Echocardiography Nomogram for Predicting Survival among Chronic Lung Disease Patients with Severe Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
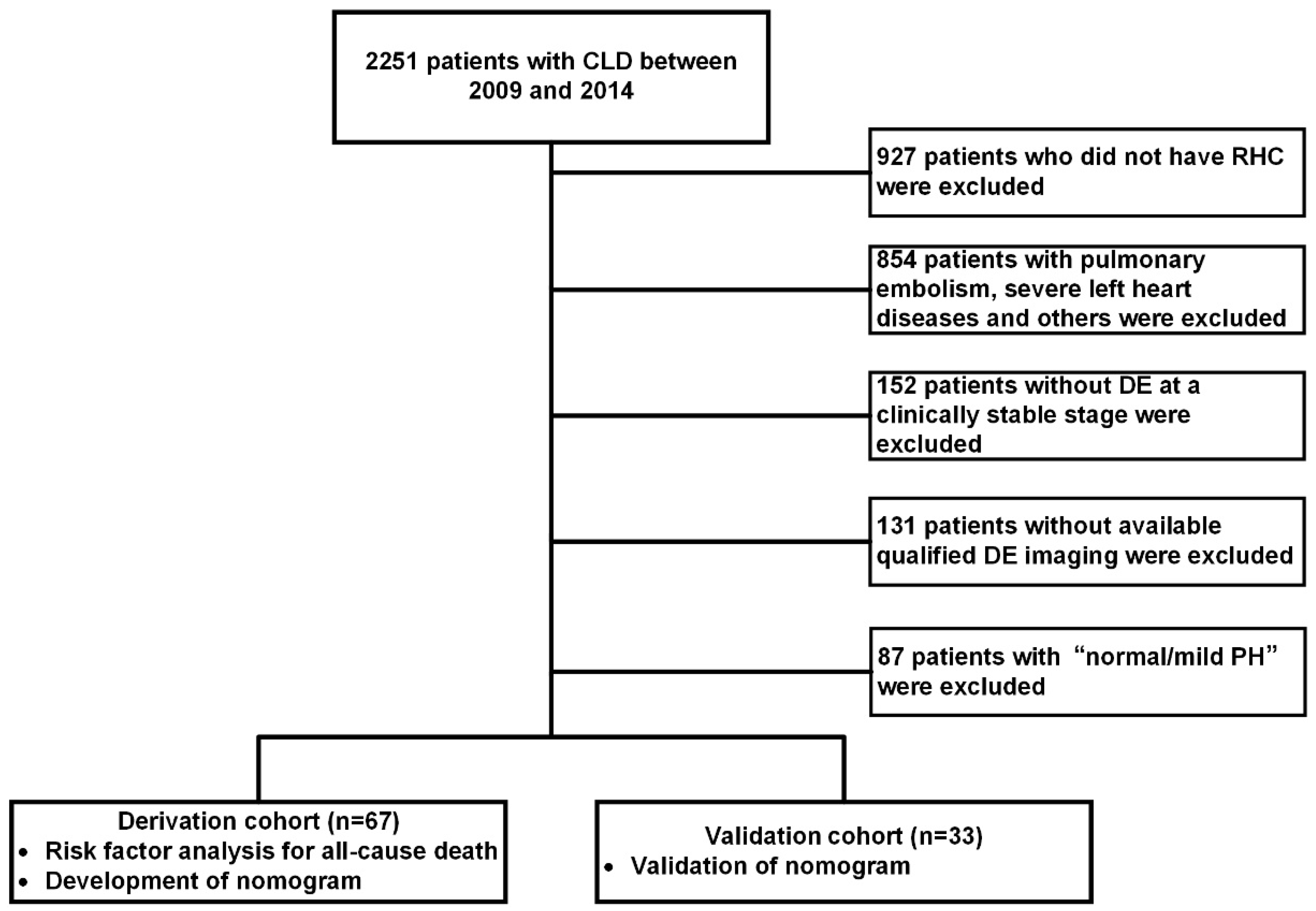
2.1. Patient Selection and Study Design
2.2. Echocardiographic Measurement
2.3. Haemodynamic Measurements
Follow-up
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Risk Score Construction in the Derivation Cohort
3.3. Nomogram for Predicting All-Cause Mortality
3.4. External Validation in the Validation Cohort
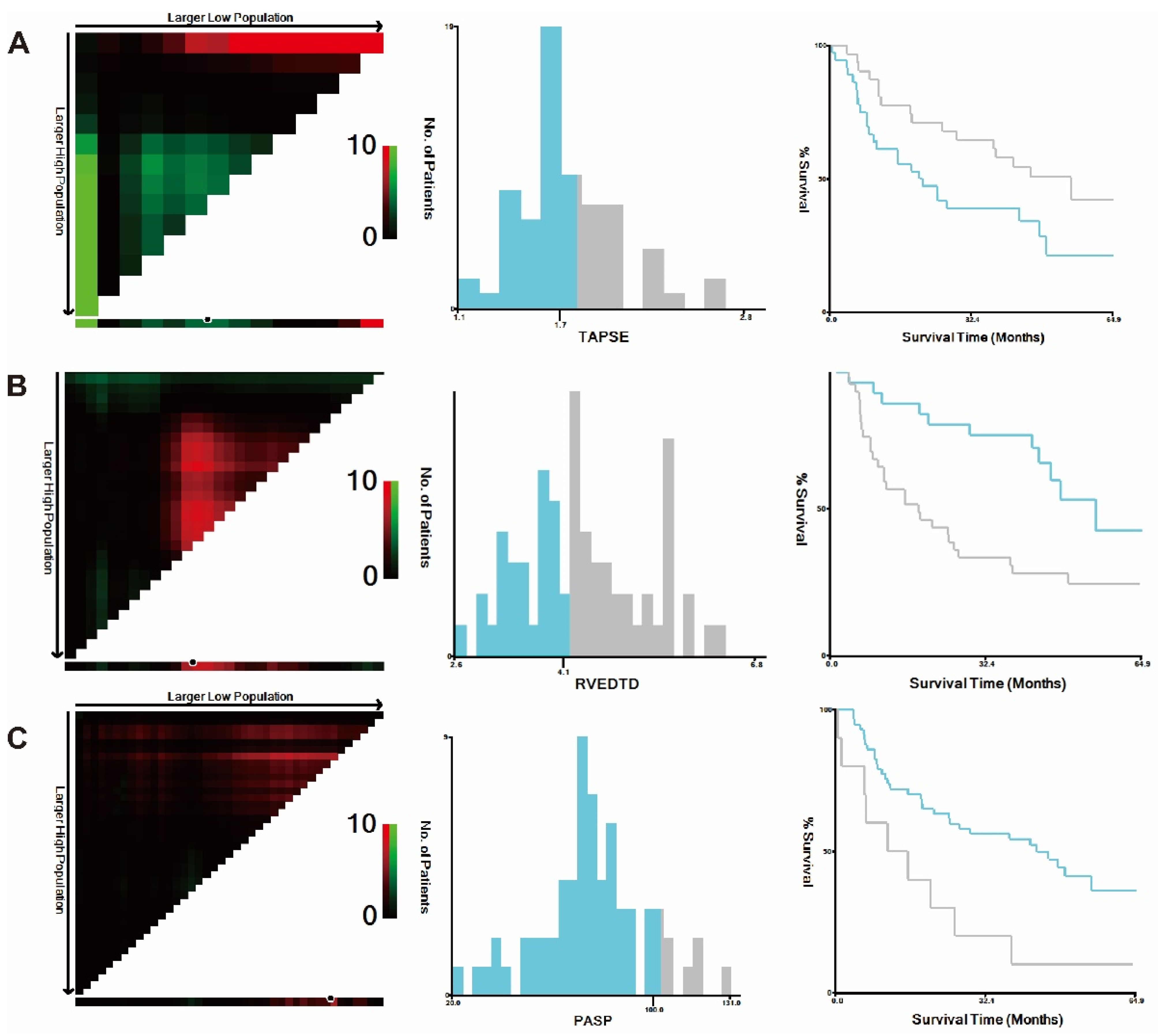
3.5. Subgroup Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLD | chronic lung diseases |
| ENDSEI | end-systolic stage left ventricular eccentricity index |
| mPAP | mean pulmonary artery pressure |
| PAd | pulmonary artery dilation |
| PAH | pulmonary arterial hypertension |
| RALD | right atrial longitudinal dimension |
| RATD | right atrial transverse dimension |
| RVEDLD | right ventricular end-diastolic longitudinal dimension |
| RVEDTD | right ventricular end-diastolic transverse dimension |
| TAPSE | tricuspid annular plane systolic excursion |
References
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Sanchez, M.A.G.; Kumar, R.K.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D34–D41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeger, W.; Adir, Y.; Barbera, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, S.; et al. Pulmonary hypertension in chronic lung diseases. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D109–D116. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Andreas, S.; Bastian, A.; Claussen, M.; Ghofrani, H.A.; Gorenflo, M.; Grohé, C.; Günther, A.; Halank, M.; Hammerl, P.; et al. Pulmonary hypertension due to chronic lung disease: Updated Recommendations of the Cologne Consensus Conference 2011. Int. J. Cardiol. 2011, 154 (Suppl. 1), S45–S53. [Google Scholar] [CrossRef]
