Clinical Characteristics and Multimodal Imaging Findings of Central Serous Chorioretinopathy in Women versus Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Study Patients
2.4. Study Protocol
2.5. Image Analyzes
2.6. Statistical Analysis
3. Results
3.1. Patients’ Demographics and Clinical Form of CSCR
3.2. Clinical and Imaging Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central Serous Chorioretinopathy: Recent Findings and New Physiopathology Hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitzmann, A.S.; Pulido, J.S.; Diehl, N.N.; Hodge, D.O.; Burke, J.P. The Incidence of Central Serous Chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology 2008, 115, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Quillen, D.A.; Gass, J.D.M.; Brod, R.D.; Gardner, T.W.; Blankenship, G.W.; Gottlieb, J.L. Central Serous Chorioretinopathy in Women. Ophthalmology 1996, 103, 72–79. [Google Scholar] [CrossRef]
- Tanaka, C.; Iwahashi, C.; Komuku, Y.; Hozumi, K.; Mitarai, K.; Gomi, F. Clinical Characteristics of Central Serous Chorioretinopathy in Patients by Age. Jpn. J. Ophthalmol. 2021, 65, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Recchia, C.A.; Yannuzzi, L.A.; Negrão, S.; Spaide, R.F.; Freund, K.B.; Rodriguez-Coleman, H.; Lenharo, M.; Iida, T. Corticosteroids and Central Serous Chorioretinopathy. Ophthalmology 2002, 109, 1834–1837. [Google Scholar] [CrossRef]
- Araki, T.; Ishikawa, H.; Iwahashi, C.; Niki, M.; Mitamura, Y.; Sugimoto, M.; Kondo, M.; Kinoshita, T.; Nishi, T.; Ueda, T.; et al. Central Serous Chorioretinopathy with and without Steroids: A Multicenter Survey. PLoS ONE 2019, 14, e0213110. [Google Scholar] [CrossRef] [Green Version]
- Kaye, R.; Chandra, S.; Sheth, J.; Boon, C.J.F.; Sivaprasad, S.; Lotery, A. Central Serous Chorioretinopathy: An Update on Risk Factors, Pathophysiology and Imaging Modalities. Prog. Retin. Eye Res. 2020, 79, 100865. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Huang, L.-Y.; Liao, W.-L.; Chou, P. Association between Central Serous Chorioretinopathy and Risk of Depression: A Population-Based Cohort Study. J. Ophthalmol. 2019, 2019, 2749296. [Google Scholar] [CrossRef]
