Real-World Journey of Unresectable Stage III NSCLC Patients: Current Dilemmas for Disease Staging and Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
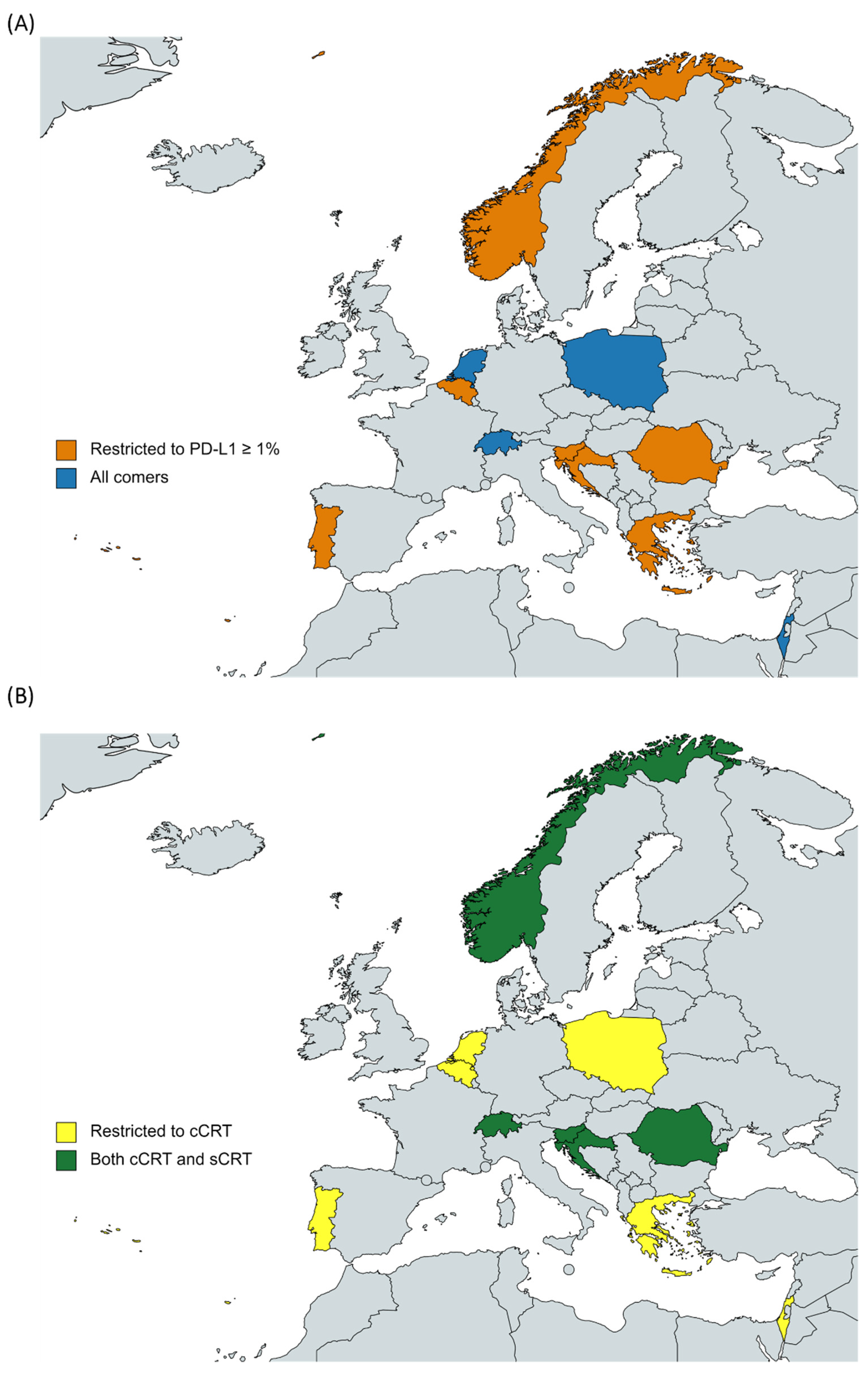
4. Discussion
- Patients should be treated within a specialized center with sufficient facilities and resources for diagnosis and disease management, including: imaging and nuclear medicine, pathology and molecular biology, medical and radiation oncology, pulmonology and thoracic surgery.
- Treatment decisions should be reached after comprehensive, in-depth discussion within this multidisciplinary team; the treatment plan must be shared with the patient.
- We suggest that each patient have a primary physician, preferably a medical oncologist, to act as a ‘reference’ for all clinical decisions and to be responsible for the patient’s treatment.
