Exploring the Developmental Potential of Human Germinal Vesicle Oocytes Aiming at Fertility Preservation: Can We Increase the Yields of Competent Oocytes through IVM Combined with Vitrification?
Abstract
:1. Introduction
2. Materials and Methods
2.1. IVM Culture Medium Selection and Fresh vs. Vitrified Stepwise-Randomized Study Design
2.1.1. IVM Culture Medium Selection
2.1.2. Fresh vs. Vitrified Comparison
2.2. GVs and Spermatozoa Resources
2.3. Oocyte Collection and Denudation
2.4. rIVM of GVs
2.5. Oocyte Vitrification and Warming
2.6. Spermatozoa Thawing
2.7. ICSI and Embryo Development Observation
2.8. Statistical Analysis
3. Results
3.1. IVM Culture Medium Selection
3.2. Fresh vs. Vitrified Comparison
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Wallberg, K.A.; Oktay, K. Options on fertility preservation in female cancer patients. Cancer Treat. Rev. 2012, 38, 354–361. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.A.; Coello, A.; Domingo, J.; Pellicer, A.; Remohí, J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil. Steril. 2016, 105, 755–764.e758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The ESHRE Guideline Group on Female Fertility Preservation; A Anderson, R.; Amant, F.; Braat, D.; D’Angelo, A.; Lopes, S.M.C.D.S.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Medicine, P.C.o.t.A.S.f.R. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef]
- Jie, H.; Zhao, M.; Alqawasmeh, O.A.M.; Chan, C.P.S.; Lee, T.L.; Li, T.; Chan, D.Y.L. In vitro rescue immature oocytes—A literature review. Hum. Fertil. 2021, 1–20. [Google Scholar] [CrossRef]
- Halvaei, I.; Ali Khalili, M.; Razi, M.H.; Nottola, S.A. The effect of immature oocytes quantity on the rates of oocytes maturity and morphology, fertilization, and embryo development in ICSI cycles. J. Assist. Reprod. Genet. 2012, 29, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Oktay, K.; Buyuk, E.; Rodriguez-Wallberg, K.A.; Sahin, G. In vitro maturation improves oocyte or embryo cryopreservation outcome in breast cancer patients undergoing ovarian stimulation for fertility preservation. Reprod. BioMedicine Online 2010, 20, 634–638. [Google Scholar] [CrossRef] [Green Version]
- Son, W.-Y.; Henderson, S.; Cohen, Y.; Dahan, M.; Buckett, W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Vuong, L.N.; Le, A.H.; Ho, V.N.A.; Pham, T.D.; Sanchez, F.; Romero, S.; De Vos, M.; Ho, T.M.; Gilchrist, R.B.; Smitz, J. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2020, 37, 347–357. [Google Scholar] [CrossRef] [Green Version]
