Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review
Abstract
:1. Introduction
2. Physician and Facility Requirements
3. Preparation for a Safe Procedure
4. EUS System
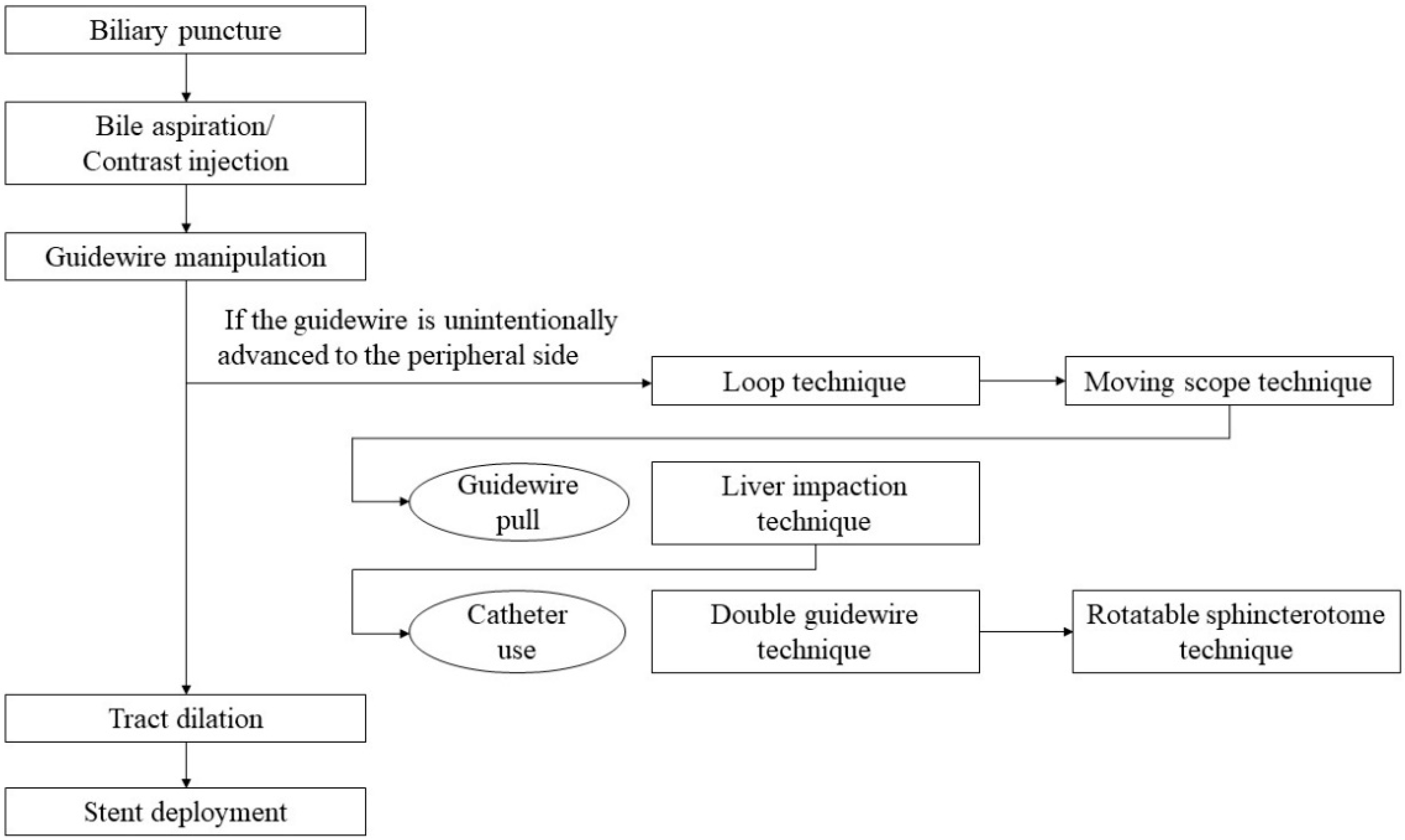
5. Step-by-Step Tutorial on EUS-HGS Procedure including Devise Selection
5.1. Selection of Bile Duct Puncture Site and Scope Position
5.2. Biliary Puncture
5.3. Contrast Injection
5.4. Guidewire Manipulation
5.5. Tract Dilation
5.6. Stent Deployment
6. Post-Procedure Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, T.H.; Choi, J.H.; Park do, H.; Song, T.J.; Kim, D.U.; Paik, W.H.; Hwangbo, Y.; Lee, S.S.; Seo, D.W.; Lee, S.K.; et al. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin. Gastroenterol. Hepatol. 2016, 14, 1011–1019.e1013. [Google Scholar] [CrossRef] [Green Version]
- Sharaiha, R.Z.; Kumta, N.A.; Desai, A.P.; DeFilippis, E.M.; Gabr, M.; Sarkisian, A.M.; Salgado, S.; Millman, J.; Benvenuto, A.; Cohen, M.; et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg. Endosc. 2016, 30, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Tombazzi, C.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic Ultrasound-Guided Biliary Drainage for Benign Biliary Diseases. Clin. Endosc. 2019, 52, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef]
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Isayama, H.; Nakai, Y.; Itoi, T.; Yasuda, I.; Kawakami, H.; Ryozawa, S.; Kitano, M.; Irisawa, A.; Katanuma, A.; Hara, K.; et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J. Hepatobil. Pancreat. Sci. 2019, 26, 249–269. [Google Scholar] [CrossRef] [Green Version]
- Hamada, T.; Isayama, H.; Nakai, Y.; Kogure, H.; Yamamoto, N.; Kawakubo, K.; Takahara, N.; Uchino, R.; Mizuno, S.; Sasaki, T.; et al. Transmural biliary drainage can be an alternative to transpapillary drainage in patients with an indwelling duodenal stent. Dig. Dis. Sci. 2014, 59, 1931–1938. [Google Scholar] [CrossRef]
- Khashab, M.A.; El Zein, M.H.; Sharzehi, K.; Marson, F.P.; Haluszka, O.; Small, A.J.; Nakai, Y.; Park, D.H.; Kunda, R.; Teoh, A.Y.; et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: An international comparative study. Endosc. Int. Open 2016, 4, E1322–E1327. [Google Scholar] [CrossRef] [Green Version]
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic Ultrasound-Guided Biliary Drainage for Unresectable Hilar Malignant Biliary Obstruction. Clin. Endosc. 2019, 52, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Kongkam, P.; Tasneem, A.A.; Rerknimitr, R. Combination of endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography-guided biliary drainage in malignant hilar biliary obstruction. Dig. Endosc. 2019, 31 (Suppl. S1), 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poincloux, L.; Rouquette, O.; Buc, E.; Privat, J.; Pezet, D.; Dapoigny, M.; Bommelaer, G.; Abergel, A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy 2015, 47, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Park, D.H. Outcomes and limitations: EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Prachayakul, V.; Thamtorawat, S.; Siripipattanamongkol, C.; Thanathanee, P. Bleeding left hepatic artery pseudoaneurysm: A complication of endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2013, 45 (Suppl. S2), E223–E224. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.P.; Rossini, L.G.; Ferrari, A.P. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy 2010, 42 (Suppl. S2), E126–E127. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, M. EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8, S35–S39. [Google Scholar] [CrossRef]