- Bunel, V.; Guyard, A.; Dauriat, G.; Danel, C.; Montani, D.; Gauvain, C.; Thabut, G.; Humbert, M.; Castier, Y.; Dorfmüller, P.; et al. Pulmonary Arterial Histologic Lesions in Patients With COPD With Severe Pulmonary Hypertension. Chest 2019, 156, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Agusti, A.; Barbera, J.A.; Celli, B.; Criner, G.; Humbert, M.; Sin, D.D.; Voelkel, N.; Olschewski, H. Pulmonary Vascular Involvement in Chronic Obstructive Pulmonary Disease. Is There a Pulmonary Vascular Phenotype? Am. J. Respir. Crit. Care Med. 2018, 198, 1000–1011. [Google Scholar] [CrossRef]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome Prediction by Quantitative Right Ventricular Function Assessment in 575 Subjects Evaluated for Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenappan, T.; Glassner, C.; Gomberg-Maitland, M. Validation of the Pulmonary Hypertension Connection Equation for Survival Prediction in Pulmonary Arterial Hypertension. Chest 2012, 141, 642–650. [Google Scholar] [CrossRef]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Ivy, D.D.; Abman, S.H. Clinical Utility of Echocardiography for the Diagnosis and Management of Pulmonary Vascular Disease in Young Children With Chronic Lung Disease. Pediatrics 2008, 121, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, S.; Jud, A.; Aurich, M.; Hess, A.; Hilbel, T.; Hardt, S.; Katus, H.A.; Mereles, D. Reliability of Noninvasive Assessment of Systolic Pulmonary Artery Pressure by Doppler Echocardiography Compared to Right Heart Catheterization: Analysis in a Large Patient Population. J. Am. Heart Assoc. 2014, 3, e001103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.R.; Criner, G.J.; Fishman, A.P.; Hassoun, P.M.; Minai, O.A.; Scharf, S.M.; Fessler, H.E. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur. Respir. J. 2007, 30, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Galie, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Raymond, R.J.; Hinderliter, A.L.; Willis, P.W.; Ralph, D.; Caldwell, E.J.; Williams, W.; Ettinger, N.A.; Hill, N.S.; Summer, W.R.; de Boisblanc, B.; et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2002, 39, 1214–1219. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Wu, C.; Pudasaini, B.; Wang, L.; Zhao, Q.-H.; Zhang, R.; Wu, W.-H.; Yuan, P.; Jing, Z.-C.; Liu, J.-M. A novel scoring index by Doppler echocardiography for predicting severe pulmonary hypertension due to chronic lung diseases: A cross-sectional diagnostic accuracy study. