Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurement of Bone Strength
2.3. Diagnosis of Vertebral Fractures
2.4. Statistics
3. Results
3.1. Patients’ Characteristics
3.2. Characteristics and Vertebral Fractures
3.3. Predictors of Vertebral Fractures
3.4. Predictors of TBS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Pares, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Meng, W.D.; Yang, J.M.; Li, S.L.; Zhong, M.N.; Hou, X.X.; Wang, R.; Long, Y.Y.; Bao, L.X.; Bao, M. The association between liver cirrhosis and fracture risk: A systematic review and meta-analysis. Clin. Endocrinol. 2018, 89, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.A.; Romeiro, F.G. Diagnosis and Management of Cirrhosis-Related Osteoporosis. Biomed. Res. Int. 2016, 2016, 1423462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, S.R.; Bates, D.; Black, D.M. Clinical use of bone densitometry: Scientific review. JAMA 2002, 288, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef] [Green Version]
- Stone, K.L.; Seeley, D.G.; Lui, L.Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef]
- Bousson, V.; Bergot, C.; Sutter, B.; Levitz, P.; Cortet, B. Trabecular bone score (TBS): Available knowledge, clinical relevance, and future prospects. Osteoporos. Int. 2012, 23, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Harvey, N.C.; Glüer, C.C.; Binkley, N.; McCloskey, E.V.; Brandi, M.L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.M.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, D.; Šteňová, E.; Lamy, O. The Trabecular Bone Score (TBS) Complements DXA and the FRAX as a Fracture Risk Assessment Tool in Routine Clinical Practice. Curr. Osteoporos. Rep. 2017, 15, 521–531. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Silva, B.C.; Sardanelli, F.; Hans, D.; Bilezikian, J.P.; Caudarella, R. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 2014, 47, 435–448. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.; Hsieh, T.J.; Ho, C.H.; Lin, C.H.; Chen, C.K. Effect of compression fracture on trabecular bone score at lumbar spine. Osteoporos. Int. 2021, 32, 961–970. [Google Scholar] [CrossRef]
- Genant, H.K.; Wu, C.Y.; van Kuijk, C.; Nevitt, M.C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jame, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021; p. 102. [Google Scholar]
