A Statistical Shape Model of the Morphological Variation of the Infrarenal Abdominal Aortic Aneurysm Neck
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements in Vascular Workstation
2.3. Preprocessing and Aortic Neck Alignment
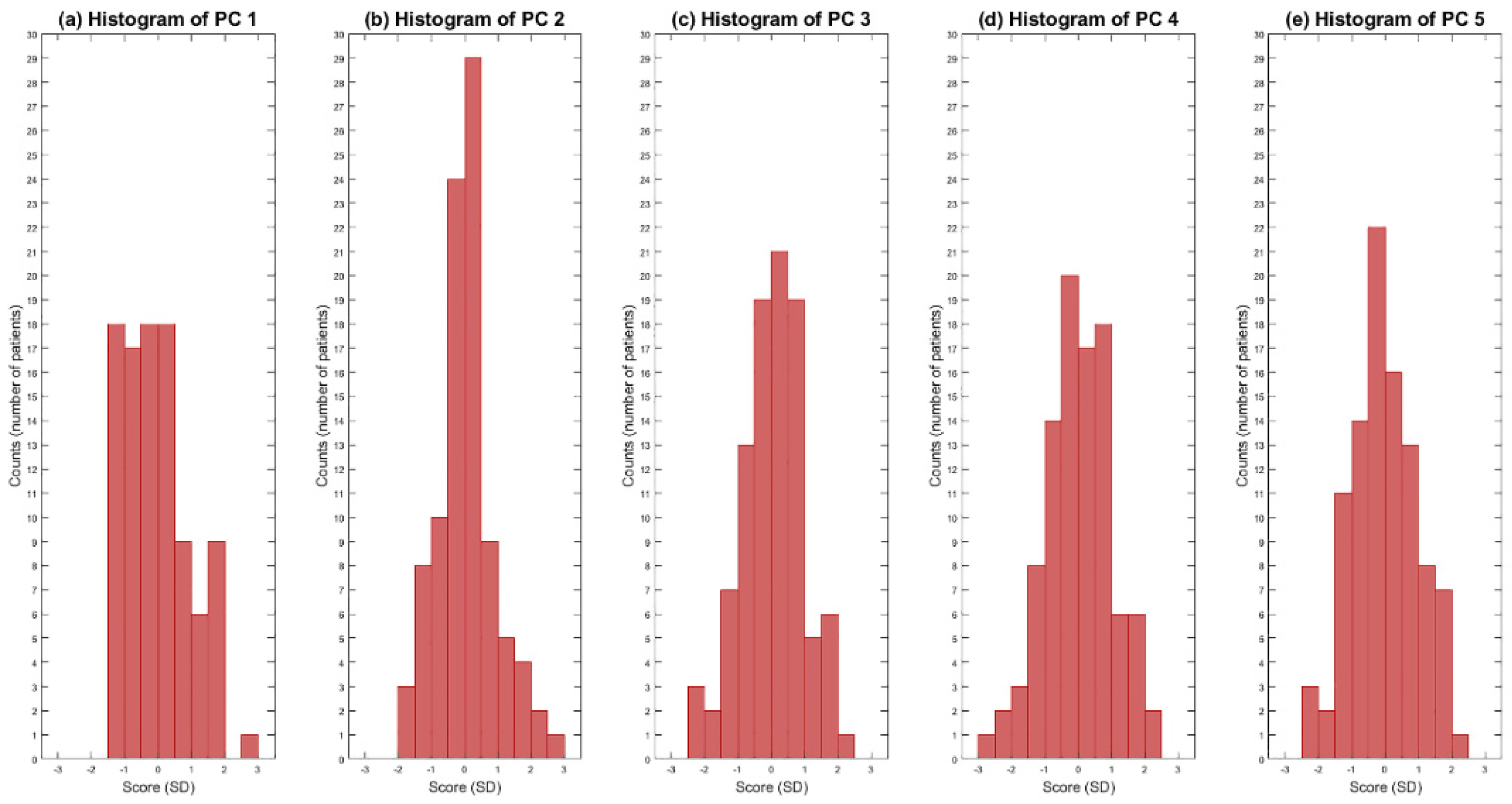
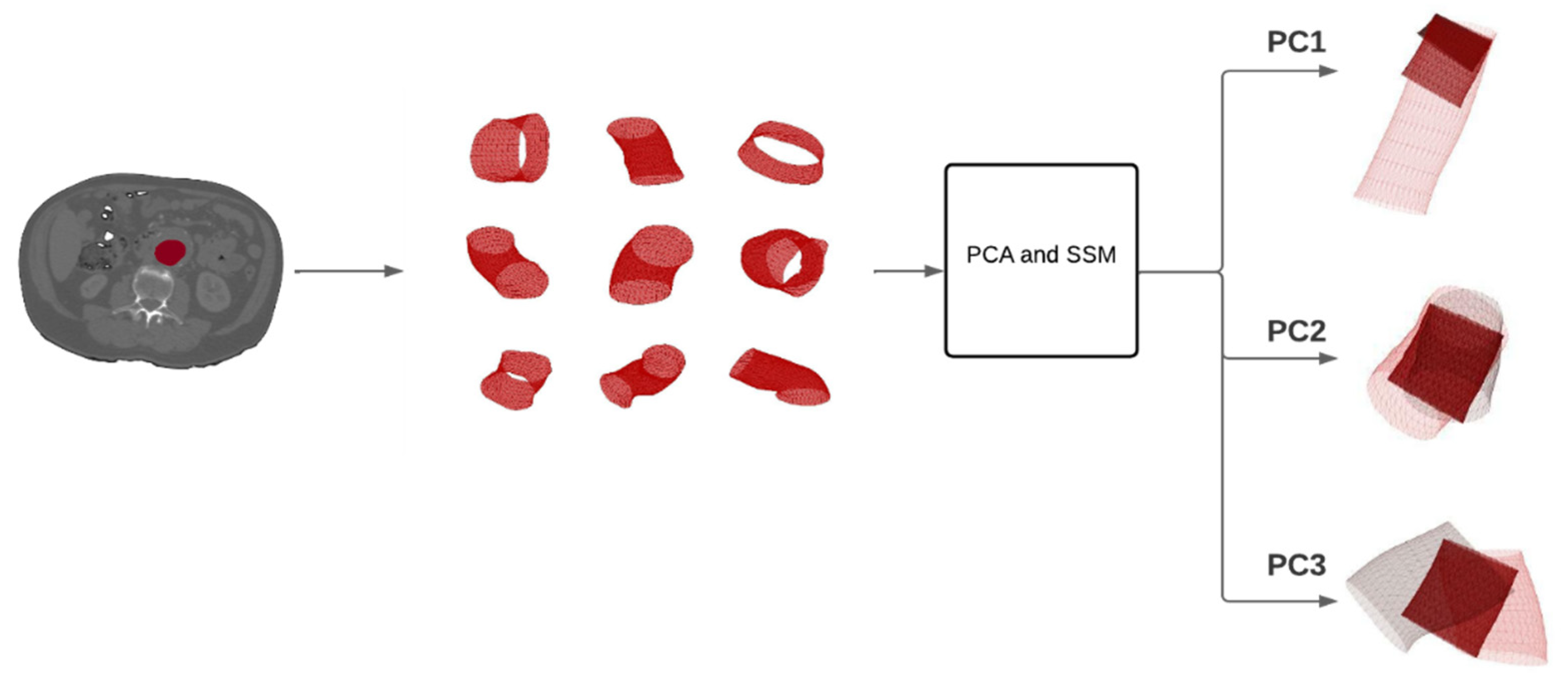
2.4. Principal Component Analysis and Statistical Shape Modelling
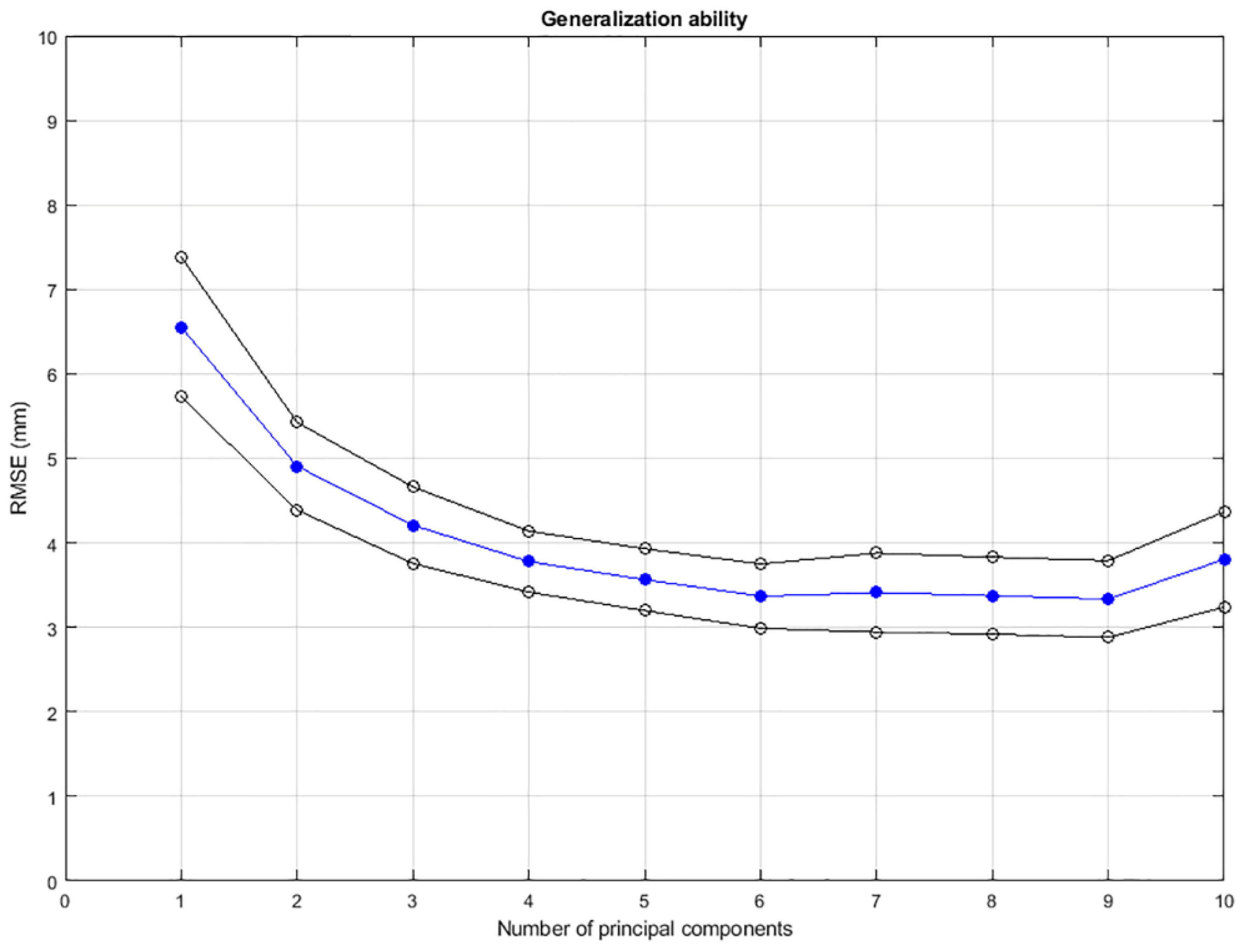
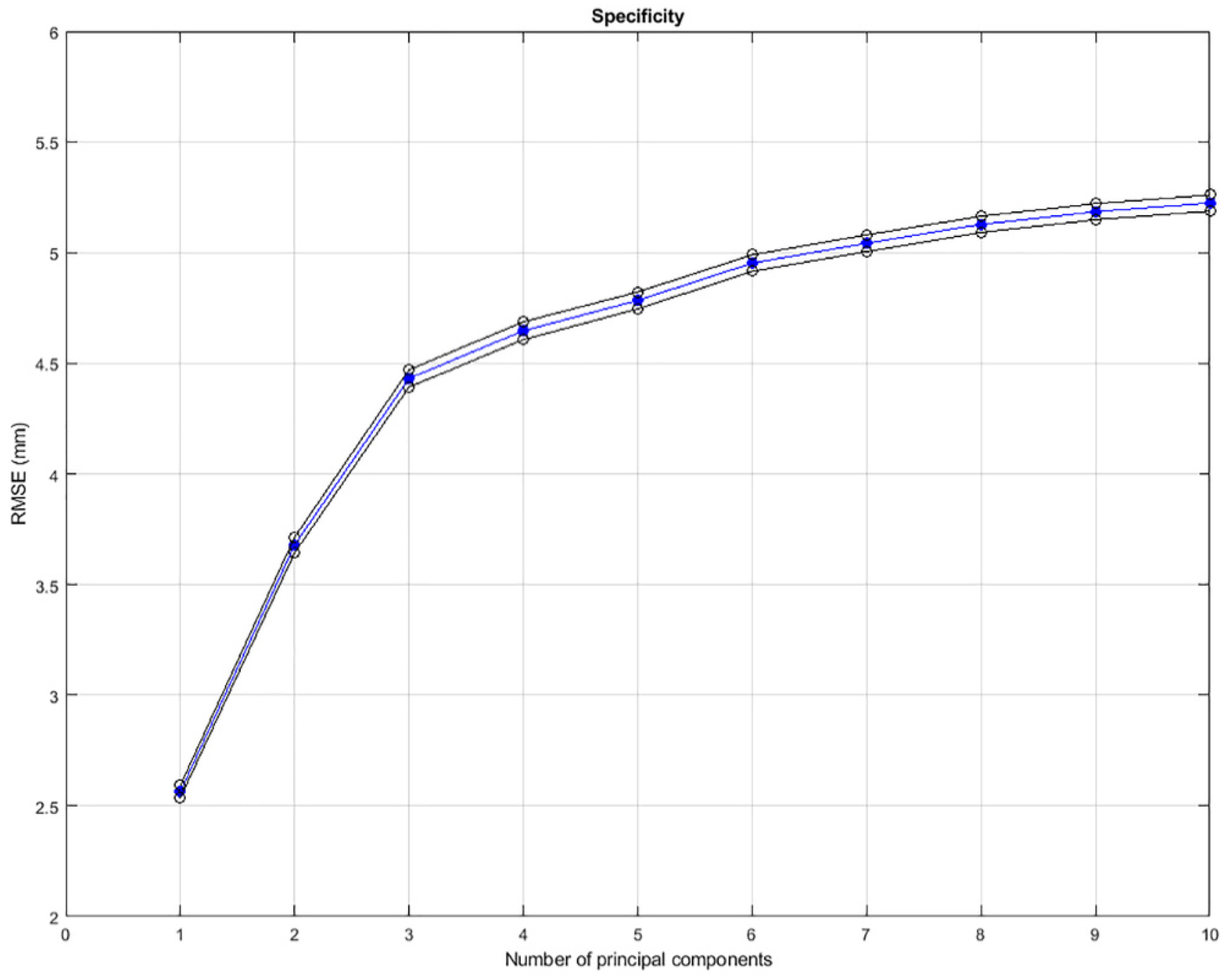
2.5. Evaluation
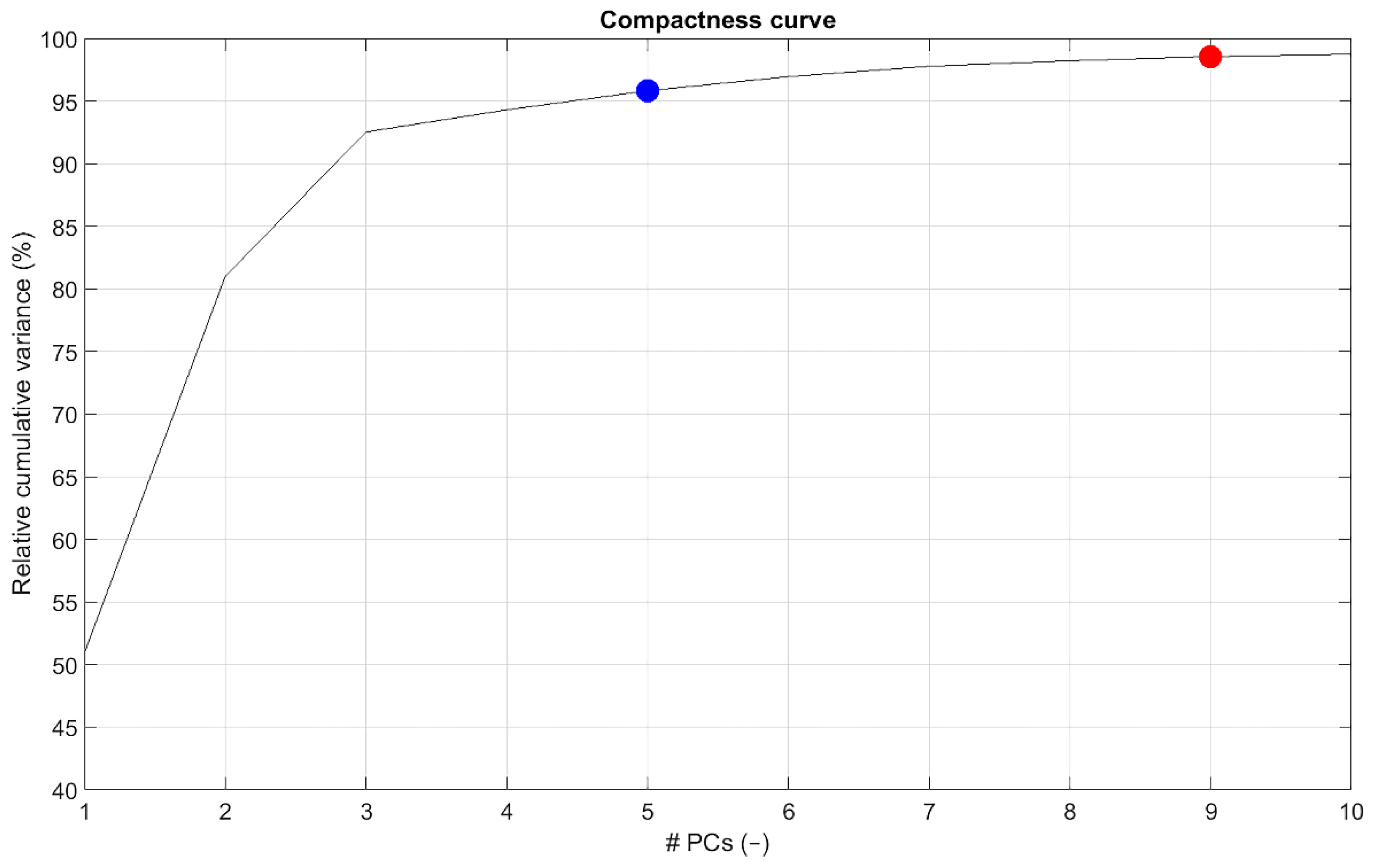
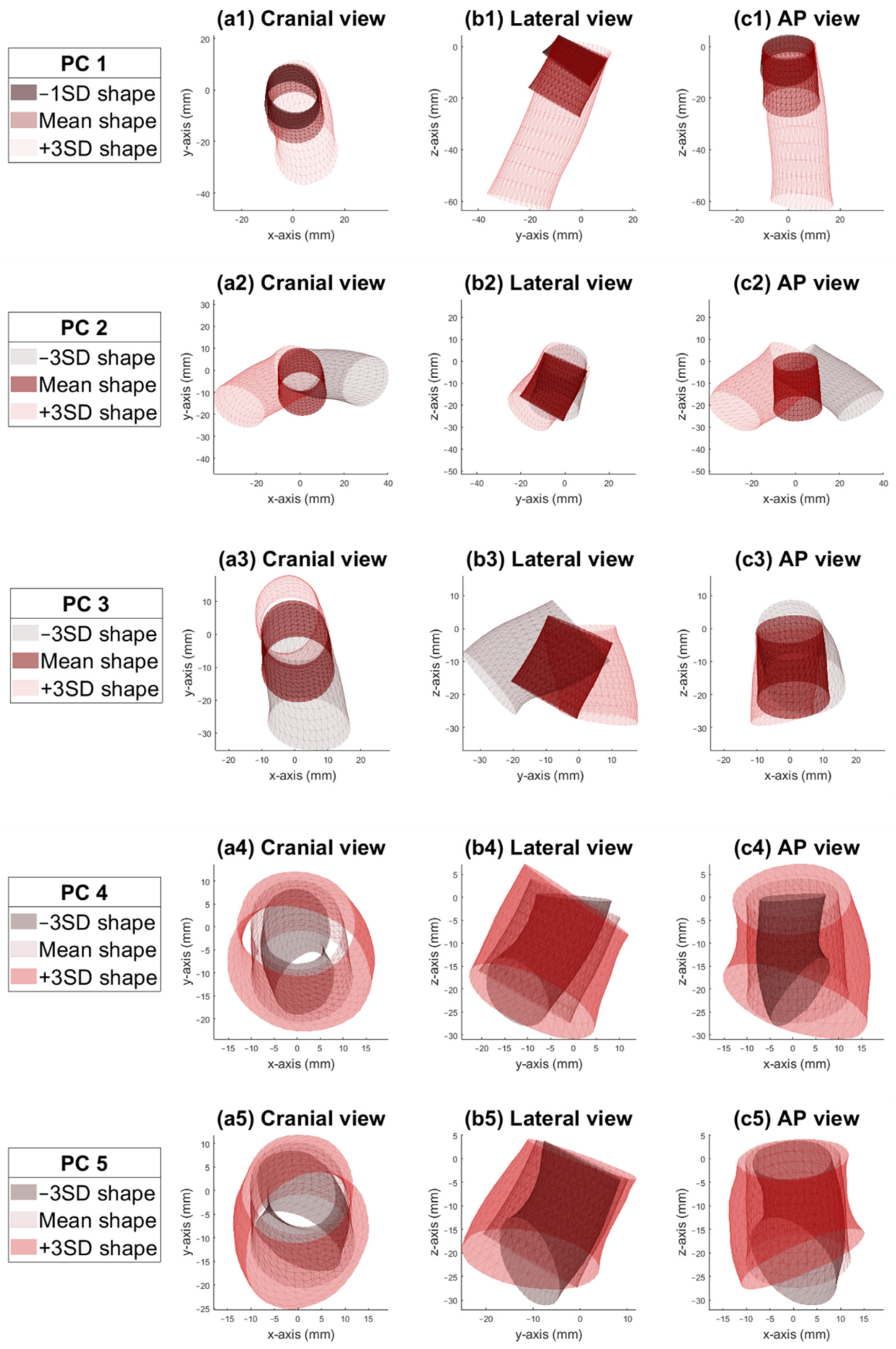
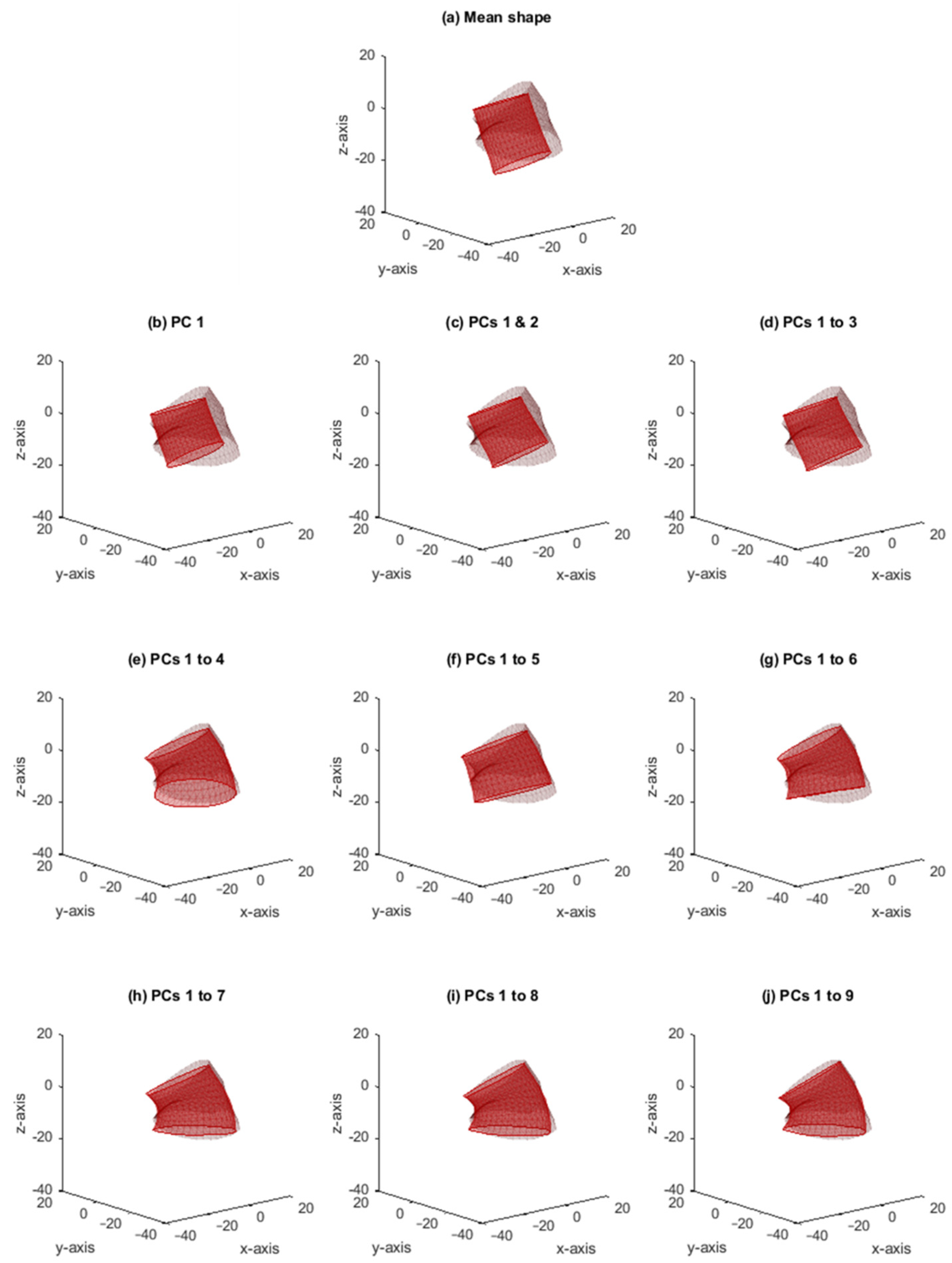
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Cerini, P.; Guzzardi, G.; Divenuto, I.; Parziale, G.; Brustia, P.; Carriero, A.; Fossaceca, R. Are Abdominal