Does Far-Infrared Therapy Improve Peritoneal Function and Reduce Recurrent Peritonitis in Peritoneal Dialysis Patients?
Abstract
:1. Introduction
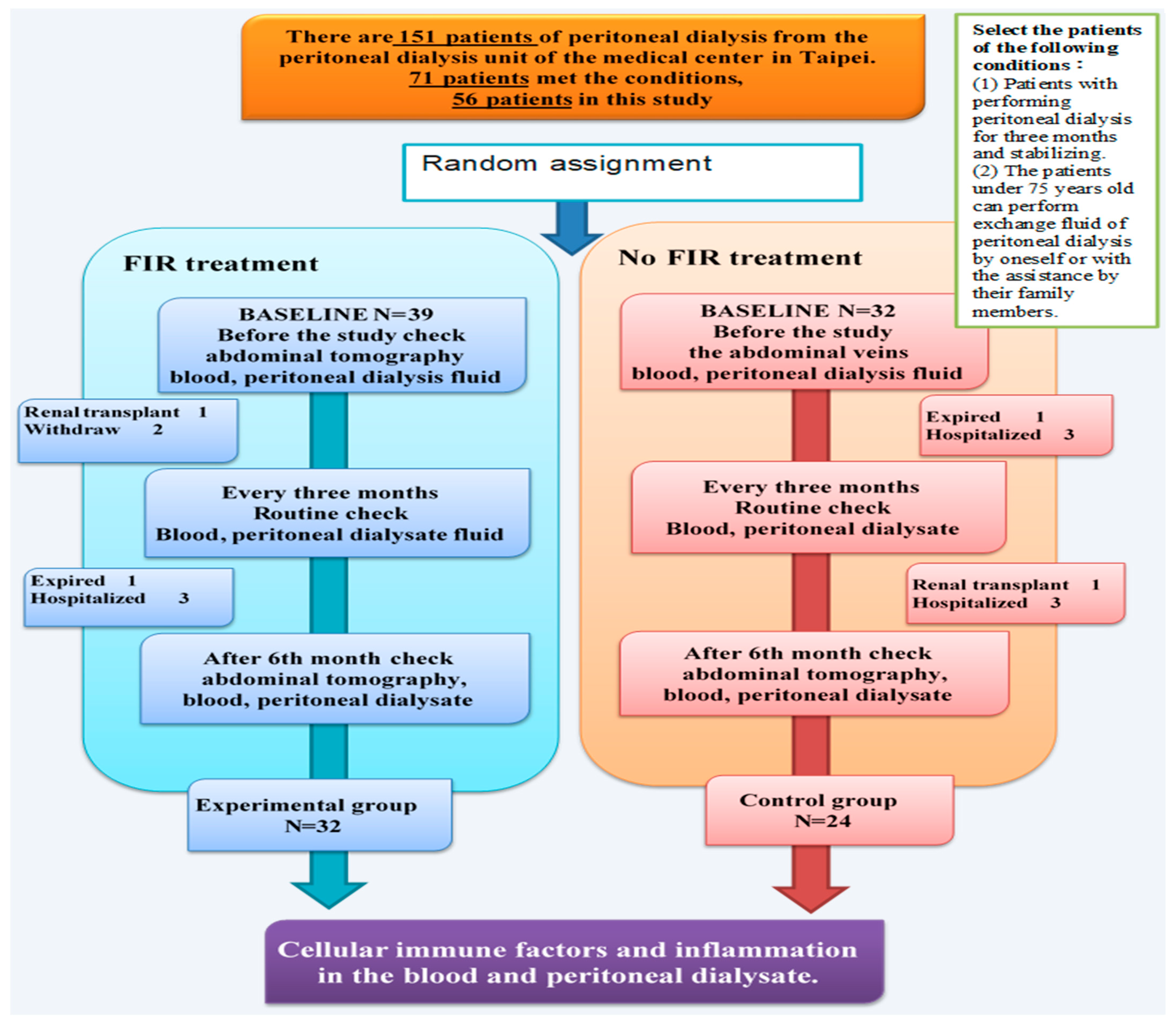
2. Materials and Methods
2.1. Research Objects
2.2. Patient Selection
- (1)
- Patients with PD stabilized for 6 months and attending daily routine PD.
- (2)
- No peritonitis at least 6 months, with 2.5% PD fluid, with an acceptable plasma creatinine ratio (D/P Cr) and peritoneal permeability, no need to use hypertonic PD fluid, and a C-reactive protein (CRP) index that did not indicate infection.
- (3)
- Patients under the age of 75 years who could perform PD fluid exchange alone or with the assistance of a family member.