- Ogura, T.; Higuchi, K. Endoscopic Ultrasound-Guided Hepaticogastrostomy: Technical Review and Tips to Prevent Adverse Events. Gut Liver 2021, 15, 196–205. [Google Scholar] [CrossRef]
- Chantarojanasiri, T.; Ratanachu-Ek, T.; Pausawasdi, N. What You Need to Know Before Performing Endoscopic Ultrasound-guided Hepaticogastrostomy. Clin. Endosc. 2021, 54, 301–308. [Google Scholar] [CrossRef]
- Okuno, N.; Hara, K.; Mizuno, N.; Kuwahara, T.; Iwaya, H.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Infectious peritonitis after endoscopic ultrasound-guided biliary drainage in a patient with ascites. Int. J. Gastrointest. Interv. 2018, 7, 40–43. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, K.; Fujisawa, T.; Ishii, S.; Suzuki, A.; Saito, H.; Takasaki, Y.; Ushio, M.; Takahashi, S.; Yamagata, W.; Tomishima, K.; et al. Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy. J. Clin. Med. 2021, 10, 3111. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Okuno, N.; Haba, S.; Kuwahara, T.; Koda, H.; Mizuno, N.; Miyano, A. How to perform EUS-guided hepaticogastrostomy easier and safer. J. Hepatobil. Pancreat. Sci. 2020, 27, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Kitano, M.; Yoshikawa, T.; Omoto, S.; Kamata, K.; Yamao, K.; Kudo, M. Hepaticogastrostomy guided by real-time contrast-enhanced harmonic endoscopic ultrasonography: A novel technique. Endoscopy 2016, 48 (Suppl. S1), E228–E229. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Samarasena, J.B.; Chang, K.J. EUS anatomy of the liver segments. Endosc. Ultrasound 2018, 7, 246–251. [Google Scholar] [CrossRef]
- Okuno, N.; Hara, K.; Mizuno, N.; Hijioka, S.; Kuwahara, T.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Risks of transesophageal endoscopic ultrasonography-guided biliary drainage. Int. J. Gastrointest. Interv. 2017, 6, 82–84. [Google Scholar] [CrossRef]
- Ogura, T.; Nishioka, N.; Ueno, S.; Yamada, T.; Yamada, M.; Imoto, A.; Hakoda, A.; Higuchi, K. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2021, 53, 369–375. [Google Scholar] [CrossRef]
- Minaga, K.; Ogura, T.; Shiomi, H.; Imai, H.; Hoki, N.; Takenaka, M.; Nishikiori, H.; Yamashita, Y.; Hisa, T.; Kato, H.; et al. Comparison of the efficacy and safety of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for malignant distal biliary obstruction: Multicenter, randomized, clinical trial. Dig. Endosc. 2019, 31, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Nakai, Y.; Oyama, H.; Kanai, S.; Noguchi, K.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Hamada, T.; et al. Double Guidewire Technique Using an Uneven Double Lumen Catheter for Endoscopic Ultrasound-Guided Interventions. Dig. Dis. Sci. 2021, 66, 1540–1547. [Google Scholar] [CrossRef]
- Oh, D.; Park, D.H.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Ther. Adv. Gastroenterol. 2017, 10, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Ogura, T.; Nishioka, N.; Yamada, T.; Yamada, M.; Ueno, S.; Higuchi, K. Risk factors for adverse events associated with bile leak during EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2020, 9, 110–115. [Google Scholar] [CrossRef]