- Liu, P.-K.; Chang, Y.-C.; Tai, M.-H.; Tsai, R.-K.; Chong, I.-W.; Wu, K.-Y.; Wu, W.-C.; Hsu, C.-Y.; Tsai, M.-J. The Association Between Central Serous Chorioretinopathy and Sleep Apnea: A Nationwide Population-Based Study. Retina 2020, 40, 2034–2044. [Google Scholar] [CrossRef]
- Bousquet, E.; Dhundass, M.; Lehmann, M.; Rothschild, P.-R.; Bayon, V.; Leger, D.; Bergin, C.; Dirani, A.; Beydoun, T.; Behar-Cohen, F. Shift Work: A Risk Factor for Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2016, 165, 23–28. [Google Scholar] [CrossRef]
- Mateo-Montoya, A.; Mauget-Faÿse, M. Helicobacter Pylori as a Risk Factor for Central Serous Chorioretinopathy: Literature Review. World J. Gastrointest Pathophysiol. 2014, 5, 355–358. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Gazouli, M.; Chatzistefanou, K.; Bakouli, A.; Moschos, M.M. The Genetic Background of Central Serous Chorioretinopathy: A Review on Central Serous Chorioretinopathy Genes. J. Genom. 2021, 9, 10–19. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid Disease. Eye 2018, 33, 14–33. [Google Scholar] [CrossRef] [Green Version]
- Castro-Navarro, V.; Behar-Cohen, F.; Chang, W.; Joussen, A.M.; Lai, T.Y.Y.; Navarro, R.; Pearce, I.; Yanagi, Y.; Okada, A.A. Pachychoroid: Current Concepts on Clinical Features and Pathogenesis. Graefes. Arch. Clin. Exp. Ophthalmol. 2020, 259, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Gemmy Cheung, C.M.; Matsumoto, H.; Kishi, S.; Boon, C.J.F.; van Dijk, E.H.C.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous Overload Choroidopathy: A Hypothetical Framework for Central Serous Chorioretinopathy and Allied Disorders. Prog. Retin. Eye Res. 2021, 86, 100973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Célérier, I.; Bousquet, E.; Jeanny, J.-C.; Jonet, L.; Savoldelli, M.; Offret, O.; Curan, A.; Farman, N.; Jaisser, F.; et al. Mineralocorticoid Receptor Is Involved in Rat and Human Ocular Chorioretinopathy. J. Clin. Investig. 2012, 122, 2672–2679. [Google Scholar] [CrossRef]