- (4)
- FIR taken at least 4 times a week.
- (1)
- Patients who underwent kidney transplantation or were hospitalized during the study period.
- (2)
- An inability to perform a complete far-infrared radiation study for 6 months.
2.3. Experimental Method
2.4. Experimental Disposal (FIR Instrument)
2.5. Centrifugal Collection of Plasma
- (1)
- Plasma collection: The complete blood cell count and differential count were acquired (CBC and DC). Blood collection tubes contained EDTA, and the plasma was centrifuged at 800× g for 15 min. The upper supernatant of the plasma was pipetted equally into 110 μL Eppendorf tubes. The remaining white blood cells in the Buffy coat were collected into another tube, and hemolysis was performed using erythrocyte lysis buffer to remove erythrocytes without cell nuclei. A 0.4 mL solution with white blood cells was separated equally into 200 μL Eppendorf tubes to collect RNA and DNA.
- (2)
- Determination of immune factors in plasma: The plasma, peritoneal dialysate, and associated supernatant were used for analysis of cytokines using Human Th1/Th2 Panels of Luminex 200 (BioRad Corporation, Madison, WI, USA). We analyzed innate immunity cytokines, including IL-1β, IL-4, IL-6, IL-12p70, IL-18, IFN-γ, and TNF-α.
2.6. Experimental Evaluation
- The study used abdominal Computed Tomography (CT) to measure the degree of stiffness of the mesenteric vessels in order to explore the changes in abdominal blood vessels. The checkpoints were as follows: before and after the FIR therapy for 6 months. According to the CT scans at the two checkpoints, it was based on mesenteric atherosclerotic plaque, lumen stenosis or occlusion, and morphological changes in bowel and mesenteric ischemia. We graded the degree of stiffness of the mesenteric vessels on a scale of 0–2, with 0 indicating normal; 1, a faint but detectable change; 2, abnormal [46].
- The study compared the effects of PD using BUN, creatinine (Cr) value, and the dialysate and plasma creatinine ratio (D/P Cr) from the blood and the peritoneal dialysate (the emptied dialysate fluid) every 3 months.After the blood and peritoneal dialysate were collected, albumin (ALB), BUN, creatinine (Cr), white blood cell (WBC), hs-CRP, and HbAlC (the first and the last time) analyses were used to assess check peritoneal permeability and inflammation.
- Evaluation of Serum Biochemical Parameters
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Evaluation of Abdominal Blood Vessels
3.3. Physiological Evaluation
3.4. Evaluation of Serum Biochemical Parameters
4. Discussion
4.1. Abdominal Vessels
4.2. The Physiological Effects of PD Due to FIR
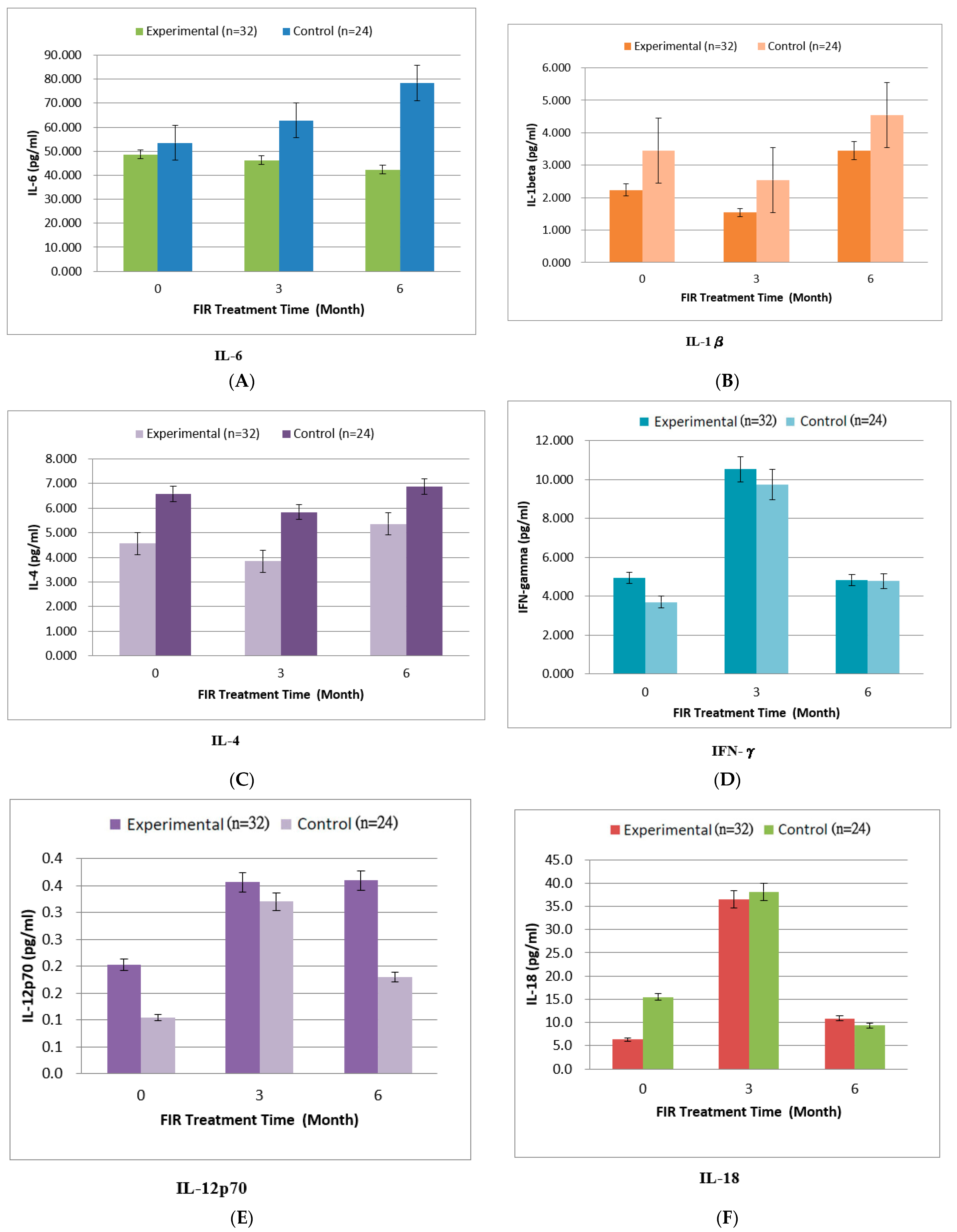
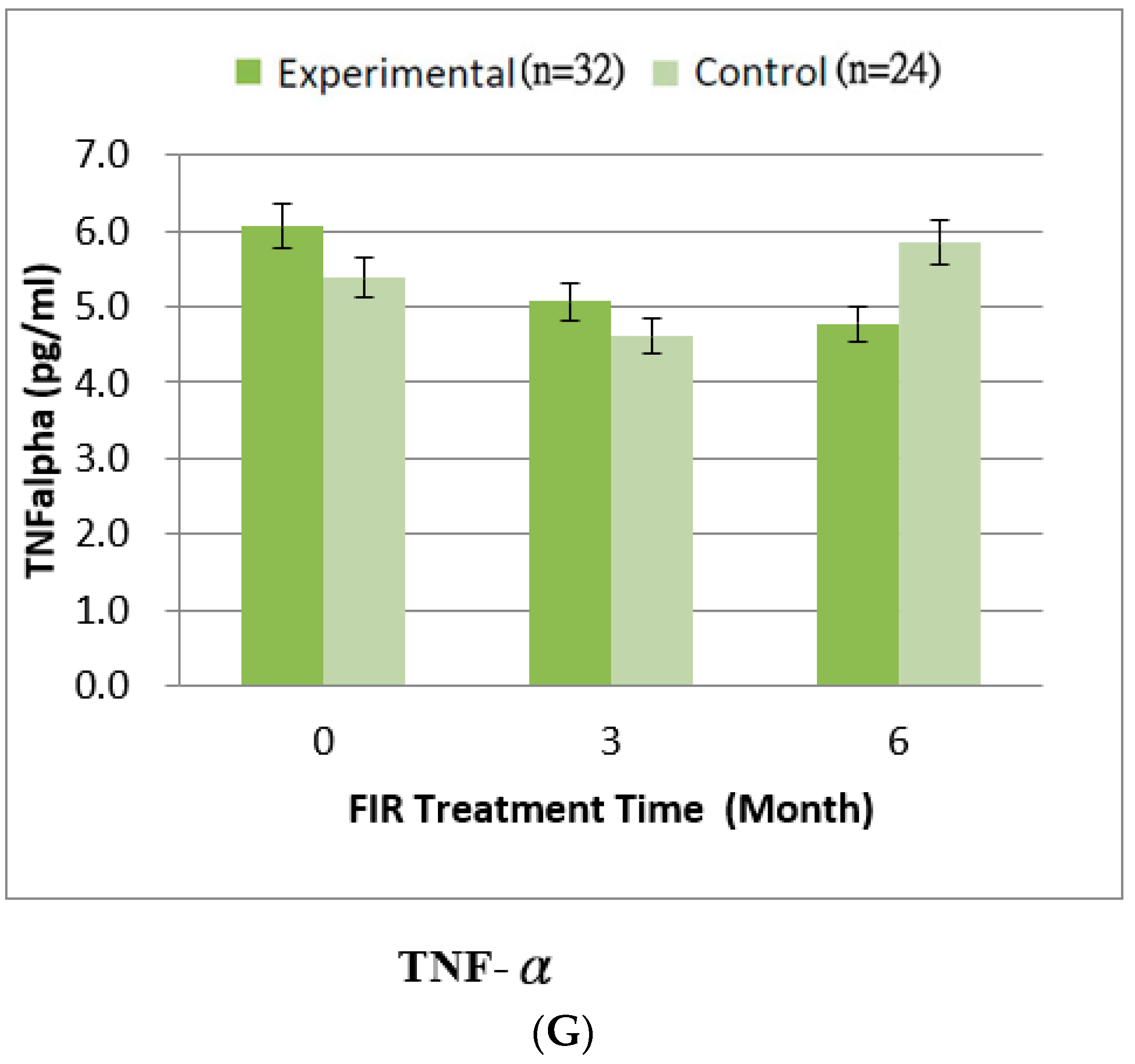
4.3. Inflammatory Cytokines