Aortic Aneurysms with Hostile Neck Really Unsuitable for EVAR? Our Experience. Radiol. Med. 2016, 121, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice–European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, G.A.; Georgiadis, G.S.; Antoniou, S.A.; Kuhan, G.; Murray, D. A Meta-Analysis of Outcomes of Endovascular Abdominal Aortic Aneurysm Repair in Patients with Hostile and Friendly Neck Anatomy. J. Vasc. Surg. 2013, 57, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stather, P.W.; Wild, J.B.; Sayers, R.D.; Bown, M.J.; Choke, E. Endovascular Aortic Aneurysm Repair in Patients with Hostile Neck Anatomy. J. Endovasc. Ther. 2013, 20, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Schuurmann, R.C.L.; van Noort, K.; Overeem, S.P.; Ouriel, K.; Jordan, W.D.; Muhs, B.E.; Mannetje, Y.T.; Reijnen, M.; Fioole, B.; Ünlü, Ç.; et al. Aortic Curvature Is a Predictor of Late Type Ia Endoleak and Migration after Endovascular Aneurysm Repair. J. Endovasc. Ther. 2017, 24, 411–417. [Google Scholar] [CrossRef]
- Brownrigg, J.R.W.; de Bruin, J.L.; Rossi, L.; Karthikesalingam, A.; Patterson, B.; Holt, P.J.; Hinchliffe, R.H.; Morgan, R.; Loftus, I.M.; Thompson, M.M. Endovascular Aneurysm Sealing for Infrarenal Abdominal Aortic Aneurysms: 30-Day Outcomes of 105 Patients in a Single Centre. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, N.F.G.; Gonçalves, F.B.; Ultee, K.; Pinto, J.P.; Josee van Rijn, M.; Raa, S.T.; Mwipatayi, P.; Böckler, D.; Hoeks, S.E.; Verhagen, H.J.M. Patients with Large Neck Diameter