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1741–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; Du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Jiang, R.; Ai, Z.-S.; Jiang, X.; Yuan, P.; Liu, D.; Zhao, Q.-H.; He, J.; Wang, L.; Gomberg-Maitland, M.; Jing, Z.-C. Intravenous fasudil improves in-hospital mortality of patients with right heart failure in severe pulmonary hypertension. Hypertens. Res. 2015, 38, 539–544. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid Annular Displacement Predicts Survival in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Haeck, M.L.; Scherptong, R.W.; Marsan, N.A.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Vliegen, H.W.; Delgado, V. Prognostic Value of Right Ventricular Longitudinal Peak Systolic Strain in Patients With Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2012, 5, 628–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, I.; Rajagopal, S.; Brucker, A.; Chiswell, K.; Christopher, B.; Alenezi, F.; Mandawat, A.; Rivera, D.; Arges, K.; Tapson, V.; et al. Clinical and Echocardiographic Predictors of Outcomes in Patients With Pulmonary Hypertension. Am. J. Cardiol. 2018, 122, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Eysmann, S.B.; Palevsky, H.I.; Reichek, N.; Hackney, K.; Douglas, P.S. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation 1989, 80, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Z.-C.; Xu, X.-Q.; Han, Z.-Y.; Wu, Y.; Deng, K.-W.; Wang, H.; Wang, Z.-W.; Cheng, X.-S.; Xu, B.; Hu, S.-S.; et al. Registry and Survival Study in Chinese Patients With Idiopathic and Familial Pulmonary Arterial Hypertension. Chest 2007, 132, 373–379. [Google Scholar] [CrossRef]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in Patients with Primary Pulmonary Hypertension. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An Evaluation of Long-term Survival From Time of Diagnosis in Pulmonary Arterial Hypertension From the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaïci, A.; Weitzenblum, E.; Cordier, J.-F.; Chabot, F.; et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010, 122, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Dai, L.-Z.; Xie, W.-P.; Yu, Z.-X.; Wu, B.-X.; Pan, L.; Yuan, P.; Jiang, X.; He, J.; Humbert, M.; et al. Survival of Chinese Patients With Pulmonary Arterial Hypertension in the Modern Treatment Era. Chest 2011, 140, 301–309. [Google Scholar] [CrossRef]
