcinoma: Surgical Resection vs. Gamma Knife Radiosurgery vs. Whole Brain Radiation Therapy. Brain Tumor Res. Treat. 2013, 1, 78–84. [Google Scholar] [CrossRef][Green Version]
- Hata, T.; Uwagawa, T.; Yanaga, K. Intracranial Bleeding during Treatment with Sorafenib for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 520–521. [Google Scholar] [CrossRef]
- Pouessel, D.; Culine, S. High frequency of intracerebral hemorrhage in metastatic renal carcinoma patients with brain metastases treated with tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor. Eur. Urol. 2008, 53, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Adhoute, X.; Penaranda, G.; Perrier, H.; Castellani, P.; Oules, V.; Bourlière, M. Sorafenib: Experience and Better Manage-ment of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer 2019, 8, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Raskett, C.M.; Gilbert, C.A.; Ross, A.H.; Altieri, D.C. Sorafenib exerts anti-glioma activity in vitro and in vivo. Neurosci. Lett. 2010, 478, 165–170. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.; Wang, W.; Liu, X.; Wan, J.; Yuan, Y.; Li, X.; Ma, L.; Liu, X. Oxidative Stress Activated by Sorafenib Alters the Temozolomide Sensitivity of Human Glioma Cells Through Autophagy and JAK2/STAT3-AIF Axis. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yamada, T.; Arai, S.; Fukuda, K.; Taniguchi, H.; Tanimoto, A.; Nishiyama, A.; Takeuchi, S.; Yamashita, K.; Ohtsubo, K.; et al. Distribution and Activity of Lenvatinib in Brain Tumor Models of Human Anaplastic Thyroid Cancer Cells in Severe Combined Immune Deficient Mice. Mol. Cancer Ther. 2019, 18, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, C.L.; Zhang, Z.M.; Lv, L.J.; Qiao, H.B.; Chen, X.J. A multi-targeted tyrosine kinase inhibitor lenvatinib for the treatment of mice with advanced glioblastoma. Mol. Med. Rep. 2017, 16, 7105–7111. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; McCully, C.; Cruz, R.; Cole, D.E.; Fox, E.; Balis, F.M.; Widemann, B.C. The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non-human primates. Investig. New Drugs 2012, 30, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kizilbash, S.H.; Laramy, J.K.; Gampa, G.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm. Res. 2018, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Z.-H.; Qu, X.-J. The Adverse Effects of Sorafenib in Patients with Advanced Cancers. Basic Clin. Pharmacol. Toxicol. 2015, 116, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Pomej, K.; Scheiner, B.; Park, D.; Bauer, D.; Balcar, L.; Meischl, T.; Mandorfer, M.; Reiberger, T.; Müller, C.; Trauner, M.; et al. Vascular Complications in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2020, 12, 2961. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhou, F.; Shao, J.H.; Wu, L.Q.; Yu, X.; Yin, X.B. Bleeding risk in cancer patients treated with sorafenib: A meta-analysis of randomized controlled trials. J. Cancer Res. Ther. 2018, 14, S948–S956. [Google Scholar] [CrossRef] [PubMed]
- Uchida-Kobayashi, S.; Kageyama, K.; Yamamoto, A.; Ikenaga, H.; Yoshida, K.; Kotani, K.; Kimura, K.; Odagiri, N.; Hagihara, A.; Fujii, H.; et al. Lenvatinib-Induced Tumor-Related Hemorrhages in Patients with Large Hepatocellular Carcinomas. Oncology 2021, 99, 186–191. [Google Scholar] [CrossRef]