- Bousquet, E.; Zhao, M.; Daruich, A.; Behar-Cohen, F. Mineralocorticoid Antagonists in the Treatment of Central Serous Chorioetinopathy: Review of the Pre-Clinical and Clinical Evidence. Exp. Eye Res. 2019, 187, 107754. [Google Scholar] [CrossRef]
- Baker, M.E.; Katsu, Y. Progesterone: An Enigmatic Ligand for the Mineralocorticoid Receptor. Biochem. Pharmacol. 2020, 177, 113976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, S.L.; Kim, J.E.; Pollack, J.S.; Merrill, P.T. Clinical Characteristics of Central Serous Chorioretinopathy in Women. Ophthalmology 2002, 109, 262–266. [Google Scholar] [CrossRef]
- Hanumunthadu, D.; Van Dijk, E.H.C.; Gangakhedkar, S.; Goud, A.; Cheung, C.M.G.; Cherfan, D.; Sarvaiya, C.; Banker, A.; Meyerle, C.; Boon, C.J.; et al. Gender Variation in Central Serous Chorioretinopathy. Eye 2018, 32, 1703–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, E.; Bonnin, S.; Mrejen, S.; Krivosic, V.; Tadayoni, R.; Gaudric, A. Optical Coherence Tomography Angiography of Flat Irregular Pigment Epithelium Detachment in Chronic Central Serous Chorioretinopathy. Retina 2017, 38, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Zakir, S.M.; Shukla, M.; Simi, Z.-U.-R.; Ahmad, J.; Sajid, M. Serum Cortisol and Testosterone Levels in Idiopathic Central Serous Chorioretinopathy. Indian J. Ophthalmol. 2009, 57, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Tufan, H.A.; Gencer, B.; Comez, A.T. Serum Cortisol and Testosterone Levels in Chronic Central Serous Chorioretinopathy. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Çiloğlu, E.; Unal, F.; Dogan, N.C. The Relationship between the Central Serous Chorioretinopathy, Choroidal Thickness, and Serum Hormone Levels. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 1111–1116. [Google Scholar] [CrossRef]