- Andersen, K.H.; Iversen, M.; Kjaergaard, J.; Mortensen, J.; Nielsen-Kudsk, J.E.; Bendstrup, E.; Videbaek, R.; Carlsen, J. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J. Heart Lung Transplant. 2012, 31, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.J.; Nathan, S.D.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. Prevalence and Outcomes of Pulmonary Arterial Hypertension in Advanced Idiopathic Pulmonary Fibrosis. Chest 2006, 129, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, J.; Andersen, K.H.; Boesgaard, S.; Iversen, M.; Steinbrüchel, D.; Andersen, C.B. Pulmonary arterial lesions in explanted lungs after transplantation correlate with severity of pulmonary hypertension in chronic obstructive pulmonary disease. J. Heart Lung Transplant. 2013, 32, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Barberà, J.A.; Blanco, I. Management of Pulmonary Hypertension in Patients with Chronic Lung Disease. Curr. Hypertens. Rep. 2015, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Weitzenblum, E.; Chaouat, A. Cor pulmonale. Chronic Respir. Disease. 2009, 6, 177–185. [Google Scholar] [CrossRef]
- Wanamaker, B.; Cascino, T.; McLaughlin, V.; Oral, H.; Latchamsetty, R.; Siontis, K.C. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythmia Electrophysiol. Rev. 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Pristera, N.; Musarra, R.; Schilz, R.; Hoit, B.D. The Role of Echocardiography in the Evaluation of Pulmonary Arterial Hypertension. Echocardiography 2015, 33, 105–116. [Google Scholar] [CrossRef]
- Arcasoy, S.M.; Christie, J.D.; Ferrari, V.A.; Sutton, M.S.J.; Zisman, D.A.; Blumenthal, N.P.; Pochettino, A.; Kotloff, R.M. Echocardiographic Assessment of Pulmonary Hypertension in Patients with Advanced Lung Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 735–740. [Google Scholar] [CrossRef] [Green Version]




| Variable | Derivation Cohort | Validation Cohort | p Value |
|---|---|---|---|
| (n = 67) | (n = 33) | ||
| Age (years) | 58.0 ± 12.9 | 61.8 ± 11.3 | 0.142 |