Have a Higher Risk of Type IA Endoleaks and Aneurysm Rupture after Standard Endovascular Aneurysm Repair. J. Vasc. Surg. 2019, 69, 783–791. [Google Scholar] [CrossRef]
- Bruse, J.L.; Khushnood, A.; McLeod, K.; Biglino, G.; Sermesant, M.; Pennec, X.; Taylor, A.M.; Hsia, T.-Y.; Schievano, S.; Taylor, A.M.; et al. How Successful Is Successful? Aortic Arch Shape after Successful Aortic Coarctation Repair Correlates with Left Ventricular Function. J. Thorac. Cardiovasc. Surg. 2017, 153, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Schoretsanitis, N.; Argyriou, C.; Georgiadis, G.S.; Lazaridis, M.K.; Georgakarakos, E. Hostile Neck in Abdominal Aortic Aneurysms. Vasc. Endovasc. Surg. 2016, 50, 208–210. [Google Scholar] [CrossRef] [Green Version]
- AbuRahma, A.F.; Yacoub, M.; Mousa, A.Y.; Abu-Halimah, S.; Hass, S.M.; Kazil, J.; AbuRahma, Z.T.; Srivastava, M.; Dean, L.S.; Stone, P.A. Aortic Neck Anatomic Features and Predictors of Outcomes in Endovascular Repair of Abdominal Aortic Aneurysms Following vs Not Following Instructions for Use. J. Am. Coll. Surg. 2016, 222, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Stather, P.W.; Sidloff, D.; Dattani, N.; Choke, E.; Bown, M.J.; Sayers, R.D. Systematic Review and Meta-Analysis of the Early and Late Outcomes of Open and Endovascular Repair of Abdominal Aortic Aneurysm. Br. J. Surg. 2013, 100, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Schanzer, A.; Greenberg, R.K.; Hevelone, N.; Robinson, W.P.; Eslami, M.H.; Goldberg, R.J.; Messina, L. Predictors of