- We encourage the designation of a ‘team coordinator’ (e.g., a nurse) to be responsible for organizing all patients’ appointments and to monitor the patient periodically after treatment.
- The multidisciplinary team should be familiar with the local country’s regulations and reimbursement issues.
- During the treatment journey, we encourage professionals to discuss with experts from other disciplines the potential adverse side-effects of the treatment (e.g., pain, gastrointestinal toxicity etc.) and how to manage them. This could contribute to improving patients’ quality of life.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cheema, P.K.; Rothenstein, J.; Melosky, B.; Brade, A.; Hirsh, V. Perspectives on Treatment Advances for Stage III Locally Advanced Unresectable Non-Small-Cell Lung Cancer. Curr. Oncol. 2019, 26, 37–42. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Casal-Mouriño, A.; Ruano-Ravina, A.; Lorenzo-González, M.; Rodríguez-Martínez, Á.; Giraldo-Osorio, A.; Varela-Lema, L.; Pereiro-Brea, T.; Barros-Dios, J.M.; Valdés-Cuadrado, L.; Pérez-Ríos, M. Epidemiology of stage III lung cancer: Frequency, diagnostic characteristics, and survival. Transl. Lung Cancer Res. 2021, 10, 506–518. [Google Scholar] [CrossRef]
- Mielgo-Rubio, X.; Rojo, F.; Mezquita-Pérez, L.; Casas, F.; Wals, A.; Juan, M.; Aguado, C.; Garde-Noguera, J.; Vicente, D.; Couñago, F. Deep diving in the PACIFIC: Practical issues in stage III non-small cell lung cancer to avoid shipwreck. World J. Clin. Oncol. 2020, 11, 898–917. [Google Scholar] [CrossRef]
- Myall, N.J.; Das, M. Advances in the Treatment of Stage III Non–Small Cell Lung Cancer. Clin. Chest Med. 2020, 41, 211–222. [Google Scholar] [CrossRef]
- Park, K.; Vansteenkiste, J.; Lee, K.H.; Pentheroudakis, G.; Zhou, C.; Prabhash, K.; Seto, T.; Voon, P.; Tan, D.; Yang, J.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann. Oncol. 2020, 31, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995, 311, 899–909. [Google Scholar] [CrossRef]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy for non-small cell lung cancer. Cochrane Database Syst. Rev. 2000, 2, CD002139. [Google Scholar] [CrossRef]
- Aupérin, A.; Le Péchoux, C.; Pignon, J.P.; Koning, C.; Jeremic, B.; Clamon, G.; Einhorn, L.; Ball, D.; Trovo, M.G.; Groen, H.J.M.; et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann. Oncol. 2006, 17, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Arellano, E.A.; Díaz, V.D.; Rodríguez, J.J.C. Current status and future directions in unresectable stage III non-small cell lung cancer. J. Clin. Transl. Res. 2020, 6, 109–120. [Google Scholar]
- Baldini, E.; Tibaldi, C.; Paoli, C.D. Chemo-radiotherapy integration in unresectable locally advanced non-small-cell lung cancer: A review. Clin. Transl. Oncol. 2020, 22, 1681–1686. [Google Scholar] [CrossRef]
- Mielgo-Rubio, X.; Calvo, V.; Luna, J.; Remon, J.; Martín, M.; Berraondo, P.; Jarabo, J.R.; Higuera, O.; Conde, E.; De Castro, J.; et al. Immunotherapy Moves to the Early-Stage Setting in Non-Small Cell Lung Cancer: Emerging Evidence and the Role of Biomarkers. Cancers 2020, 12, 3459. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Alrifai, D.; McDonald, F.; Forster, M. Beyond chemoradiotherapy: Improving treatment outcomes for patients with stage III unresectable non-small-cell lung cancer through immuno-oncology and durvalumab (Imfinzi®▼, AstraZeneca UK Limited). Br. J. Cancer 2020, 123, 18–27. [Google Scholar] [CrossRef]
- Inoue, H.; Okamoto, I. Immune Checkpoint Inhibitors for the Treatment of Unresectable Stage III Non–Small Cell Lung Cancer: Emerging Mechanisms and Perspectives. Lung Cancer Targets Ther. 2020, 10, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC—An Update From the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC—Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.G.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes with Durvamulab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J. Clin. Oncol. 2021, 39, 8511. [Google Scholar] [CrossRef]