4.4. Overall Assessment
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamie, P.D.; Julia, B.L. Clinical aspects of diabetic nephropathy. In Schrier’s Diseases of the Kidney; Robert, W.S., Eric, G.N., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; p. 165975. [Google Scholar]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [PubMed]
- Yang, X.; Yu, X.Q. Approaches to the protection of peritoneum in patients on long-term peritoneal dialysis. Chin. J. Blood Purif. 2010, 9, 523–525. [Google Scholar]
- Muskiet, M.; Smits, M.; Morsink, L.M.; Diamant, M. The gut–renal axis: Do incretin-based agents confer renoprotection in diabetes? Nat. Rev. Nephrol. 2013, 10, 88–103. [Google Scholar] [CrossRef] [PubMed]
- For the Diabetes TW, Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002, 287, 2563–2569. [Google Scholar] [CrossRef]
- Fineberg, D.; Jandeleit-Dahm, K.A.M.; Cooper, M.E. Diabetic nephropathy: Diagnosis and treatment. Nat. Rev. Endocrinol. 2013, 9, 713–723. [Google Scholar] [CrossRef]
- Sowinski, K.M.; Churchwell, M.D.; Decker, B.S. Hemodialysis and Peritoneal Dialysis. In Pharmacotherapy: A Pathophysiologic Approach, 11th ed.; DiPiro, J.T., Yee, G.C., Posey, L.M., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Mehrotra, R. Metabolic Effects of Peritoneal Dialysis. In Handbook of Dialysis Therapy, 5th ed.; Nissenson, A.R., Fine, R.N., Eds.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Wieslander, A.P.; Nordin, M.K.; Martinson, E.; Kjellstrand, P.T.; Boberg, U.C. Heat Sterilized PD-Fluids Impair Growth and Inflammatory Responses of Cultured Cell Lines and Human Leukocytes. Clin. Nephrol. 1993, 39, 343–348. [Google Scholar]
- Chang, C.N.; Niu, C.Y.; Tan, A.C.; Chan, C.H.; Chen, C.F.; Chen, T.H.; Li, S.Y.; Chen, Y.T.; Chen, F.Y.; Liu, W.S.; et al. The Effect of Far-Infrared Therapy on the Peritoneal Expression of Glucose Degradation Products in Diabetic Patients on Peritoneal Dialysis. Int. J. Mol. Sci. 2021, 22, 3732. [Google Scholar] [CrossRef]
- Jiang, S.K.; Feng, S.; Shi, Y.B. Association between serum leptin levels and peritoneal dialysis: A meta-analysis. Exp. Ther. Med. 2015, 10, 300–308. [Google Scholar] [CrossRef]
- Witowski, J.; Wisniewska, J.; Korybalska, K.; Bender, T.O.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jörres, A. Prolonged Exposure to Glucose Degradation Products Impairs Viability and Function of Human Peritoneal Mesothelial Cells. J. Am. Soc. Nephrol. 2001, 12, 2434–2441. [Google Scholar] [CrossRef]