- Vila, J.J.; Perez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, M.A.; Perez-Millan, A.; Gonzalez-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; De la Serna, C.; Aparicio, J.R.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012, 76, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Committee, A.T.; Hwang, J.H.; Aslanian, H.R.; Thosani, N.; Goodman, A.; Manfredi, M.; Navaneethan, U.; Pannala, R.; Parsi, M.A.; Smith, Z.L.; et al. Devices for use with EUS. VideoGIE 2017, 2, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Seldinger, S.I. Catheter Replacement of the Needle in Percutaneous Arteriography: A new technique. Acta Radiol. 2010, 39, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Karia, K.; Gabr, M.; Desai, A.; Doshi, R.; Gaidhane, M.; Sharaiha, R.Z.; Kahaleh, M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J. Gastroenterol. 2016, 22, 2256–2270. [Google Scholar] [CrossRef] [Green Version]
- Ishiwatari, H.; Satoh, T.; Sato, J.; Fujie, S.; Kaneko, J.; Matsubayashi, H.; Ono, H. Bent needle technique as a rescue for bile duct puncture in endoscopic ultrasonography-guided intrahepatic biliary drainage. Endoscopy 2019, 51, E103–E104. [Google Scholar] [CrossRef] [Green Version]
- Ishiwatari, H.; Satoh, T.; Sato, J.; Kaneko, J.; Matsubayashi, H.; Yabuuchi, Y.; Kishida, Y.; Yoshida, M.; Ito, S.; Kawata, N.; et al. Bile aspiration during EUS-guided hepaticogastrostomy is associated with lower risk of postprocedural adverse events: A retrospective single-center study. Surg. Endosc. 2021, 35, 6836–6845. [Google Scholar] [CrossRef]
- Kanno, Y.; Ito, K.; Sakai, T.; Okano, H. Novel combination of a 0.018-inch guidewire, dedicated thin dilator, and 22-gauge needle for EUS-guided hepaticogastrostomy. VideoGIE 2020, 5, 355–358. [Google Scholar] [CrossRef]
- Ogura, T.; Ueno, S.; Okuda, A.; Nishioka, N.; Higuchi, K. EUS-guided hepaticogastrostomy for hepaticojejunostomy stricture using a 22G needle and a mechanical dilator (with video). J. Hepatobil. Pancreat. Sci. 2021. [Google Scholar] [CrossRef]
- Ueno, S.; Ogura, T.; Higuchi, K. Moving scope technique for guidewire insertion during endoscopic ultrasound-guided hepaticogastrostomy. Dig. Endosc. 2021, 33, e109–e110. [Google Scholar] [CrossRef]
- Ogura, T.; Masuda, D.; Takeuchi, T.; Fukunishi, S.; Higuchi, K. Liver impaction technique to prevent shearing of the guidewire during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2015, 47, E583–E584. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Kubota, Y.; Makiyama, H.; Sato, S.; Ban, T. Uneven double-lumen cannula for rescue guidewire technique in endoscopic ultrasonography-guided hepaticogastrostomy. Endoscopy 2017, 49, E264–E265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, S.; Oka, M.; Nagoshi, S. Rotatable sphincterotome as a salvage for guidewire manipulation in endoscopic ultrasound-guided hepaticogastrostomy. Dig. Endosc. 2021, 33, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.; Benias, P.C.; Kumbhari, V. Initial clinical experience of a steerable access device for EUS-guided biliary drainage. Gastrointest. Endosc. 2020, 91, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Marrache, M.K.; Al-Sabban, A.; Itani, M.; Farha, J.; Fayad, L.; Khashab, M.A.; Kumbhari, V. Endoscopic ultrasound-guided rendezvous ERCP using a steerable access device. Endoscopy 2020, 52, E355–E356. [Google Scholar] [CrossRef] [Green Version]