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the development and future of oocyte IVM in clinical practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Wallberg, K.A.; Marklund, A.; Lundberg, F.; Wikander, I.; Milenkovic, M.; Anastacio, A.; Sergouniotis, F.; Wånggren, K.; Ekengren, J.; Lind, T.; et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non-oncologic indications in Sweden-Trends in patients’ choices and benefit of the chosen methods after long-term follow up. Acta Obstet. Gynecol. Scand. 2019, 98, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-X.; Chian, R.-C. Fertility Preservation with Immature and in Vitro Matured Oocytes. Semin. Reprod. Med. 2009, 27, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeck, L.L.; Wortham, J.W., Jr.; Witmyer, J.; Sandow, B.A.; Acosta, A.A.; Garcia, J.E.; Jones, G.S.; Jones, H.W., Jr. Maturation and fertilization of morphologically immature human oocytes in a program of in vitro fertilization. Fertil. Steril. 1983, 39, 594–602. [Google Scholar] [CrossRef]
- Tucker, M.J.; Wright, G.; Morton, P.C.; Massey, J.B. Birth after cryopreservation of immature oocytes with subsequent in vitro maturation. Fertil. Steril. 1998, 70, 578–579. [Google Scholar] [CrossRef]
- Magli, M.C.; Jones, G.M.; Lundin, K.; van den Abbeel, E. Atlas of human embryology: From oocytes to preimplantation embryos. Preface. Hum. Reprod. 2012, 27 (Suppl S1), i1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, S.C.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample Size Calculations in Clinical Research, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2008; p. 89. [Google Scholar]
- Martin-Palomino Olid, N.; García, D.; Rodríguez, A.; Vassena, R. Could fertility clinics offer a sizable improvement of live birth rates by maturing post-GVBD oocytes in vitro? J. Assist. Reprod. Genet. 2019, 36, 1927–1934. [Google Scholar] [CrossRef]
- Peinado, I.; Moya, I.; Sáez-Espinosa, P.; Barrera, M.; García-Valverde, L.; Francés, R.; Torres, P.; Gómez-Torres, M.J. Impact of Maturation and Vitrification Time of Human GV Oocytes on the Metaphase Plate Configuration. Int. J. Mol. Sci. 2021, 22, 1125. [Google Scholar] [CrossRef]
- Kasapi, E.; Asimakopoulos, B.; Chatzimeletiou, K.; Petousis, S.; Panagiotidis, Y.; Prapas, N.; Nikolettos, N. Vitrification of Human Germinal Vesicle Oocytes: Before or after In Vitro Maturation? Int. J. Fertil. Steril. 2017, 11, 85–92. [Google Scholar] [CrossRef]
- Segovia, Y.; Victory, N.; Peinado, I.; García-Valverde, L.M.; García, M.; Aizpurua, J.; Monzó, A.; Gómez-Torres, M.J. Ultrastructural characteristics of human oocytes vitrified before and after in vitro maturation. J. Reprod. Dev. 2017, 63, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.; Paynter, S.J.; Fuller, B.J.; Shaw, R.W. Differential effects of cryopreservation on nuclear or cytoplasmic maturation in vitro in immature mouse oocytes from stimulated ovaries. Hum. Reprod. 1998, 13, 971–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siristatidis, C.; Sergentanis, T.N.; Vogiatzi, P.; Kanavidis, P.; Chrelias, C.; Papantoniou, N.; Psaltopoulou, T. In Vitro Maturation in Women with vs. without Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0134696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalom-Paz, E.; Almog, B.; Shehata, F.; Huang, J.; Holzer, H.; Chian, R.C.; Son, W.Y.; Tan, S.L. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod. Biomed. Online 2010, 21, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, N. Fertility preservation in female cancer patients: An overview. J. Hum. Reprod. Sci. 2015, 8, 3–13. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Z.; Han, D.; Cao, Y.; Zhou, P.; Wei, Z.; Lv, M.; Chen, D. Gene expression profiling of human blastocysts from in vivo and ‘rescue IVM’ with or without melatonin treatment. Mol. Med. Rep. 2017, 16, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, N.; Haaf, T. Epigenetic disturbances in in vitro cultured gametes and embryos: Implications for human assisted reproduction. Fertil. Steril. 2013, 99, 632–641. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, P.; Yan, Z.; Yang, M.; Huo, Y.; Nie, Y.; Zhu, X.; Qiao, J.; Yan, L. Single-cell multiomics sequencing reveals the functional regulatory landscape of early embryos. Nat. Commun. 2021, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
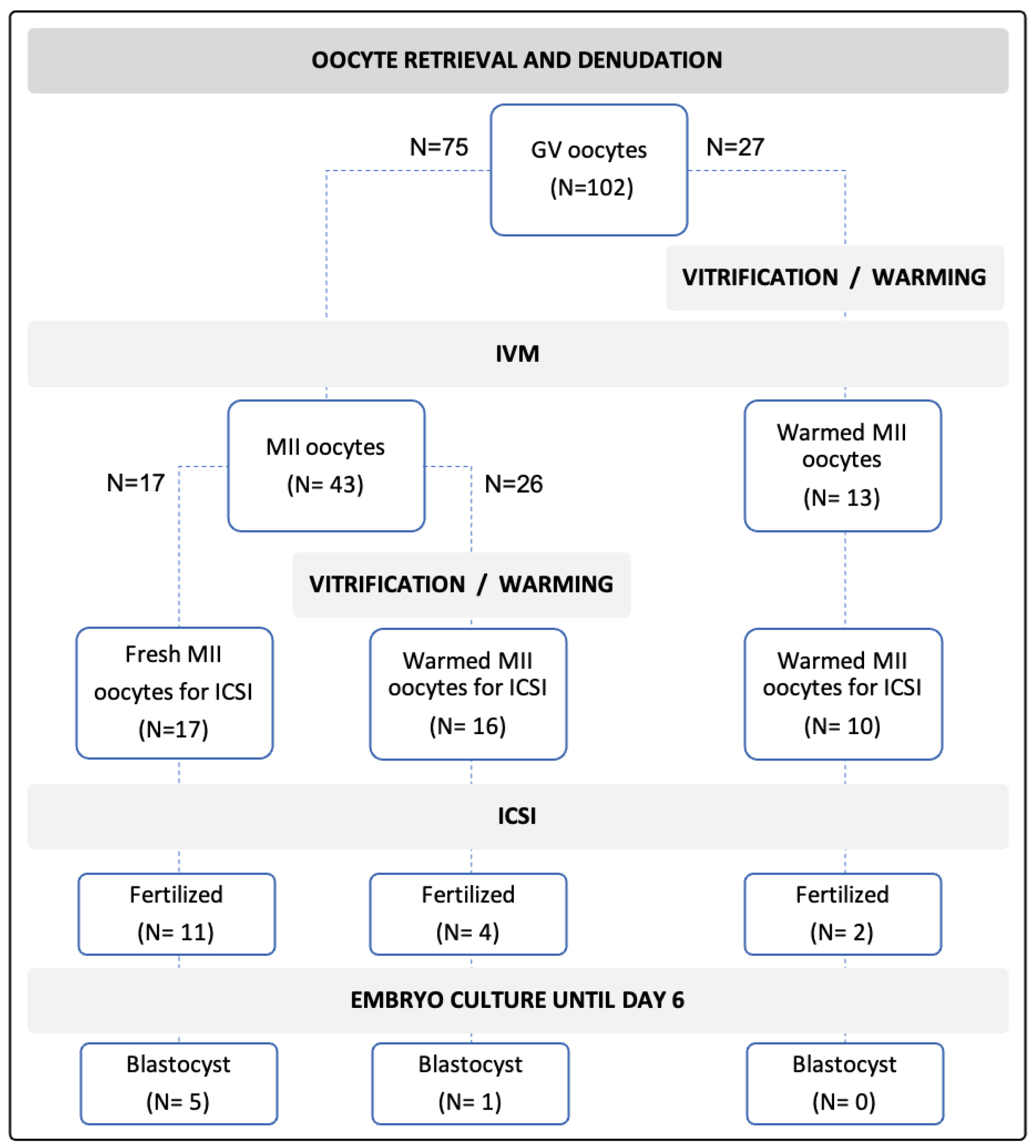
| Outcomes | Control: Fresh rIVM-Fresh ICSI | Fresh rIVM- V/W-ICSI | V/W- rIVM-ICSI |
|---|---|---|---|
| Maturity rates | 43/75 (57.3%) | 13/27 (48.1%) | |
| Survival after V/W | - | 16/26 (61.5%) | NA |
| Fertilized/injection | 11/17 (64.7%) | 4/16 (25.0%) * | 2/10 (20.0%) * |
| Developed blastocysts/fertilized oocytes | 5/11 (45.4%) | 1/4 (25.0%) | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Phoon, J.; Barbunopulos, L.; Sheikhi, M.; Palomares, A.R.; Rodriguez-Wallberg, K.A. Exploring the Developmental Potential of Human Germinal Vesicle Oocytes Aiming at Fertility Preservation: Can We Increase the Yields of Competent Oocytes through IVM Combined with Vitrification? J. Clin. Med. 2022, 11, 1703. https://doi.org/10.3390/jcm11061703
Hao X, Phoon J, Barbunopulos L, Sheikhi M, Palomares AR, Rodriguez-Wallberg KA. Exploring the Developmental Potential of Human Germinal Vesicle Oocytes Aiming at Fertility Preservation: Can We Increase the Yields of Competent Oocytes through IVM Combined with Vitrification? Journal of Clinical Medicine. 2022; 11(6):1703. https://doi.org/10.3390/jcm11061703
Chicago/Turabian StyleHao, Xia, Jessie Phoon, Lina Barbunopulos, Mona Sheikhi, Arturo Reyes Palomares, and Kenny A. Rodriguez-Wallberg. 2022. "Exploring the Developmental Potential of Human Germinal Vesicle Oocytes Aiming at Fertility Preservation: Can We Increase the Yields of Competent Oocytes through IVM Combined with Vitrification?" Journal of Clinical Medicine 11, no. 6: 1703. https://doi.org/10.3390/jcm11061703
APA StyleHao, X., Phoon, J., Barbunopulos, L., Sheikhi, M., Palomares, A. R., & Rodriguez-Wallberg, K. A. (2022). Exploring the Developmental Potential of Human Germinal Vesicle Oocytes Aiming at Fertility Preservation: Can We Increase the Yields of Competent Oocytes through IVM Combined with Vitrification? Journal of Clinical Medicine, 11(6), 1703. https://doi.org/10.3390/jcm11061703