| Male, n (%) | 38 (56.7) | 24 (84.8) | 0.187 |
| BSA (m2) | 1.60 ± 0.2 | 1.59 ± 0.1 | 0.797 |
| Aetiology, n (%) | |||
| COPD | 52 (77.6) | 24 (72.4) | 0.490 |
| Interstitial lung disease | 8 (11.9) | 2 (6.1) | 0.339 |
| Asthma | 3 (4.5) | 0 | 0.549 |
| Pneumoconiosis | 2 (3.0) | 1 (3.0) | 1.000 |
| CPFE | 3 (4.5) | 1 (3.0) | 0.928 |
| Post tubercular sequelae | 1 (1.5) | 0 | 1.000 |
| Sleep-disordered breathing | 2 (3.0) | 1 (3.0) | 1.000 |
| Chest wall abnormalities | 1 (1.5) | 0 | 1.000 |
| Comorbidities, n (%) | |||
| Systemic hypertension | 10 (14.9) | 4 (12.1) | 1.000 |
| Diabetes mellitus | 2 (3.0) | 1 (3.0) | 1.000 |
| Coronary heart disease | 1 (1.5) | 1 (3.0) | 1.000 |
| Malignant tumour | 1 (1.5) | 0 | 1.000 |
| Arrhythmia | 3 (4.5) | 2 (6.1) | 1.000 |
| Hyperlipidaemia | 0 | 1 (3.0) | 0.330 |
| Previous cerebral infarction | 1 (1.5) | 0 | 1.000 |
| Pulmonary function test | |||
| FEV1% predicted | 32.3 (24.0, 44.0) | 35.5 (35.6, 49.4) | 0.127 |
| FVC% predicted | 54.9 (40.9, 63.4) | 60.1 (57.9, 72.7) | 0.081 |
| FEV1/FVC | 47.4 (42.9, 56.1) | 47.7 (47.1, 56.1) | 0.823 |
| RV% predicted | 197.9 (137.9, 251.4) | 116.4 (155.7, 206.3) | 0.220 |
| TLC% predicted | 115.5 (92.0, 131.7) | 108.8 (98.7, 117.6) | 0.440 |
| DLco% predicted | 40.9 (27.9, 56.2) | 40.9 (36.4, 61.6) | 1.000 |
| Haemodynamics | |||
| mRAP, mmHg | 7.0 (4.8, 9.3) | 7.0 (5.6, 8.5) | 0.541 |
| mPAP, mm Hg | 46.0 (42.0, 55.0) | 44.0 (41.8, 49.2) | 0.108 |
| PAWP, mm Hg | 9.0 (6.0, 13.0) | 8.0 (7.6, 10.2) | 0.348 |
| CO, L/min | 4.6 (4.0, 5.7) | 4.5 (4.1, 5.3) | 0.139 |
| CI, L/min/m2 | 3.1 (2.6, 3.7) | 2.7 (2.5, 3.1) | 0.035 |
| PVR, Wood units | 8.0 (6.3, 10.2) | 8.0 (7.1, 9.1) | 0.895 |
| Echocardiography | |||
| LVEF, % | 70.9 ± 9.0 | 68.9 ± 9.9 | 0.315 |
| LA, cm | 3.1 ± 0.6 | 3.5 ± 0.7 | 0.799 |
| LVDED, cm | 4.0 (3.5, 4.4) | 3.9 (3.7, 4.2) | 0.629 |
| LVSED, cm | 2.3 ± 0.6 | 2.4 ± 0.6 | 0.949 |
| RATD, cm | 4.8 (4.3, 5.5) | 4.9 (4.8, 5.6) | 0.200 |
| RALD, cm | 5.2 (4.3, 5.9) | 5.0 (5.4, 5.9) | 0.175 |
| RVEDTD, cm | 4.3 (3.8, 5.0) | 4.3 (4.0, 4.9) | 0.670 |
| RVEDLD, cm | 6.6 ± 0.9 | 6.8 ± 0.9 | 0.271 |
| PASP, mmHg | 76.2 ± 22.6 | 74.1 ± 24.4 | 0.533 |
| TAPSE, cm | 1.7 (1.5, 1.9) | 1.8 (1.6, 1.8) | 0.921 |