- Witowski, J.; Jörres, A.; Korybalska, K.; Ksiazek, K.; Wisniewska-Elnur, J.; Bender, T.O.; Passlick-Deetjen, J.; Breborowicz, A. Glucose Degradation Products in Peritoneal Dialysis Fluids: Do They Harm? Kidney Int. 2003, 63, S148–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witowski, J.; Bender, T.O.; Gahl, G.M.; Frei, U.; Jörres, A. Glucose Degradation Products and Peritoneal Membrane Function. Perit. Dial. Int. 2001, 21, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, A.; Hutchison, A.J. Peritoneal Dialysis Solutions. In Handbook of Dialysis Therapy, 5th ed.; Nissenson, A.R., Fine, R.N., Eds.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Hsu, Y.H.; Chen, Y.W.; Cheng, C.Y.; Lee, S.L.; Chiu, T.H.; Chen, C.H. Detecting the limits of the biological effects of far-infrared radiation on epithelial cells. Sci. Rep. 2019, 9, 11586. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.A. Multi-watt near-infrared light therapy as a neuroregenerative treatment for traumatic brain injury. Neural Regen. Res. 2016, 11, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.K. In Vitro and In Vivo Studies of the Biological Effects of Bioceramic (a Material of Emitting High Performance Far-Infrared Ray) Irradiation. Chin. J. Physiol. 2015, 58, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, Y.; Teraoka, F.; Matsumoto, T.; Madachi, A.; Toki, F.; Uda, E.; Hase, R.; Takahashi, J.; Matsuura, N. Effects of far infrared ray on Hela cells and WI-38 cells. Int. Congr. Ser. 2003, 1255, 339–341. [Google Scholar] [CrossRef]
- Ou, S.M.; Hu, F.H.; Yang, W.C.; Lin, C.C. Far-Infrared Therapy as a Novel Treatment for Encapsulating Peritoneal Sclerosis. Am. J. Gastroenterol. 2014, 109, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Miyata, M.; Eto, H.; Shirasawa, T.; Hamada, N.; Ikeda, Y.; Biro, S.; Otsuji, Y.; Tei, C. Repeated Thermal Therapy Up-Regulates Endothelial Nitric Oxide Synthase and Augments Angiogenesis in a Mouse Model of Hindlimb Ischemia. Circ. J. 2006, 70, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.-Y.; Chiu, J.-H.; Yang, S.-D.; Hsu, Y.-C.; Lui, W.-Y.; Wu, C.-W. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol. Photoimmunol. Photomed. 2006, 22, 78–86. [Google Scholar] [CrossRef]
- Chang, Y. The Effect of Far Infrared Radiation Therapy on Inflammation Regulation in Lipopolysaccharide-induced Peritonitis in Mice. SAGE Open Med. 2018, 6, 2050312118798941. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.; Kabaya, M. Biological activities caused by far-infrared radiation. Int. J. Biometeorol. 1989, 33, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, F.G.; Rasker, J.J.; Floors, M.; Landkroon, R.; van Rennes, B.; Zwijnenberg, J.; van de Laar, M.A.; Koel, G.J. Infrared sauna in patients with rheumatoid arthritis and ankylosing spondylitis. A pilot study showing good tolerance, short-term improvement of pain and stiffness, and a trend towards long-term beneficial effects. Clin. Rheumatol. 