- Lima, T.B.; Santos, L.A.A.; Nunes, H.R.C.; Silva, G.F.; Caramori, C.A.; Qi, X.; Romeiro, F.G. Safety and efficacy of risedronate for patients with esophageal varices and liver cirrhosis: A non-randomized clinical trial. Sci. Rep. 2019, 9, 18958. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Treatment of osteoporotic fracture. Lancet 1984, 1, 27–33. [Google Scholar] [CrossRef]
- NIH Consensus. Osteoporosis prevention, diagnosis, and therapy. NIH Consens. Statement 2000, 17, 1–45. [Google Scholar]
- Siffert, R.S.; Luo, G.M.; Cowin, S.C.; Kaufman, J.J. Dynamic relationships of trabecular bone density, architecture, and strength in a computational model of osteopenia. Bone 1996, 18, 197–206. [Google Scholar] [CrossRef]
- Winzenrieth, R.; Dufour, R.; Pothuaud, L.; Hans, D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: Analyzing the odds of vertebral fracture. Calcif. Tissue Int. 2010, 86, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Rabier, B.; Héraud, A.; Grand-Lenoir, C.; Winzenrieth, R.; Hans, D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone 2010, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.; Fidler, E.; Libber, J.; Aubry-Rozier, B.; Hans, D.; Binkley, N. Spine trabecular bone score subsequent to bone mineral density improves fracture discrimination in women. J. Clin. Densitom. 2014, 17, 60–65. [Google Scholar] [CrossRef]
- Hans, D.; Goertzen, A.L.; Krieg, M.A.; Leslie, W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J. Bone Miner. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef]
- Iki, M.; Tamaki, J.; Kadowaki, E.; Sato, Y.; Dongmei, N.; Winzenrieth, R.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: The Japanese Population-Based Osteoporosis (JPOS) cohort study. J. Bone Miner. Res. 2014, 29, 399–407. [Google Scholar] [CrossRef]
- Briot, K.; Paternotte, S.; Kolta, S.; Eastell, R.; Reid, D.M.; Felsenberg, D.; Glüer, C.C.; Roux, C. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: The OPUS study. Bone 2013, 57, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Iki, M.; Tamaki, J.; Sato, Y.; Winzenrieth, R.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Age-related normative values of trabecular bone score (TBS) for Japanese women: The Japanese Population-based Osteoporosis (JPOS) study. Osteoporos. Int. 2015, 26, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Osteoporosis: Overlooked in men for too long. Lancet Diabetes Endocrinol. 2021, 9, 1. [CrossRef]
- Prasad, D.; Nguyen, M.H. Chronic hepatitis, osteoporosis, and men: Under-recognised and underdiagnosed. Lancet Diabetes Endocrinol. 2021, 9, 141. [Google Scholar] [CrossRef]