- Prachayakul, V.; Aswakul, P. A novel technique for endoscopic ultrasound-guided biliary drainage. World J. Gastroenterol. 2013, 19, 4758–4763. [Google Scholar] [CrossRef]
- Paik, W.H.; Park, D.H.; Choi, J.H.; Choi, J.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Lee, J.B. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J. Gastroenterol. 2014, 20, 5051–5059. [Google Scholar] [CrossRef]
- Park, D.H.; Jeong, S.U.; Lee, B.U.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest. Endosc. 2013, 78, 91–101. [Google Scholar] [CrossRef]
- Amano, M.; Ogura, T.; Onda, S.; Takagi, W.; Sano, T.; Okuda, A.; Miyano, A.; Masuda, D.; Higuchi, K. Prospective clinical study of endoscopic ultrasound-guided biliary drainage using novel balloon catheter (with video). J. Gastroenterol. Hepatol. 2017, 32, 716–720. [Google Scholar] [CrossRef]
- Kanno, Y.; Ito, K.; Koshita, S.; Ogawa, T.; Masu, K.; Masaki, Y.; Noda, Y. Efficacy of a newly developed dilator for endoscopic ultrasound-guided biliary drainage. World J. Gastrointest. Endosc. 2017, 9, 304–309. [Google Scholar] [CrossRef]
- Honjo, M.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Sofuni, A.; Nagakawa, Y.; Iwasaki, H.; Kanai, T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc. Ultrasound 2018, 7, 376–382. [Google Scholar] [CrossRef]
- Kawakami, H.; Kubota, Y. Novel wire-guided fine-gauge bougie dilator for transpapillary or endoscopic ultrasonography-guided biliary drainage. Endoscopy 2017, 49, E75–E77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, T.; Nakai, Y.; Iwashita, T.; Higuchi, K.; Itoi, T. Novel fine gauge electrocautery dilator for endoscopic ultrasound-guided biliary drainage: Experimental and clinical evaluation study (with video). Endosc. Int. Open 2019, 7, E1652–E1657. [Google Scholar] [CrossRef]
- Burmester, E.; Niehaus, J.; Leineweber, T.; Huetteroth, T. EUS-cholangio-drainage of the bile duct: Report of 4 cases. Gastrointest. Endosc. 2003, 57, 246–251. [Google Scholar] [CrossRef]
- Giovannini, M.; Dotti, M.; Bories, E.; Moutardier, V.; Pesenti, C.; Danisi, C.; Delpero, J.R. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy 2003, 35, 1076–1078. [Google Scholar] [CrossRef]
- Ramirez-Luna, M.A.; Tellez-Avila, F.I.; Giovannini, M.; Valdovinos-Andraca, F.; Guerrero-Hernandez, I.; Herrera-Esquivel, J. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cancer. Endoscopy 2011, 43, 826–830. [Google Scholar] [CrossRef]
- Attasaranya, S.; Netinasunton, N.; Jongboonyanuparp, T.; Sottisuporn, J.; Witeerungrot, T.; Pirathvisuth, T.; Ovartlarnporn, B. The Spectrum of Endoscopic Ultrasound Intervention in Biliary Diseases: A Single Center’s Experience in 31 Cases. Gastroenterol. Res. Pract. 2012, 2012, 680753. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, C.; Luigiano, C.; Fuccio, L.; Polifemo, A.M.; Ferrara, F.; Ghersi, S.; Bassi, M.; Billi, P.; Maimone, A.; Cennamo, V.; et al. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: A case series. Endoscopy 2011, 43, 438–441. [Google Scholar] [CrossRef]