- Kotani, K.; Uchida-Kobayashi, S.; Yoshida, K.; Kawamura, E.; Fujii, H.; Hagihara, A.; Enomoto, M.; Tamori, A.; Kawada, N. Lenvatinib-Induced Tumor-Related Hemorrhage in Patients with Unresectable Hepatocellular Carcinoma. Am. J. Gastroenterol. 2021, 116, 631. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mahapatra, S.; Bandyopadhyay, D.; Samanta, S.; Chakraborty, S.; Philpotts, L.L.; Jahangir, E.; Roy, B. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: A network meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 157, 103186. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Moriguchi, M.; Aramaki, T.; Mitsuya, K.; Asakura, K.; Sawada, A.; Endo, M.; Nakasu, Y. Brain metastasis from hepatocellular carcinoma: The impact of radiotherapy on control of intracranial hemorrhage. Hepatol. Res. 2015, 45, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Falkson, S.R.; Bhambhvani, H.P.; Hayden Gephart, M. Hepatocellular Carcinoma Brain Metastases: A Single-Institution Experience. World Neurosurg. 2020, 140, e27–e32. [Google Scholar] [CrossRef]
- Moravan, M.J.; Fecci, P.E.; Anders, C.K.; Clarke, J.M.; Salama, A.K.; Adamson, J.D.; Floyd, S.R.; Torok, J.A.; Salama, J.K.; Sampson, J.H.; et al. Current multidisciplinary management of brain metastases. Cancer 2020, 126, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal avoidance dsuring whole-brain radiotherapy plus memantine for patients with brain metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Patel, A.J.; Suki, D.; Hatiboglu, M.A.; Abouassi, H.; Shi, W.; Wildrick, D.M.; Lang, F.F.; Sawaya, R. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J. Neurosurg. 2010, 113, 181–189. [Google Scholar] [CrossRef]
- Azoulay, M.; Santos, F.; Shenouda, G.; Petrecca, K.; Oweida, A.; Guiot, M.C.; Owen, S.; Panet-Raymond, V.; Souhami, L.; Abdulkarim, B.S. Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: Results from a single institution. J. Neuro-Oncol. 2017, 132, 419–426. [Google Scholar] [CrossRef]

| n | ||
|---|---|---|
| Age, median (IQR) | 59 (49–63) | |
| Sex, n (%) | Male | 34 (87) |
| Female | 5 (13) | |
| Child-Pugh classification, n (%) | A | 23 (59) |
| B | 16 (41) | |
| KPS, n (%) | >80 | 8 (21) |
| 70–80 | 19 (49) | |
| <70 | 12 (30) | |
| Alcohol history, n (%) | 11 (28) | |
| Hepatitis, n (%) | hepatitis B virus | 29 (74) |
| hepatitis C virus | 10 (26) | |
| Non B-Non C | 5 (13) | |
| Extracranial metastasis, n (%) | 34 (87) | |
| Brain metastasis number, n (%) | 1 | 19 (49) |
| 2 | 9 (23) | |
| >3 | 11 (28) | |
| Brain metastasis size (cm), mean ± SD | 3.2 ± 1.5 | |
| Brain metastasis location | Supratentorium | 34 (87) |
| Infratentorium | 5 (13) | |
| Timing to brain metastasis, n (%) | Synchronous | 3 (8) |
| Metachronous | 36 (92) | |
| TKI therapy, n (%) | Sorafenib | 22 (56) |
| Lenvatinib | 7 (18) | |
| None antiangiogenic | 10 (26) | |
| Brain metastasis treatment, n (%) | Surgery + WBRT | 15 (38) |
| WBRT | 24 (62) | |
| Localregional therapy, n (%) | TACE or HAIC | 21 (54) |
| Liver resection | 24 (62) | |
| n | Tumor Bleeding | % | p Value | ||
|---|---|---|---|---|---|