- Leib, E.; Winzenrieth, R.; Aubry-Rozier, B.; Hans, D. Vertebral microarchitecture and fragility fracture in men: A TBS study. Bone 2014, 62, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Aubry-Rozier, B.; Lix, L.M.; Morin, S.N.; Majumdar, S.R.; Hans, D. Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: The Manitoba Bone Density Program. Bone 2014, 67, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.B.; Holloway-Kew, K.L.; Hans, D.; Kotowicz, M.A.; Hyde, N.K.; Pasco, J.A. Reference Ranges for Trabecular Bone Score in Australian Men and Women: A Cross-Sectional Study. JBMR Plus 2019, 3, e10133. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Sharifi, F.; Heshmat, R.; Ostovar, A.; Ebrahimpur, M.; Sheidaei, A.; Nabipour, I.; Larijani, B. The reference value of trabecular bone score (TBS) in the Iranian population. J. Diabetes Metab. Disord. 2020, 19, 493–498. [Google Scholar] [CrossRef] [PubMed]
| Total | Men | Women | p-Value † | |
|---|---|---|---|---|
| Characteristic | (n = 275) | (n = 147) | (n = 128) | |
| Age (years) | 72 (12) | 72 (11) | 71 (13) | 0.467 |
| Body mass index (kg/m2) | 24.0 (4.5) | 23.6 (4.0) | 24.4 (5.0) | 0.115 |
| Diabetes mellitus | 83 (30) | 49 (33) | 34 (27) | 0.238 |
| Etiology | ||||
| Cryptogenic/HBV/HCV/Alcohol/Others | 92/51/43/43/46 | 48/31/25/34/9 | 44/20/18/9/37 | <0.001 |
| Hepatocellular carcinoma | 138 (50) | 98 (67) | 40 (31) | <0.001 |
| Child–Pugh class | ||||
| A/B/C | 202/57/16 | 114/25/8 | 88/32/8 | 0.241 |
| MELD score | 8 (7–10) | 8 (7–9) | 8 (7–10) | 0.535 |
| Total bilirubin (mg/dL) | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) | 1.1 (0.7–1.5) | 0.086 |
| Creatinine (mg/dL) | 0.74 (0.62–0.92) | 0.84 (0.69–1.03) | 0.64 (0.54–0.76) | <0.001 |
| INR | 1.04 (0.99–1.15) | 1.03 (0.98–1.10) | 1.09 (0.99–1.19) | 0.060 |
| Albumin (g/dL) | 3.8 (3.3–4.1) | 3.8 (3.4–4.2) | 3.7 (3.3–4.1) | 0.431 |
| Sodium (mEq/L) | 139 (137–140) | 139 (137–140) | 139 (138–141) | 0.041 |
| Calcium (mg/dL) | 9.1 (8.8–9.4) | 9.1 (8.8–9.4) | 9.2 (8.8–9.5) | 0.448 |
| Phosphorus (mg/dL) | 3.7 (6.8) | 3.2 (0.5) | 3.4 (0.5) | 0.001 |
| 25(OH)D (ng/mL) | 13 (10–18) | 15 (11–20) | 12 (9–15) | <0.001 |
| BMD (g/cm2) | 0.92 (0.20) | 0.99 (0.20) | 0.84 (0.17) | <0.001 |
| BMD T-score | −0.71 (1.69) | −0.14 (1.67) | −1.37 (1.47) | <0.001 |
| TBS | 1.35 (0.09) | 1.37 (0.08) | 1.31 (0.09) | <0.001 |
| Vertebral fracture | 62 (23) | 20 (14) | 42 (33) | <0.001 |