- Artifon, E.L.; Marson, F.P.; Gaidhane, M.; Kahaleh, M.; Otoch, J.P. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest Endosc. 2015, 81, 950–959. [Google Scholar] [CrossRef]
- Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Koshita, S.; Kanno, Y.; Ogawa, T.; Masu, K.; Hashimoto, S.; et al. Metallic stent deployment in endosonography-guided biliary drainage: Long-term follow-up results in patients with bilio-enteric anastomosis. Dig. Endosc. 2012, 24, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Bories, E.; Pesenti, C.; Caillol, F.; Lopes, C.; Giovannini, M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007, 39, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Nakai, Y.; Isayama, H.; Koike, K. Tandem stent placement as a rescue for stent misplacement in endoscopic ultrasonography-guided hepaticogastrostomy. Dig. Endosc. 2013, 25, 340–341. [Google Scholar] [CrossRef]
- Okuno, N.; Hara, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Yamao, K. Stent migration into the peritoneal cavity following endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2015, 47 (Suppl. S1), E311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamata, K.; Takenaka, M.; Minaga, K.; Omoto, S.; Miyata, T.; Yamao, K.; Imai, H.; Kudo, M. Stent migration during EUS-guided hepaticogastrostomy in a patient with massive ascites: Troubleshooting using additional EUS-guided antegrade stenting. Arab J. Gastroenterol. 2017, 18, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Sodarat, P.; Luangsukrerk, T.; Kongkam, P.; Seabmuangsai, O.; Wachiramatharuch, C. Surgical hepaticogastrostomy as a method for resolving stent migration in endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2021, 53, E350–E351. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Sun, S.; Liu, X.; Wang, S.; Ge, N.; Wang, G. Endoscopic ultrasound-guided repositioning of a migrated metal hepatogastrostomy stent using foreign body forceps. Endoscopy 2016, 48 (Suppl. S1), E28–E29. [Google Scholar] [CrossRef] [Green Version]
- Minaga, K.; Kitano, M.; Yamashita, Y.; Nakatani, Y.; Kudo, M. Stent migration into the abdominal cavity after EUS-guided hepaticogastrostomy. Gastrointest. Endosc. 2017, 85, 263–264. [Google Scholar] [CrossRef]
- van Geenen, E.J.M.; Siersema, P.D. Stent migration into the abdominal cavity after EUS-guided hepaticogastrostomy. Gastrointest. Endosc. 2018, 87, 617–618. [Google Scholar] [CrossRef]
- Yang, M.J.; Kim, J.H.; Kim, D.J.; Hwang, J.C.; Yoo, B.M. Hepatobiliary and Pancreatic: EUS-guided reintervention for extraluminal stent migration after EUS-guided hepaticogastrostomy. J. Gastroenterol. Hepatol. 2018, 33, 772. [Google Scholar] [CrossRef]