- Luchak, A.; Solomon, L.A.; Kanagalingam, T.; Vijeyakumaran, M.; Rowe, B.H.; Cameron, L. Comparative Efficacy of Glucocorticoid Receptor Agonists on Th2 Cell Function and Attenuation by Progesterone. BMC Immunol. 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Barrett Mueller, K.; Lu, Q.; Mohammad, N.N.; Luu, V.; McCurley, A.; Williams, G.H.; Adler, G.K.; Karas, R.H.; Jaffe, I.Z. Estrogen Receptor Inhibits Mineralocorticoid Receptor Transcriptional Regulatory Function. Endocrinology 2014, 155, 4461–4472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, E.; Provost, J.; Zola, M.; Spaide, R.F.; Mehanna, C.; Behar-Cohen, F. Mid-Phase Hyperfluorescent Plaques Seen on Indocyanine Green Angiography in Patients with Central Serous Chorioretinopathy. J. Clin. Med. 2021, 10, 4525. [Google Scholar] [CrossRef]
- Shiragami, C.; Takasago, Y.; Osaka, R.; Kobayashi, M.; Ono, A.; Yamashita, A.; Hirooka, K. Clinical Features of Central Serous Chorioretinopathy with Type 1 Choroidal Neovascularization. Am. J. Ophthalmol. 2018, 193, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, J.; Schworm, B.; Priglinger, S.G. The Pachychoroid Disease Spectrum-and the Need for a Uniform Classification System. Ophthalmol. Retina 2019, 3, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
| Women (n = 75 Patients, 86 Eyes) | Men (n = 75 Patients, 98 Eyes) | p Value | |
|---|---|---|---|
| Follow-up, mean ± SD, months | 33.7 ± 25.4 | 32.7 ± 30.2 | 0.48 * |
| Age, mean ± SD, years | 52.2 ± 11.6 | 45.7 ± 8.9 | <0.001 * |
| Pregnancy, n (%) | 4 (5.3%) | ||
| Menopause, n (%) | 43 (57.3%) | ||
| Corticosteroid intake, n (%) | 42 (56%) | 30 (40%) | 0.05 † |
| Bilateral CSCR, n (%) | 12 (16%) | 26 (34.7%) | 0.009† |
| Acute/recurrent CSCR, n (%) | 24 (27.9%) | 26 (26.5%) | 0.83 † |
| Complex (persistent/chronic) CSCR, n (%) | 62 (72.1%) | 72 (73.5%) |
| Women (n = 75 Patients, 86 Eyes) | Men (n = 75 Patients, 98 Eyes) | p Value | |
|---|---|---|---|
| Best-Corrected Visual Acuity at baseline, logMAR, mean ± SD (Snellen) | 0.21 ± 0.24 (20/32) | 0.22 ± 0.3 (20/32) | 0.42 * |
| Spherical equivalent ** mean ± SD | 0.6 ± 1.8 | 0.3 ± 1.2 | 0.04 * |
| OCT findings | |||