2009, 28, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, A.; Koga, Y.; Hattanmaru, M.; Minagoe, S.; Tei, C. The Effects of Repeated Thermal Therapy for Patients with Chronic Pain. Psychother. Psychosom. 2005, 74, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Seo, Y.; Kim, Y.-W.; Kim, S.; Bae, H.; Choi, J.; Lim, I.; Bang, H.; Kim, J.-H.; Ko, J.-H. Far-infrared radiation stimulates platelet-derived growth factor mediated skeletal muscle cell migration through extracellular matrix-integrin signaling. Korean J. Physiol. Pharmacol. 2019, 23, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyokawa, H.; Matsui, Y.; Uhara, J.; Tsuchiya, H.; Teshima, S.; Nakanishi, H.; Kwon, A.-H.; Azuma, Y.; Nagaoka, T.; Ogawa, T.; et al. Promotive Effects of Far-Infrared Ray on Full-Thickness Skin Wound Healing in Rats. Exp. Biol. Med. 2003, 228, 724–729. [Google Scholar] [CrossRef]
- Capon, A.; Mordon, S. Can thermal lasers promote skin wound healing? Am. J. Clin. Dermatol. 2003, 4, 1–12. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Lin, Y.-F.; Chen, C.-H.; Chiu, Y.-J.; Chiu, H.-W. Far infrared promotes wound healing through activation of Notch1 signaling. Klin. Wochenschr. 2017, 95, 1203–1213. [Google Scholar] [CrossRef]
- Chiu, H.-W.; Chen, C.-H.; Chang, J.-N.; Chen, C.-H.; Hsu, Y.-H. Far-infrared promotes burn wound healing by suppressing NLRP3 inflammasome caused by enhanced autophagy. Klin. Wochenschr. 2016, 94, 809–819. [Google Scholar]
- Ikeda, Y.; Biro, S.; Kamogawa, Y.; Yoshifuku, S.; Eto, H.; Orihara, K.; Yu, B.; Kihara, T.; Miyata, M.; Hamasaki, S. Repeated Sauna Therapy Increases Arterial Endothelial Nitric Oxide Synthase Expression and Nitric Oxide Production in Cardiomyopathic Hamsters. Circ. J. 2005, 69, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Yang, C.-C.; Hsiao, L.-D.; Chen, S.-Y.; Yang, C.-M. Heme Oxygenase-1 Induction by Carbon Monoxide Releasing Molecule-3 Suppresses Interleukin-1β-Mediated Neuroinflammation. Front. Mol. Neurosci. 2017, 10, 387. [Google Scholar] [CrossRef]
- Chen, S.-C.; Lee, M.-Y.; Huang, J.-C.; Kuo, I.-C.; Mai, H.-C.; Kuo, P.-L.; Chang, J.-M.; Hwang, S.-J.; Chen, H.-C. Association of Far-Infrared Radiation Therapy and Ankle-Brachial Index of Patients on Hemodialysis with Peripheral Artery Occlusive Disease. Int. J. Med. Sci. 2016, 13, 970–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Liu, X.M.; Peyton, K.; Wang, H.; Yang, W.C.; Lin, S.J.; Durante, W. Far Infrared Therapy Inhibits Vascular Endothelial Inflammation via the Induction of Heme Oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.H.; Wu, K.D.; Lee, L.S.; Wang, H.; Liu, C.F. Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patient. Am. J. Chin. Med. 2009, 37, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.M.; Cheng, X.W.; Lee, S.; Lee, K.H.; Cho, H.; Kang, J.H.; Kim, W. Preconditioning with far-infrared irradiation enhances proliferation, cell survival, and migration of rat bone marrow-derived stem cells via CXCR4-ERK pathways. Sci Rep. 2017, 7, 13718. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhou, H.; Liang, H.; Li, C. Regulation of ERK and AKT pathways by hepatitis B virus X protein via the Notch1 pathway in hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 1449–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, A.; Maiello, M.R.; D’Alessio, A.; Pergameno, M.; Normanno, N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets 2012, 16, S17–S27. [Google Scholar] [CrossRef]
- Lin, C.C.; Chung, M.Y.; Yang, W.C.; Lin, S.J.; Lee, P.C. Length polymorphisms of hemeoxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: A novel physicogenomic study. Nephrol. Dial. Transplant. 2013, 28, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.O.; Lodwick, D.; Williams, B. Vascular endothelial growth factor and microvascular permeability. Microcirculation 1999, 6, 83–96. [Google Scholar] [CrossRef]
- Salm, D.C.; Belmonte, L.A.O.; Emer, A.A.; Leonel, L.D.S.; de Brito, R.N.; da Rocha, C.C.; Martins, T.C.; dos Reis, D.C.; Moro, A.R.P.; Mazzardo-Martins, L.; et al. Aquatic exercise and Far Infrared (FIR) modulates pain and blood cytokines in fibromyalgia patients: A double-blind, randomized, placebo-controlled pilot study. J. Neuroimmunol. 2019, 337, 577077. [Google Scholar] [CrossRef]
- Lai, C.-C.; Fang, H.-C.; Mar, G.-Y.; Liou, J.-C.; Tseng, C.-J.; Liu, C.-P. Post-angioplasty Far Infrared Radiation Therapy Improves 1-Year Angioplasty-Free Hemodialysis Access Patency of Recurrent Obstructive Lesions. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Yang, W.-C.; Chen, M.-C.; Liu, W.-S.; Yang, C.-Y.; Lee, P.-C. Effect of Far Infrared Therapy on Arteriovenous Fistula Maturation: An Open-Label Randomized Controlled Trial. Am. J. Kidney Dis. 2013, 62, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Jagirdar, R.M.; Bozikas, A.; Zarogiannis, S.G.; Bartosova, M.; Schmitt, C.P.; Liakopoulos, V. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. Int. J. Mol. Sci. 2019, 20, 5765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.J. Progress in multi-slice CT diagnosis of atherosclerotic mesenteric ischemia. World Chin. J. Dig. 2017, 25, 2770–2775. [Google Scholar] [CrossRef]
- Brown, E.A.; Bargman, J.; van Biesen, W.; Chang, M.-Y.; Finkelstein, F.O.; Hurst, H.; Johnson, D.W.; Kawanishi, H.; Lambie, M.; de Moraes, T.P.; et al. Length of Time on Peri-toneal Dialysis and Encapsulating Peritoneal Sclerosis—Position Paper for ISPD: 2017 Update. Perit. Dial. Int. 2017, 37, 362–374. [Google Scholar] [CrossRef]