- Nakai, Y.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; Kogure, H.; et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest. Endosc. 2020, 92, 623–631.e621. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Yamamoto, K.; Sano, T.; Onda, S.; Imoto, A.; Masuda, D.; Takagi, W.; Fukunishi, S.; Higuchi, K. Stent length is impact factor associated with stent patency in endoscopic ultrasound-guided hepaticogastrostomy. J. Gastroenterol. Hepatol. 2015, 30, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Choi, J.H.; Lee, S.S.; Cho, H.D.; Seo, D.W.; Park, S.H.; Lee, S.K.; Kim, M.H.; Park, D.H. A pilot proof-of-concept study of a modified device for one-step endoscopic ultrasound-guided biliary drainage in a new experimental biliary dilatation animal model. World J. Gastroenterol. 2014, 20, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Preliminary report on a new hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2014, 80, 707–711. [Google Scholar] [CrossRef]
- Park, D.H.; Lee, T.H.; Paik, W.H.; Choi, J.H.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 1461–1466. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, S.S.; Oh, D.; Song, T.J.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2017, 85, 1067–1075. [Google Scholar] [CrossRef]
- De Cassan, C.; Bories, E.; Pesenti, C.; Caillol, F.; Godat, S.; Ratone, J.P.; Delpero, J.R.; Ewald, J.; Giovannini, M. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: Results of a retrospective monocentric study. Endosc. Ultrasound 2017, 6, 329–335. [Google Scholar] [CrossRef]
- Leung Ki, E.L.; Napoleon, B. EUS-specific stents: Available designs and probable lacunae. Endosc. Ultrasound 2019, 8, S17–S27. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, S.S. Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound? J. Clin. Med. 2020, 9, 3595. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016, 48, 1125–1128. [Google Scholar] [CrossRef]
- Ogura, T.; Okuda, A.; Higuchi, K. Endoscopic ultrasound-guided hepaticogastrostomy for hepaticojejunostomy stricture using a one-step stent deployment technique (with video). J. Hepatobil. Pancreat. Sci. 2021, 28, e34–e35. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Yamada, M.; Nishioka, N.; Yamada, T.; Higuchi, K. One-step stent deployment of EUS-guided hepaticogastrostomy using a novel covered metal stent with a fine-gauge stent delivery system (with video). Endosc. Ultrasound 2020, 9, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Maehara, K.; Hijioka, S.; Nagashio, Y.; Ohba, A.; Maruki, Y.; Suzuki, H.; Sone, M.; Okusaka, T.; Saito, Y. Endoscopic ultrasound-guided hepaticogastrostomy or hepaticojejunostomy without dilation using a stent with a thinner delivery system. Endosc. Int. Open 2020, 8, E1034–E1038. [Google Scholar] [CrossRef] [PubMed]
- Okuno, N.; Hara, K.; Mizuno, N.; Kuwahara, T.; Iwaya, H.; Ito, A.; Kuraoka, N.; Matsumoto, S.; Polmanee, P.; Niwa, Y. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: A prospective clinical study. J. Gastroenterol. Hepatol. 2018, 33, 1413–1421. [Google Scholar] [CrossRef]
- Ogura, T.; Kitano, M.; Okuda, A.; Itonaga, M.; Ueno, S.; Yamashita, Y.; Nishioka, N.; Ashida, R.; Miyano, A.; Higuchi, K. Endoscopic ultrasonography-guided hepaticogastrostomy using a novel laser-cut type partially covered self-expandable metal stent (with video). Dig. Endosc. 2021, 33, 1188–1193. [Google Scholar] [CrossRef]
- Miyano, A.; Ogura, T.; Yamamoto, K.; Okuda, A.; Nishioka, N.; Higuchi, K. Clinical Impact of the Intra-scope Channel Stent Release Technique in Preventing Stent Migration During EUS-Guided Hepaticogastrostomy. J. Gastrointest. Surg. 2018, 22, 1312–1318. [Google Scholar] [CrossRef]