| PAd, cm | 2.9 (2.6, 3.2) | 3.1 (2.9, 3.4) | 0.208 |
| ENDSEI | 1.3 (1.0, 1.5) | 1.1 (1.1, 1.3) | 0.158 |
| TV s’, cm/s | 12.0 (9.0, 13.0) | 11.2 (10.1, 11.6) | 0.608 |
| PASP/TAPSE, mmHg/cm | 44.3 ± 14.4 | 44.4 ± 16.3 | 0.975 |
| Blood gas analysis | |||
| pH | 7.39 (7.35, 7.42) | 7.40 (7.38, 7.42) | 0.189 |
| PaO2, mmHg | 58 (46, 60) | 59.5 (50.8, 69.5) | 0.591 |
| PaCO2, mmHg | 50.3 (39.7, 60.1) | 46.5 (37.7, 58.6) | 0.236 |
| SaO2, % | 87.0 (79.3, 91.7) | 93.8 (83.1, 93.8) | 0.136 |
| PAH-targeted therapy | |||
| PDE5I | 47 (70.1) | 30 (90.1) | 0.127 |
| ERA | 5 (7.5) | 1 (3.3) | 0.661 |
| Prostacyclin | 6 (9.0) | 1 (3.3) | 0.420 |
| None | 9 (13.4) | 2 (6.1) | 0.330 |
| Traditional treatment | |||
| Oxygen | 67 (100) | 33 (100) | 1.000 |
| ICS/LABA | 59 (88.1) | 30 (90.9) | 1.000 |
| Variable * | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR [95% CI] | p Value | HR [95% CI] | p Value | β-Coefficient | Weighted Scores | |
| Age, years | 1.581 (0.779, 3.206) | 0.204 | ||||
| Sex | 0.691 (0.370, 1.290) | 0.246 | ||||
| BSA | 1.004 (0.263, 3.833) | 1.004 | ||||
| PaO2, mm Hg | 0.983 (0.963, 1.004) | 0.119 | ||||
| PaCO2, mm Hg | 1.010 (0.984, 1.036) | 0.471 | ||||
| SaO2, % | 0.973 (0.940, 1.008) | 0.125 | ||||
| Lactic acid, mmol/L | 1.355 (0.891, 2.062) | 0.155 | ||||
| Echocardiography | ||||||
| LVEF, % | 0.531 (0.223, 1.268) | 0.154 | ||||
| LA, cm | 0.666 (0.350, 1.265) | 0.214 | ||||
| LVDED, cm | 0.608 (0.310, 1.193) | 0.148 | ||||
| LVSED, cm | 0.408 (0.220, 0.756) | 0.004 | ||||
| RATD, cm | 0.485 (0.201, 1.168) | 0.107 | ||||
| RALD, cm | 1.588 (0.809, 3.118) | 0.179 | ||||
| RVEDTD, cm | 2.583 (1.311, 5.091) | 0.006 | 2.248 (1.062, 4.759) | 0.034 | 0.810 | 1 |
| RVEDLD, cm | 1.363 (0.738, 2.519) | 0.322 | ||||
| PASP, mmHg | 2.599 (1.232, 5.485) | 0.012 | 5.039 (2.002, 12.680) | 0.001 | 1.617 | 2 |
| TAPSE, cm | 0.514 (0.273, 0.969) | 0.040 | 0.414 (0.196, 0.873) | 0.021 | −0.882 | −1 |
| PAd, cm | 0.537 (0.253, 1.295) | 0.181 | ||||
| ENDSEI | 2.342 (1.071, 5.123) | 0.033 | ||||
| Pulmonary function test | ||||||
| FEV1% pred | 0.753 (0.390, 1.455) | 0.399 | ||||
| FVC% pred | 0.674 (0.362, 1.257) | 0.215 | ||||
| FEV1/FVC | 0.427 (0.202, 0.904) | 0.026 | ||||