- Li, P.K.; Szeto, C.C.; Piraino, B.; Bernardini, J.; Figueiredo, A.E.; Gupta, A.; Johnson, D.W.; Kuijper, E.J.; Lye, W.C.; Salzer, W.; et al. Peritoneal dialysis-related infections recommendations. Perit Dial. Int. 2010, 30, 393–423. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Hong, S.-Y. Infectious complications of peritoneal dialysis. Kidney Dial. 2014, 26, 75–78. [Google Scholar]
- Huang, P.-H.; Chen, J.-W.; Lin, C.-P.; Chen, Y.-H.; Wang, C.-H.; Leu, H.-B.; Lin, S.-J. Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions. Cardiovasc. Diabetol. 2012, 11, 99. [Google Scholar] [CrossRef] [Green Version]
- Nicola, R.S.; Jason, J.A. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar]
- Fu, Q.; Li, Z.; Ye, J.; Li, Z.; Fu, F.; Lin, S.L.; Chang, C.A.; Yang, H.; Song, J. Magnetic targeted near-infrared II PA/MR imaging guided photothermal therapy to trigger cancer immunotherapy. Theranostics 2020, 10, 4997–5010. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. Thematernal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Cheng, F.-Y.; Chao, Y.-F.C.; Liu, C.-Y.; Chang, Y. Effects of far-infrared therapy on foot circulation among hemodialysis patients with diabetes mellitus. Biol. Res. Nurs. 2020, 22, 403–411. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Experimental Group (n = 32) | Control Group (n = 24) | p | ||
|---|---|---|---|---|---|
| 0 | 6 (Months) | 0 | 6 (Months) | ||
| Degree of stiffness (Variation grade) | 0.73 ± 0.16 | 0.72 ± 0.27 | 0.73 ± 0.19 | 0.74 ± 0.25 | 0.256 |
| Parameters | Experimental Group (n = 32) | Control Group (n = 24) | p | ||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 0 | 3 | 6 | ||
| Age (years) | 54.53 ± 12.56 | 58.31 ± 8.19 | 0.115 | ||||
| Gender (M/F) | 15/17 | 9/15 | 0.116 | ||||
| Body weight | 57.63 ± 13.04 | 63.46 ± 16.41 | 0.425 | ||||
| BMI (Kg/m2) | 25.52 ± 4.98 | 24.82 ± 5.55 | 0.591 | ||||
| Years of PD | 11.46 ± 3.54 | 8.76 ± 5.18 | 0.253 | ||||
| Peritoneal function | |||||||
| Peritoneal Kt/V | 1.95 ± 0.26 | 2.03 ± 0.22 | 2.14 ± 0.17 | 2.11 ± 0.47 | 1.95 ± 0.46 | 1.82 ± 0.58 | 0.097 |
| Peritoneal weekly CCr (L/week/m2) | 61.67 ± 14.72 | 58.24 ± 12.11 | 56.61 ± 13.28 | 58.51 ± 15.34 | 62.14 ± 10.40 | 65.25 ± 14.41 | 0.173 |
| Serum biochemistry | |||||||
| Glucose (mg/dL) | 105.27 ± 29.48 | 111.27 ± 28.37 | 99.40 ± 12.25 | 98.85 ± 10.24 | 99.31 ± 9.75 | 105.08 ± 13.72 | <0.001 ** |
| WBC | 6.51 ± 2.26 | 5.90 ± 1.97 | 6.68 ± 2.24 | 8.28 ± 2.74 | 7.59 ± 1.84 | 7.89 ± 2.01 | 0.365 |
| HbA1c (%) | 9.93 ± 1.51 | 9.79 ± 1.65 | 10.21 ± 1.91 | 9.68 ± 1.46 | 10.22 ± 1.69 | 10.25 ± 1.85 | 0.757 |
| BUN (mg/dL) | 63.67 ± 14.77 | 62.27 ± 13.03 | 61.13 ± 12.22 | 71.85 ± 13.38 | 64.15 ± 20.40 | 60.01 ± 12.45 | 0.42 |