- Uchida, D.; Kawamoto, H.; Kato, H.; Goto, D.; Tomoda, T.; Matsumoto, K.; Yamamoto, N.; Horiguchi, S.; Tsutsumi, K.; Okada, H. The intra-conduit release method is useful for avoiding migration of metallic stents during EUS-guided hepaticogastrostomy (with video). J. Med. Ultrason 2018, 45, 399–403. [Google Scholar] [CrossRef]
- Shima, Y.; Isayama, H.; Ito, Y.; Hamada, T.; Nakai, Y.; Tsujino, T.; Nakata, R.; Koike, K. Crisscross anchor-stents to prevent metal stent migration during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2014, 46 (Suppl. S1), E563. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Isayama, H.; Ishii, S. “ClipFlap” anchoring method for endoscopic ultrasonography-guided hepaticogastrostomy with a covered self-expandable metallic stent. Dig. Endosc. 2020, 32, 628. [Google Scholar] [CrossRef]
- Fine, C.; Rivory, J.; Forestier, J.; Saurin, J.C.; Sosa-Valencia, L.; Ponchon, T.; Pioche, M. Endoscopic management of gastric wall bleeding and stent blood clot occlusion after endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2016, 48, E351–E352. [Google Scholar] [CrossRef] [Green Version]




















| EG-580UT (Fujifilm) | GF-UCT260 (Olympus) | EG38-J10UT (Pentax) | ||
|---|---|---|---|---|
| Endoscopic Functions | Viewing direction | Forward oblique viewing 40° | Forward oblique viewing 55° | Forward oblique viewing 45° |
| Observation range | 3–100 mm | 3–100 mm | 3–100 mm | |
| Field of view | 140° | 100° | 120° | |
| Distal end diameter | 13.9 mm | 14.6 mm | 14.3 mm | |
| Insertion tube diameter | 12.4 mm | 12.6 mm | 12.8 mm | |
| Bending capacity up/down | 150°/150° | 130°/90° | 160°/130° | |
| Bending capacity left/right | 120°/120° | 90°/90° | 120°/120° | |
| Working channel diameter | 3.8 mm | 3.7 mm | 4.0 mm | |
| Working length | 1250 mm | 1250 mm | 1250 mm | |
| Total length | 1550 mm | 1555 mm | 1566 mm | |
| Ultrasound Functions | Dedicated processor | SU-1 | EU-ME2 | None |
| Sound method | Electronic curved linear array | Electronic curved linear array | Electronic curved linear array | |
| Scanning area | 150° | 180° | 150° | |
| Frequency | 5–12 MHz | 5–12 MHz | 5–13 MHz | |
| Scanning mode | B-Mode, M-Mode, Color Doppler, Power Doppler, Pulse Doppler | B-Mode, Color Flow Mode, Power Flow Mode | Depends on ultrasoundplatforms(ARIETTA series) |
| Adverse Event | Incidence |
|---|---|
| Overall | 18.2% |
| Bleeding | 3.7% |
| Bile leak | 2.8% |
| Biloma | 2.6% |
| Stent migration | 1.6% |
| Stent misplacement | 1.2% |
| Intrahepatic hematoma | 1.2% |
| Sepsis | 1.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, S.; Nakagawa, K.; Suda, K.; Otsuka, T.; Oka, M.; Nagoshi, S. Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review. J. Clin. Med. 2022, 11, 1591. https://doi.org/10.3390/jcm11061591
Matsubara S, Nakagawa K, Suda K, Otsuka T, Oka M, Nagoshi S. Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review. Journal of Clinical Medicine. 2022; 11(6):1591. https://doi.org/10.3390/jcm11061591
Chicago/Turabian StyleMatsubara, Saburo, Keito Nakagawa, Kentaro Suda, Takeshi Otsuka, Masashi Oka, and Sumiko Nagoshi. 2022. "Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review" Journal of Clinical Medicine 11, no. 6: 1591. https://doi.org/10.3390/jcm11061591