- Mir, N.A. Guideline Concordance with Durvalumab in Unresectable Stage III Non-Small Cell Lung Cancer: A Single Center Veterans Hospital Experience. Fed. Pract. 2020, 38, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Spitaleri, G.; Gyawali, B.; De Marinis, F. Immunotherapy in Non–Small-Cell Lung Cancer Patients With Performance Status 2: Clinical Decision Making With Scant Evidence. J. Clin. Oncol. 2019, 37, 1863–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musen, M.A.; Rohn, J.A.; Fagan, L.M.; Shortliffe, E.H. Knowledge engineering for a clinical trial advice system: Uncovering errors in protocol specification. Bull. Cancer 1987, 74, 291–296. [Google Scholar] [PubMed]
- Ross, J.; Tu, S.; Carini, S.; Sim, I. Analysis of Eligibility Criteria Complexity in Clinical Trials. Summit Transl. Bioinform. 2010, 2010, 46–50. [Google Scholar] [PubMed]
- Sharma, N.S. Patient centric approach for clinical trials: Current trend and new opportunities. Perspect. Clin. Res. 2015, 6, 134–138. [Google Scholar] [CrossRef]
- McDonald, F.; Mornex, F.; Garassino, M.C.; Filippi, A.R.; Christoph, D.; Haakensen, V.D.; Agbarya, A.; Van den Heuvel, M.; Vercauter, P.; Chouaid, C.; et al. PACIFIC-R: Real-world characteristics of unresectable Stage III NSCLC patients treated with Durvamulab after chemoradiotherapy. J. Thorac. Oncol. 2021, 16, S738–S739. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Agarwal, R.; Dhooria, S.; Prasad, K.T.; Aggarwal, A.N. Role of EBUS TBNA in staging of lung cancer: A clinician’s perspective. J. Cytol. 2019, 36, 61–64. [Google Scholar] [CrossRef]
- Leiro-Fernández, V.; Mouronte-Roibás, C.; García-Rodríguez, E.; Botana-Rial, M.; Ramos-Hernández, C.; Torres-Durán, M.; Ruano-Raviña, A.; Fernández-Villar, A.; On behalf of the Lung Cancer Group at the Álvaro Cunqueiro Hospital in Vigo. Predicting delays in lung cancer diagnosis and staging. Thorac. Cancer 2019, 10, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubens, M.A.; Davies, M. NCCN Guidelines Updates: New Immunotherapy Strategies for Improving Outcomes in Non-Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 574–578. [Google Scholar]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Budjan, J. Whole-body MRI for lung cancer staging: A step in the right direction. Lancet Respir. Med. 2019, 7, 471–472. [Google Scholar] [CrossRef] [Green Version]
- Filippi, A.R.; Di Muzio, J.; Badellino, S.; Mantovani, C.; Ricardi, U. Locally-advanced non-small cell lung cancer: Shall immunotherapy be a new chance? J. Thorac. Dis. 2018, 10, S1461–S1467. [Google Scholar] [CrossRef]
- De Waele, M.; Hendriks, J.; Lauwers, P.; Hertoghs, M.; Carp, L.; Salgado, R.; Van Schil, P. Restaging the mediastinum in non-small cell lung cancer after induction therapy: Non-invasive versus invasive procedures. Acta Chir. Belg. 2011, 111, 161–164. [Google Scholar] [CrossRef]
- Frąk, M.; Krawczyk, P.; Kalinka, E.; Milanowski, J. Molecular and Clinical Premises for the Combination Therapy Consisting of Radiochemotherapy and Immunotherapy in Non-Small Cell Lung Cancer Patients. Cancers 2021, 13, 1222. [Google Scholar] [CrossRef]
- Prasad, R.N.; Williams, T.M. A narrative review of toxicity of chemoradiation and immunotherapy for unresectable, locally advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2020, 9, 2040–2050. [Google Scholar] [CrossRef]
- Shibata, Y.; Murakami, S. Safety evaluation of durvalumab for the treatment of non-small-cell lung cancer. Expert Opin. Drug Saf. 2020, 19, 653–659. [Google Scholar] [CrossRef]
- Han, J.; Tian, K.; Yang, J.; Gong, Y. Durvalumab vs placebo consolidation therapy after chemoradiotherapy in stage III non-small-cell lung cancer: An updated PACIFIC trial-based cost-effectiveness analysis. Lung Cancer 2020, 146, 42–49. [Google Scholar] [CrossRef]