| RV% pred | 1.197 (0.615, 2.330) | 0.597 | ||||
| TLC% pred | 1.354 (0.660, 2.780) | 0.409 | ||||
| DLco% pred | 0.437 (0.217, 0.879) | 0.020 | ||||
| Variables | Value |
|---|---|
| TAPSE ≥ 1.8 cm | −1 |
| LVSED < 2.3 cm | 0 |
| RVEDTD ≥ 4.2 cm | +1 |
| RVEDTD < 4.2 cm | 0 |
| PASP ≥ 103 mm Hg | +2 |
| PASP < 103 mm Hg | 0 |
| Total Scores | −1~3 |
| Variable * | HR [95% CI] | p Value |
|---|---|---|
| Lactic acid, mmol/L | 2.877 (1.103, 7.503) | 0.031 |
| Echocardiography | ||
| LVSED, cm | 0.460 (0.231, 0.915) | 0.027 |
| RVEDTD, cm | 3.708 (1.156, 9.069) | 0.004 |
| PASP, mmHg | 1.432 (0.717, 2.858) | 0.309 |
| TAPSE, cm | 2.040 (0.986, 4.218) | 0.055 |
| ENDSEI | 2.267 (1.121, 4.587) | 0.023 |
| Pulmonary function test | ||
| FVC% pred | 0.467 (0.226, 0.966) | 0.040 |
| FEV1/FVC | 0.473 (0.224, 0.997) | 0.049 |
| DLco% pred | 0.260 (0.100, 0.676) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Wang, L.; Zhao, Q.-H.; Wu, C.; Yuan, P.; Wang, S.; Zhang, R.; Gong, S.-G.; Wu, W.-H.; He, J.; et al. Echocardiography Nomogram for Predicting Survival among Chronic Lung Disease Patients with Severe Pulmonary Hypertension. J. Clin. Med. 2022, 11, 1603. https://doi.org/10.3390/jcm11061603
Jiang R, Wang L, Zhao Q-H, Wu C, Yuan P, Wang S, Zhang R, Gong S-G, Wu W-H, He J, et al. Echocardiography Nomogram for Predicting Survival among Chronic Lung Disease Patients with Severe Pulmonary Hypertension. Journal of Clinical Medicine. 2022; 11(6):1603. https://doi.org/10.3390/jcm11061603
Chicago/Turabian StyleJiang, Rong, Lan Wang, Qin-Hua Zhao, Cheng Wu, Ping Yuan, Shang Wang, Rui Zhang, Su-Gang Gong, Wen-Hui Wu, Jing He, and et al. 2022. "Echocardiography Nomogram for Predicting Survival among Chronic Lung Disease Patients with Severe Pulmonary Hypertension" Journal of Clinical Medicine 11, no. 6: 1603. https://doi.org/10.3390/jcm11061603
APA StyleJiang, R., Wang, L., Zhao, Q.-H., Wu, C., Yuan, P., Wang, S., Zhang, R., Gong, S.-G., Wu, W.-H., He, J., Qiu, H.-L., Luo, C.-J., Liu, J.-M., & Jing, Z.-C. (2022). Echocardiography Nomogram for Predicting Survival among Chronic Lung Disease Patients with Severe Pulmonary Hypertension. Journal of Clinical Medicine, 11(6), 1603. https://doi.org/10.3390/jcm11061603