Abdominal Aortic Aneurysm Sac Enlargement After Endovascular Repair. Circulation 2011, 123, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Marone, E.M.; Freyrie, A.; Ruotolo, C.; Michelagnoli, S.; Antonello, M.; Speziale, F.; Veroux, P.; Gargiulo, M.; Gaggiano, A. Expert Opinion on Hostile Neck Definition in Endovascular Treatment of Abdominal Aortic Aneurysms (a Delphi Consensus). Ann. Vasc. Surg. 2020, 62, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Semper-Hogg, W.; Fuessinger, M.A.; Schwarz, S.; Ellis, E.; Cornelius, C.-P.; Probst, F.; Metzger, M.C.; Schlager, S. Virtual Reconstruction of Midface Defects Using Statistical Shape Models. J. Cranio-Maxillofac. Surg. 2017, 45, 461–466. [Google Scholar] [CrossRef]
- Fuessinger, M.A.; Schwarz, S.; Neubauer, J.; Cornelius, C.-P.; Gass, M.; Poxleitner, P.; Zimmerer, R.; Metzger, M.C.; Schlager, S. Virtual Reconstruction of Bilateral Midfacial Defects by Using Statistical Shape Modeling. J. Cranio-Maxillofac. Surg. 2019, 47, 1054–1059. [Google Scholar] [CrossRef]
- IJpma, F.F.A.; Meesters, A.M.L.; Merema, B.B.J.; ten Duis, K.; de Vries, J.-P.P.M.; Banierink, H.; Wendt, K.W.; Kraeima, J.; Witjes, M.J.H. Feasibility of Imaging-Based 3-Dimensional Models to Design Patient-Specific Osteosynthesis Plates and Drilling Guides. JAMA Netw. Open 2021, 4, e2037519. [Google Scholar] [CrossRef]
- Tuszynski, J. Triangle/Ray Intersection. Available online: https://www.mathworks.com/Matlabcentral/Fileexchange/33073-Triangle-Ray-Intersection (accessed on 13 February 2022).