| Total (n = 275) | Men (n = 147) | Women (n = 128) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Vertebral Fracture | Vertebral Fracture | p-Value † | No Vertebral Fracture | Vertebral Fracture | p-Value † | No Vertebral Fracture | Vertebral Fracture | p-Value † | |
| Characteristic | (n = 213) | (n = 62) | (n = 127) | (n = 20) | (n = 86) | (n = 42) | |||
| Age (years) | 70 (12) | 78 (8) | <0.001 | 71 (11) | 78 (8) | 0.005 | 67 (13) | 78 (9) | <0.001 |
| Body mass index (kg/m2) | 23.8 (4.0) | 24.6 (5.9) | 0.186 | 23.4 (3.7) | 24.4 (5.9) | 0.324 | 24.3 (4.4) | 24.7 (6.0) | 0.611 |
| Diabetes mellitus | 64 (30) | 19 (31) | 1.000 | 43 (34) | 6 (30) | 0.804 | 21 (24) | 13 (31) | 0.523 |
| Etiology | |||||||||
| cryptogenic/HBV/HCV/alcohol/others | 71/42/28/36/36 | 9/9/15/7/22 | 0.245 | 41/30/20/27/9 | 7/1/5/7/0 | 0.160 | 30/12/8/9/27 | 14/8/10/0/10 | 0.046 |
| Hepatocellular carcinoma | 106 (50) | 32 (52) | 0.885 | 84 (66) | 14 (70) | 0.804 | 22 (26) | 18 (43) | 0.067 |
| Child–Pugh class | |||||||||
| A/B/C | 160/38/15 | 42/19/1 | 0.045 | 98/21/8 | 16/4/0 | 0.662 | 62/17/7 | 26/15/1 | 0.098 |
| MELD score | 8 (7–10) | 8 (7–10) | 0.673 | 8 (7–9) | 7 (7–10) | 0.862 | 8 (7–10) | 8 (7–10) | 0.410 |
| Total bilirubin (mg/dL) | 1.0 (0.7–1.5) | 0.9 (0.5–1.3) | 0.107 | 0.9 (0.7–1.4) | 0.7 (0.5–1.1) | 0.065 | 1.1 (0.8–1.5) | 1.1 (0.5–1.5) | 0.221 |
| Creatinine (mg/dL) | 0.73 (0.62–0.91) | 0.76 (0.64–0.97) | 0.648 | 0.84 (0.69–0.98) | 0.91 (0.80–1.17) | 0.305 | 0.62 (0.54–0.70) | 0.72 (0.57–0.80) | 0.239 |
| INR | 1.06 (0.99–1.17) | 1.03 (0.99–1.14) | 0.593 | 1.04 (0.98–1.12) | 1.03 (1.00–1.06) | 0.607 | 1.10 (0.99–1.21) | 1.04 (0.98–1.15) | 0.127 |
| Albumin (g/dL) | 3.8 (3.3–4.3) | 3.8 (3.4–4.0) | 0.923 | 3.8 (3.3–4.3) | 3.8 (3.5–4.1) | 0.545 | 3.7 (3.3–4.3) | 3.8 (3.3–4.0) | 0.703 |
| Sodium (mEq/L) | 139 (137–140) | 139 (137–140) | 0.965 | 139 (137–140) | 139 (137–140) | 0.556 | 139 (138–141) | 139 (138–141) | 0.954 |
| Calcium (mg/dL) | 9.1 (8.8–9.4) | 9.1 (8.9–9.4) | 0.778 | 9.1 (8.8–9.4) | 9.0 (8.9–9.3) | 0.701 | 9.2 (8.8–9.5) | 9.2 (8.8–9.5) | 0.699 |
| Phosphorus (mg/dL) | 3.3 (0.5) | 3.4 (0.4) | 0.017 | 3.2 (0.5) | 3.4 (0.4) | 0.059 | 3.4 (0.5) | 3.5 (0.4) | 0.539 |
| 25(OH)D (ng/mL) | 13 (10–18) | 12 (9–18) | 0.176 | 15 (11–21) | 15 (10–20) | 0.774 | 12 (10–215 | 11 (9–16) | 0.782 |
| BMD (g/cm2) | 0.95 (0.19) | 0.83 (0.20) | <0.001 | 1.00 (0.20) | 0.97 (0.22) | 0.567 | 0.88 (0.17) | 0.76 (0.15) | <0.001 |