| Choroidal thickness (µm), mean ± SD | 432.4 ± 104.2 | 473.8 ± 83.7 | 0.008 * |
| Pigment epithelium detachment, n (%) | |||
| At least one dome-shaped PED | 19 (22.1%) | 25 (25.5%) | 0.59 † |
| At least one flat irregular PED | 59 (68.6%) | 55 (56.1%) | 0.08 † |
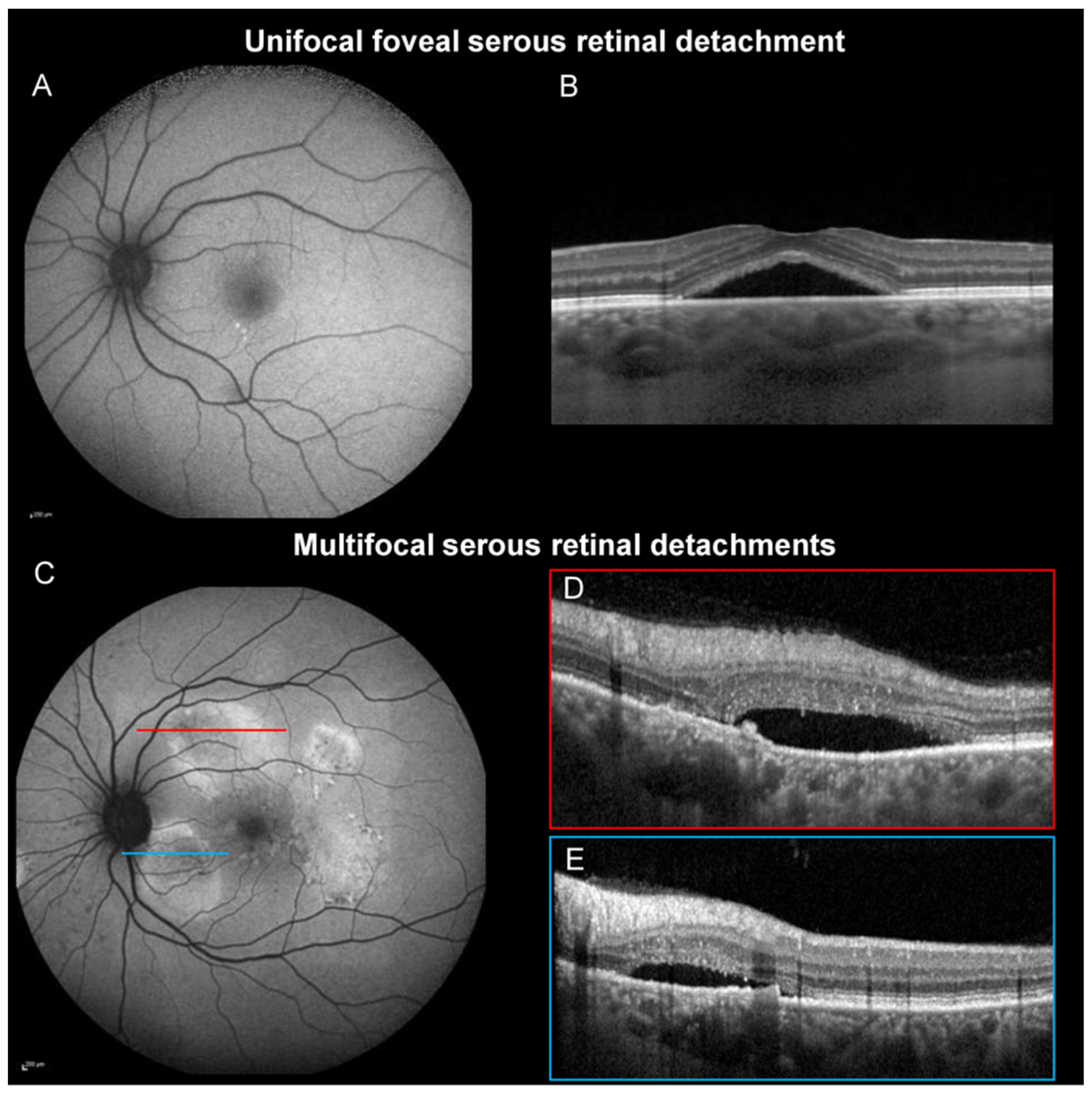
| Unifocal foveal SRD, n (%) | 63 (73.3%) | 46 (46.9%) | <0.001† |
| Multifocal SRDs, n (%) | 16 (18.6%) | 43 (43.9%) | <0.001† |
| Autofluorescence findings | |||
| Multifocal hyper-/hypo-autofluorescent area showing RPE damage, n (%) | 36 (41.9%) | 60 (61.2%) | 0.009† |
| Gravitational tracks, n (%) | 14 (16.3%) | 29 (29.6%) | 0.03† |
| Fluorescein angiography | |||
| Focal leakage, n (%) | 42 (56.8%) | 54 (57.5%) | 0.9 † |
| Indocyanine green angiography ‡ | |||
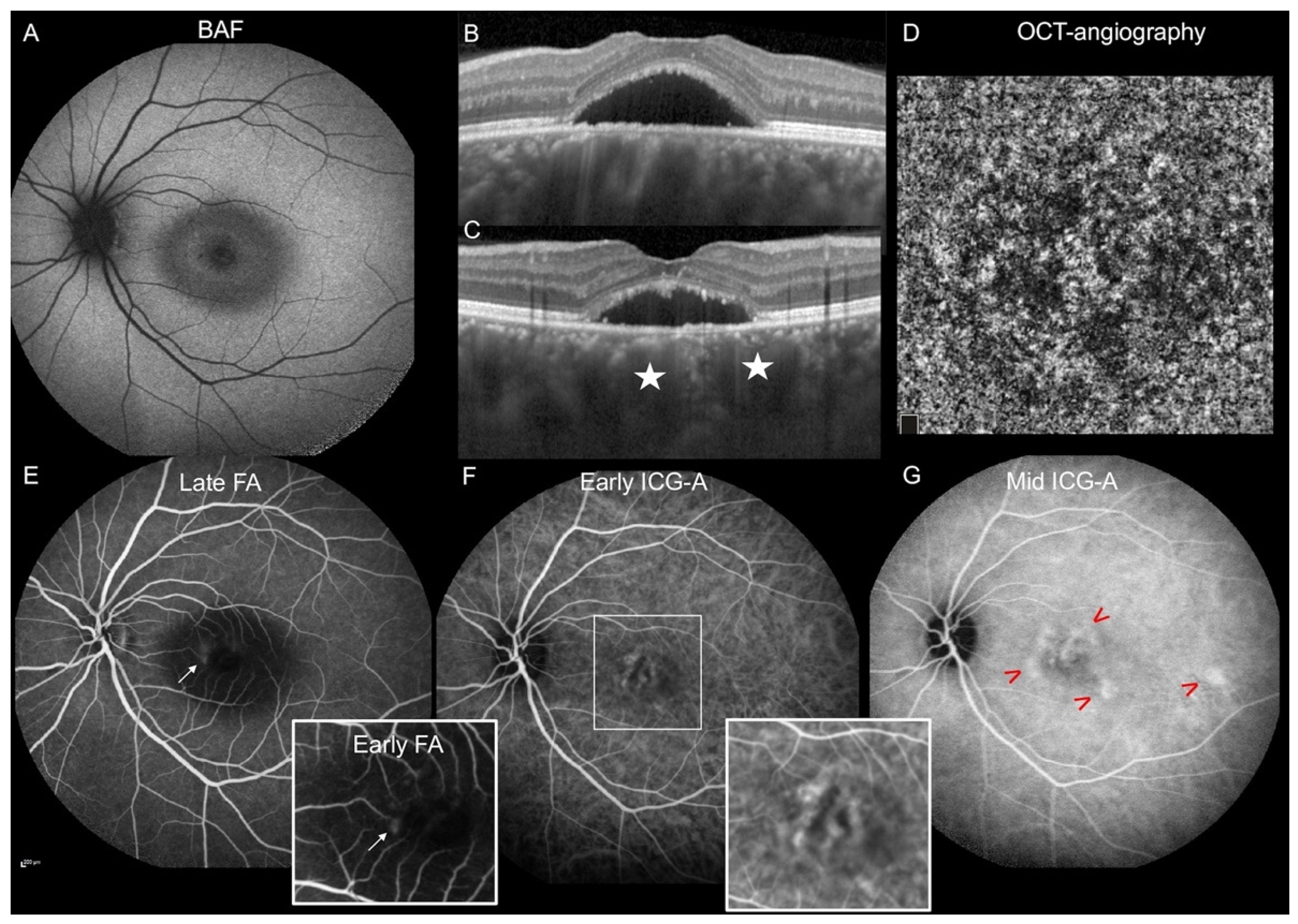
| Hyperfluorescent plaques during the mid-phase, n (%) | 36 (48%) | 65 (72.2%) | 0.001† |
| OCT-angiography *** | |||
| Type 1 macular neovascularization, n (%) | 23/64 (35.9%) | 8/60 (13.3%) | 0.004† |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousquet, E.; Torres-Villaros, H.; Provost, J.; Elalouf, M.; Gigon, A.; Mantel, I.; Timsit, A.; Behar-Cohen, F. Clinical Characteristics and Multimodal Imaging Findings of Central Serous Chorioretinopathy in Women versus Men. J. Clin. Med. 2022, 11, 1706. https://doi.org/10.3390/jcm11061706
Bousquet E, Torres-Villaros H, Provost J, Elalouf M, Gigon A, Mantel I, Timsit A, Behar-Cohen F. Clinical Characteristics and Multimodal Imaging Findings of Central Serous Chorioretinopathy in Women versus Men. Journal of Clinical Medicine. 2022; 11(6):1706. https://doi.org/10.3390/jcm11061706
Chicago/Turabian StyleBousquet, Elodie, Héloïse Torres-Villaros, Julien Provost, Martine Elalouf, Anthony Gigon, Irmela Mantel, Aurélie Timsit, and Francine Behar-Cohen. 2022. "Clinical Characteristics and Multimodal Imaging Findings of Central Serous Chorioretinopathy in Women versus Men" Journal of Clinical Medicine 11, no. 6: 1706. https://doi.org/10.3390/jcm11061706