- Van Haver, A.; Mahieu, P.; Claessens, T.; Li, H.; Pattyn, C.; Verdonk, P.; Audenaert, E.A. A Statistical Shape Model of Trochlear Dysplasia of the Knee. Knee 2014, 21, 518–523. [Google Scholar] [CrossRef]
- Casier, S.J.; Van den Broecke, R.; Van Houcke, J.; Audenaert, E.; De Wilde, L.F.; Van Tongel, A. Morphologic Variations of the Scapula in 3-Dimensions: A Statistical Shape Model Approach. J. Shoulder Elb. Surg. 2018, 27, 2224–2231. [Google Scholar] [CrossRef]
- Khanduja, V.; Baelde, N.; Dobbelaere, A.; Van Houcke, J.; Li, H.; Pattyn, C.; Audenaert, E.A. Patient-Specific Assessment of Dysmorphism of the Femoral Head-Neck Junction: A Statistical Shape Model Approach. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 12, 765–772. [Google Scholar] [CrossRef]
- Manu Shape Model Builder. Available online: Https://www.mathworks.com/Matlabcentral/Fileexchange/49940-Shape-Model-Builder (accessed on 13 February 2022).
- Styner, M.A.; Rajamani, K.T.; Nolte, L.-P.; Zsemlye, G.; Székely, G.; Taylor, C.J.; Davies, R.H. Evaluation of 3D Correspondence Methods for Model Building. In Proceedings of the Biennial International Conference on Information Processing in Medical Imaging, Ambleside, UK, 20–25 July 2003. [Google Scholar]
- Wang, J.; Shi, C. Automatic Construction of Statistical Shape Models Using Deformable Simplex Meshes with Vector Field Convolution Energy. Biomed. Eng. Online 2017, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Derycke, L.; Sénémaud, J.; Perrin, D.; Avril, S.; Desgranges, P.; Albertini, J.-N.; Cochennec, F.; Haulon, S. Patient Specific Computer Modelling for Automated Sizing of Fenestrated Stent Grafts. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 237–246. [Google Scholar] [CrossRef]
- Lareyre, F.; Adam, C.; Carrier, M.; Raffort, J. Automated Segmentation of the Human Abdominal Vascular System Using a Hybrid Approach Combining Expert System and Supervised Deep Learning. J. Clin. Med. 2021, 10, 3347. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, M.; Martin, C.; Elefteriades, J.A.; Sun, W. A Machine Learning Approach to Investigate the Relationship between Shape Features and Numerically Predicted Risk of Ascending Aortic Aneurysm. Biomech. Model. Mechanobiol. 2017, 16, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- de Bruijne, M.; van Ginneken, B.; Viergever, M.A.; Niessen, W.J. Interactive Segmentation of Abdominal Aortic Aneurysms in CTA Images. Med. Image Anal. 2004, 8, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huysmans, T.; Sijbers, J.; Brigitte, V. Automatic Construction of Correspondences for Tubular Surfaces. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 636–651. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Veldhuizen, W.A.; Schuurmann, R.C.L.; IJpma, F.F.A.; Kropman, R.H.J.; Antoniou, G.A.; Wolterink, J.M.; de Vries, J.-P.P.M. A Statistical Shape Model of the Morphological Variation of the Infrarenal Abdominal Aortic Aneurysm Neck. J. Clin. Med. 2022, 11, 1687. https://doi.org/10.3390/jcm11061687
van Veldhuizen WA, Schuurmann RCL, IJpma FFA, Kropman RHJ, Antoniou GA, Wolterink JM, de Vries J-PPM. A Statistical Shape Model of the Morphological Variation of the Infrarenal Abdominal Aortic Aneurysm Neck. Journal of Clinical Medicine. 2022; 11(6):1687. https://doi.org/10.3390/jcm11061687
Chicago/Turabian Stylevan Veldhuizen, Willemina A., Richte C. L. Schuurmann, Frank F. A. IJpma, Rogier H. J. Kropman, George A. Antoniou, Jelmer M. Wolterink, and Jean-Paul P. M. de Vries. 2022. "A Statistical Shape Model of the Morphological Variation of the Infrarenal Abdominal Aortic Aneurysm Neck" Journal of Clinical Medicine 11, no. 6: 1687. https://doi.org/10.3390/jcm11061687
APA Stylevan Veldhuizen, W. A., Schuurmann, R. C. L., IJpma, F. F. A., Kropman, R. H. J., Antoniou, G. A., Wolterink, J. M., & de Vries, J.-P. P. M. (2022). A Statistical Shape Model of the Morphological Variation of the Infrarenal Abdominal Aortic Aneurysm Neck. Journal of Clinical Medicine, 11(6), 1687. https://doi.org/10.3390/jcm11061687