| Patients never received TKI | 10 | 7 | 70 | Reference | |
| Patients received TKI | Continuously | 13 | 8 | 61.5 | >0.99 |
| Started after surgery or WBRT | 6 | 4 | 66.7 | >0.99 | |
| Withdrawal | 10 | 7 | 70 | >0.99 |
| n | Hazard Ratio | 95% CI | p Value | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 39 | 0.99 | 0.96 | 1.03 | 0.77 | |
| Sex | Male | 34 | ||||
| Female | 5 | 0.98 | 0.37 | 2.56 | 0.96 | |
| Child-Pugh calssification | A | 23 | ||||
| B | 16 | 3.56 | 1.7 | 7.44 | 0.001 | |
| Karnofsky Performance Score | 39 | 0.96 | 0.94 | 0.99 | 0.003 | |
| Alcohol history | 11 | 1.35 | 0.65 | 2.81 | 0.42 | |
| Hepatitis B infection | Not carrier | 10 | ||||
| Carrier | 29 | 1.01 | 0.47 | 2.18 | 0.98 | |
| Hepatitis C infection | Not carrier | 29 | ||||
| Carrier | 10 | 1.25 | 0.58 | 2.7 | 0.58 | |
| Extracranial metastasis | 34 | 1.86 | 0.63 | 5.53 | 0.26 | |
| Brain metastasis number | 1 | 19 | ||||
| 2 | 9 | 1.67 | 0.7 | 4 | 0.25 | |
| >3 | 11 | 2.24 | 0.98 | 5.1 | 0.06 | |
| Brain metastasis size | 39 | 0.93 | 0.75 | 1.15 | 0.48 | |
| Timing to brain metastasis | Synchronous | 3 | ||||
| Metachronous | 34 | 0.95 | 0.29 | 3.13 | 0.93 | |
| Brain metastasis location | Supratentorium | 34 | ||||
| Infratentorium | 5 | 3.3 | 1.2 | 9.2 | 0.023 | |
| TKI therapy | Sorafenib or Lenvatinib | 29 | 0.28 | 0.13 | 0.62 | 0.002 |
| Metastasis treatment | WBRT | 25 | ||||
| Surgery + WBRT | 12 | 0.4 | 0.19 | 0.83 | 0.014 | |
| Localregional therapy | 31 | 0.61 | 0.27 | 1.36 | 0.23 | |
| n | Adjust Odds Ratio | 95% CI | p Value | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| TKI therapy | Sorafenib or Lenvatinib | 29 | 0.26 | 0.12 | 0.6 | 0.001 |
| Metastasis treatment | WBRT | 25 | ||||
| Surgery + WBRT | 12 | 0.45 | 0.21 | 0.97 | 0.041 | |
| Karnofsky Performance Score | 39 | 0.97 | 0.94 | 0.99 | 0.006 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perng, P.-S.; Lai, Y.-H.; Lee, P.-H.; Huang, C.-C.; Hsu, H.-H.; Lee, J.-S. Safety and Efficacy of Sorafenib and Lenvatinib in Patients Who Underwent Surgery or Whole-Brain Radiotherapy for Brain Metastasis of Hepatocellular Carcinoma. J. Clin. Med. 2022, 11, 1536. https://doi.org/10.3390/jcm11061536
Perng P-S, Lai Y-H, Lee P-H, Huang C-C, Hsu H-H, Lee J-S. Safety and Efficacy of Sorafenib and Lenvatinib in Patients Who Underwent Surgery or Whole-Brain Radiotherapy for Brain Metastasis of Hepatocellular Carcinoma. Journal of Clinical Medicine. 2022; 11(6):1536. https://doi.org/10.3390/jcm11061536
Chicago/Turabian StylePerng, Pang-Shuo, Yu-Hsuan Lai, Po-Hsuan Lee, Chi-Chen Huang, Hao-Hsiang Hsu, and Jung-Shun Lee. 2022. "Safety and Efficacy of Sorafenib and Lenvatinib in Patients Who Underwent Surgery or Whole-Brain Radiotherapy for Brain Metastasis of Hepatocellular Carcinoma" Journal of Clinical Medicine 11, no. 6: 1536. https://doi.org/10.3390/jcm11061536
APA StylePerng, P.-S., Lai, Y.-H., Lee, P.-H., Huang, C.-C., Hsu, H.-H., & Lee, J.-S. (2022). Safety and Efficacy of Sorafenib and Lenvatinib in Patients Who Underwent Surgery or Whole-Brain Radiotherapy for Brain Metastasis of Hepatocellular Carcinoma. Journal of Clinical Medicine, 11(6), 1536. https://doi.org/10.3390/jcm11061536