| Creatinine (mg/dL) | 9.07 ± 3.48 | 7.79 ± 4.25 | 6.23 ± 0.68 | 12.02 ± 1.69 | 10.22 ± 1.69 | 9.16 ± 0.21 | 0.039 * |
| Albumin (g/dL) | 3.68 ± 0.30 | 3.57 ± 0.38 | 3.73 ± 0.43 | 3.56 ± 0.42 | 3.67 ± 0.40 | 3.73 ± 0.56 | 0.048 * |
| eGFR | 4.01 ± 0.75 | 3.99 ± 0.73 | 4.12 ± 0.78 | 4.21 ± 1.41 | 3.96 ± 0.91 | 3.73 ± 0.78 | 0.043 * |
| Phosphate (mg/dL) | 4.96 ± 1.02 | 4.83 ± 0.91 | 5.01 ± 0.68 | 5.13 ± 1.43 | 5.02 ± 1.61 | 5.36 ± 1.12 | 0.58 |
| T-P | 6.52 ± 0.71 | 6.48 ± 0.54 | 6.47 ± 0.66 | 6.44 ± 0.52 | 6.47 ± 0.58 | 6.36 ± 0.65 | 0.314 |
| hs-CRP (mg/dL) | 1.31 ± 2.14 | 0.51 ± 0.54 | 0.75 ± 1.37 | 0.98 ± 1.49 | 1.00 ± 2.21 | 0.41 ± 0.36 | <0.001 ** |
| Parameters | Experimental Group (n = 32) | Control Group (n = 24) | p | ||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 0 | 3 | 6 | ||
| IL-1beta | 2.23 ± 0.44 | 1.54 ± 0.14 | 3.45 ± 0.75 | 3.45 ± 0.39 | 2.54 ± 0.42 | 4.55 ± 1.12 | 0.175 |
| IL-4 | 4.56 ± 1.75 | 3.84 ± 0.87 | 5.36 ± 2.44 | 6.58 ± 1.98 | 5.84 ± 1.58 | 6.87 ± 2.12 | 0.156 |
| IL-6 | 48.6 ± 9.49 | 46.2 ± 12.35 | 42.3 ± 11.63 | 53.5 ± 11.64 | 58.8 ± 19.42 | 65.4 ± 21.75 | 0.061 |
| IFN-γ | 4.93 ± 1.44 | 10.52 ± 2.86 | 4.83 ± 1.43 | 3.69 ± 0.94 | 9.73 ± 2.53 | 4.78 ± 2.14 | 0.124 |
| IL-12p70 | 0.20 ± 0.09 | 0.36 ± 1.14 | 0.36 ± 0.09 | 0.10 ± 0.04 | 0.32 ± 0.053 | 0.18 ± 0.07 | 0.093 |
| TNF-alpha | 6.06 ± 2.23 | 5.07 ± 2.12 | 4.78 ± 1.47 | 5.39 ± 2.18 | 4.61 ± 1.97 | 5.85 ± 2.11 | 0.254 |
| IL-18 | 6.35 ± 2.12 | 36.48 ± 8.9 | 10.88 ± 2.47 | 15.51 ± 4.23 | 38.11± 11.64 | 9.34 ± 3.11 | 0.213 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Chang, J.-T.; Lee, M.-Y.; Huang, M.-Z.; Chao, Y.-F.C.C.; Shih, Y.-L.; Hwang, Y.-R. Does Far-Infrared Therapy Improve Peritoneal Function and Reduce Recurrent Peritonitis in Peritoneal Dialysis Patients? J. Clin. Med. 2022, 11, 1624. https://doi.org/10.3390/jcm11061624
Chang Y, Chang J-T, Lee M-Y, Huang M-Z, Chao Y-FCC, Shih Y-L, Hwang Y-R. Does Far-Infrared Therapy Improve Peritoneal Function and Reduce Recurrent Peritonitis in Peritoneal Dialysis Patients? Journal of Clinical Medicine. 2022; 11(6):1624. https://doi.org/10.3390/jcm11061624
Chicago/Turabian StyleChang, Yuanmay, Jui-Ting Chang, Mei-Yi Lee, Mei-Zen Huang, Yann-Fen C. C. Chao, Yung-Luen Shih, and Yao-Rong Hwang. 2022. "Does Far-Infrared Therapy Improve Peritoneal Function and Reduce Recurrent Peritonitis in Peritoneal Dialysis Patients?" Journal of Clinical Medicine 11, no. 6: 1624. https://doi.org/10.3390/jcm11061624
APA StyleChang, Y., Chang, J.-T., Lee, M.-Y., Huang, M.-Z., Chao, Y.-F. C. C., Shih, Y.-L., & Hwang, Y.-R. (2022). Does Far-Infrared Therapy Improve Peritoneal Function and Reduce Recurrent Peritonitis in Peritoneal Dialysis Patients? Journal of Clinical Medicine, 11(6), 1624. https://doi.org/10.3390/jcm11061624