| BMD T-score | −0.47 (1.63) | −1.53 (1.67) | <0.001 | −0.10 (1.64) | −0.35 (1.84) | 0.537 | −1.02 (1.45) | −2.10 (1.26) | <0.001 |
| TBS | 1.37 (0.08) | 1.28 (0.09) | <0.001 | 1.38 (0.08) | 1.31 (0.08) | <0.001 | 1.34 (0.08) | 1.27 (0.09) | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age | 1.10 (1.06–1.14) | <0.001 | 1.10 (1.06–1.15) | <0.001 |
| Sex (female) | 3.10 (1.70–5.64) | <0.001 | 0.79 (0.31–2.02) | 0.616 |
| Child–Pugh score | 0.99 (0.83–1.18) | 0.887 | 0.94 (0.70–1.26) | 0.682 |
| MELD score | 0.98 (0.88–1.08) | 0.672 | 1.02 (0.87–1.21) | 0.785 |
| BMD † | 1.55 (1.26–1.90) | <0.001 | 1.04 (0.81–1.33) | 0.758 |
| TBS † | 2.14 (1.69–2.73) | <0.001 | 2.22 (1.49–3.03) | <0.001 |
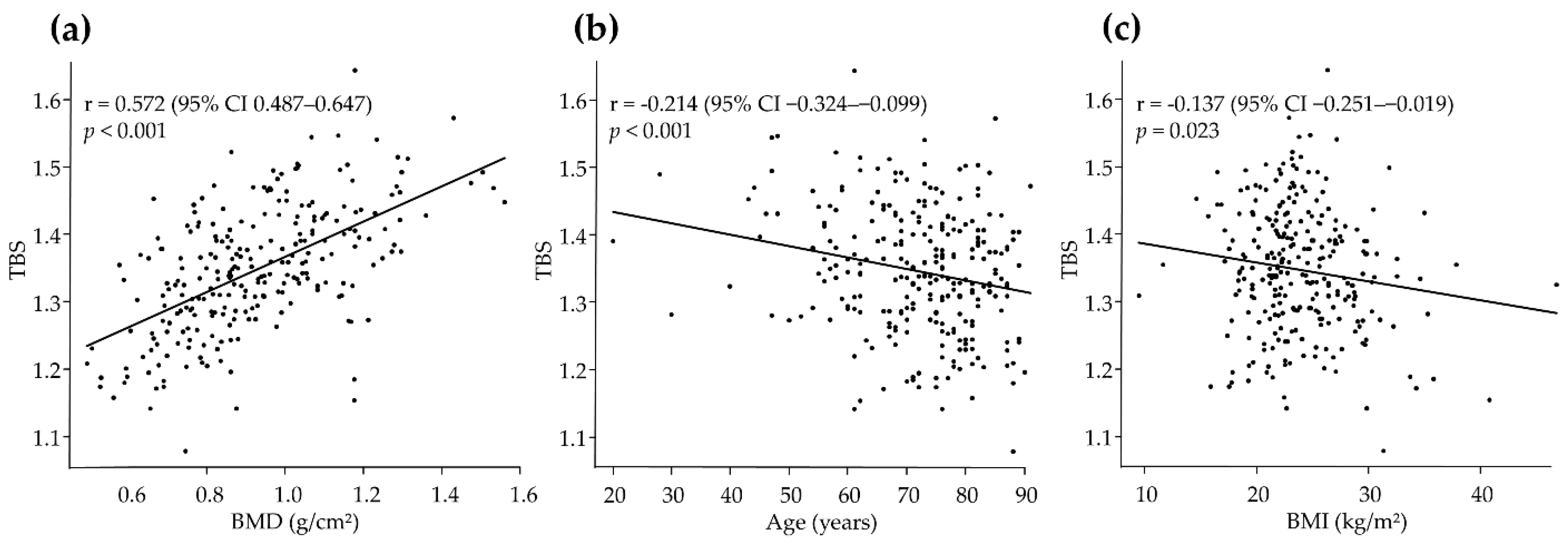
| Predictors | Partial Regression Coefficient (B) | Standard Error | T-Value | p-Value | Standardized Partial Regression Coefficient (β) | VIF |
|---|---|---|---|---|---|---|
| Total | ||||||
| Age | −0.002 | <0.001 | −4.43 | <0.001 | −0.211 | 1.07 |
| BMI | −0.005 | <0.001 | −5.31 | <0.001 | −0.251 | 1.06 |
| BMD | 0.266 | 0.022 | 12.20 | <0.001 | 0.583 | 1.08 |
| Men | ||||||
| Age | −0.002 | <0.001 | −3.65 | <0.001 | −0.237 | 1.10 |
| BMI | −0.008 | 0.001 | −6.30 | <0.001 | −0.404 | 1.07 |
| BMD | 0.240 | 0.026 | 9.10 | <0.001 | 0.591 | 1.10 |
| Women | ||||||
| Age | −0.002 | <0.001 | −2.49 | 0.014 | −0.204 | 1.28 |
| BMI | −0.002 | 0.001 | −1.52 | 0.131 | −0.116 | 1.11 |
| BMD | 0.290 | 0.045 | 6.44 | <0.001 | 0.529 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogiso, Y.; Hanai, T.; Nishimura, K.; Miwa, T.; Maeda, T.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis. J. Clin. Med. 2022, 11, 1562. https://doi.org/10.3390/jcm11061562
Ogiso Y, Hanai T, Nishimura K, Miwa T, Maeda T, Imai K, Suetsugu A, Takai K, Shimizu M. Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis. Journal of Clinical Medicine. 2022; 11(6):1562. https://doi.org/10.3390/jcm11061562
Chicago/Turabian StyleOgiso, Yui, Tatsunori Hanai, Kayoko Nishimura, Takao Miwa, Toshihide Maeda, Kenji Imai, Atsushi Suetsugu, Koji Takai, and Masahito Shimizu. 2022. "Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis" Journal of Clinical Medicine 11, no. 6: 1562. https://doi.org/10.3390/jcm11061562
APA StyleOgiso, Y., Hanai, T., Nishimura, K., Miwa, T., Maeda, T., Imai, K., Suetsugu, A., Takai, K., & Shimizu, M. (2022). Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis. Journal of Clinical Medicine, 11(6), 1562. https://doi.org/10.3390/jcm11061562