- Armeni, P.; Borsoi, L.; Fornaro, G.; Jommi, C.; Grossi, F.; Costa, F. Cost-effectiveness and Net Monetary Benefit of Durvalumab Consolidation Therapy Versus No Consolidation Therapy After Chemoradiotherapy in Stage III Non–small Cell Lung Cancer in the Italian National Health Service. Clin. Ther. 2020, 42, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Cho, B.C.; Gray, J.E.; Paz-Ares, L.G.; Ozguroglu, M.; Villegas, A.E.; Daniel, D.B.; Vicente, D.; Murakami, S.; Hui, R.; et al. First subsequent treatment after discontinuation of Durvamulab in unresectable, stage III NSCLC patients from PACIFIC. J. Clin. Oncol. 2021, 37, 9054. [Google Scholar] [CrossRef]
- Metro, G.; Signorelli, D. Immune checkpoints inhibitors rechallenge in non-small-cell lung cancer: Different scenarios with different solutions? Lung Cancer Manag. 2019, 8, LMT18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inno, A.; Roviello, G.; Ghidini, A.; Luciani, A.; Catalano, M.; Gori, S.; Petrelli, F. Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit. Rev. Oncol. 2021, 165, 103434. [Google Scholar] [CrossRef]
| Variable | Category | Total n (%) |
|---|---|---|
| Clinical staging | ||
| PET scan | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| When PET scan is performed | At diagnosis | 7 (63.6%) |
| Candidates for surgery or radical CRT | 4 (36.4%) | |
| Baseline brain MRI in all patients | Yes | 8 (72.7%) |
| No | 3 (27.3%) | |
| Pathological staging | ||
| EBUS | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| When EBUS is performed | At diagnosis | 6 (54.6%) |
| Mediastinal nodes verification | 5 (45.4%) | |
| Mediastinoscopy | Yes | 7 (63.6%) |
| No | 4 (36.4%) | |
| PD-L1 evaluation | Yes | 9 (81.8%) |
| No | 2 (18.2%) | |
| EGFR/ALK evaluation | Yes | 7 (63.6%) |
| No | 4 (36.4%) |
| Variable | Category | Total n (%) |
|---|---|---|
| Multidisciplinary team treatment decision | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| Difference between unresectable IIIA or IIIB | Yes | 4 (36.4%) |
| No | 7 (63.6%) | |
| Neoadjuvant ChT | Yes | 8 (72.7%) |
| No | 3 (27.3%) | |
| When neoadjuvant ChT is used before surgery * (a) | Potentially operable cases | 5 (62.5%) |
| Bulky mediastinal mass | 2 (25.0%) | |
| Tumor size | 1 (12.5%) | |
| Clinical trials | 1 (12.5%) | |
| Definition of cCRT | Simultaneous use of ChT and RT at D1 of cycle 1 | 3 (27.3%) |
| At least 2 cycles of ChT administered during the RT, where induction chemotherapy is allowed | 6 (54.5%) | |
| At least 1cycle of ChT administered during the RT, where induction chemotherapy is allowed | 2 (18.2%) | |
| Reason for using induction ChT before CRT * | RT delay | 5 (71.4%) |
| PS | 1 (14.3%) | |
| Tumor size | 1 (14.3%) | |
| Reasons for not receiving CRT * (b) | PS | 9 (81.8%) |
| Comorbidities | 5 (45.5%) | |
| Access | 3 (27.3%) | |
| Tumor size | 1 (9.1%) | |
| Age | 2 (18.2%) | |
| Reasons for not receiving CRT as scheduled * | Adverse events | 6 (75.0%) |
| PS | 2 (25.0%) | |
| Is patients’ age a qualifying factor for cCRT? | Yes | 4 (36.4%) |
| No | 5 (45.5%) | |
| Sometimes | 2 (18.2%) | |
| Is patients’ PS a qualifying factor for cCRT? | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| Recommend CRT for patients with stage IIIC | Yes | 4 (36.4%) |
| Whenever possible | 7 (63.6%) | |
| Recommend CRT in molecular aberrations | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| RT delay hinders cCRT qualification | Yes | 6 (54.6%) |
| No | 5 (45.4%) | |
| Use RT—IMRT | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| Use RT—3D-CRT | Yes | 5 (45.4%) |
| No | 6 (54.6%) | |
| Use platinum-based ChT protocols | Yes | 11 (100.0%) |
| No | 0 (0.0%) | |
| Use etoposide-based ChT protocols | Yes | 9 (81.8%) |
| No | 2(18.2%) | |
| AEs during and after cCRT * | Pneumonitis | 8 (72.7%) |
| Hematological toxicity | 4 (36.4%) | |
| Esophagitis | 4 (36.4%) | |
| Is there an AEs risk management plan during cCRT? | Yes | 4 (36.4%) |
| No | 7 (63.6%) |
| Variable | Category | Total n (%) |
|---|---|---|
| Timing of first evaluation after RT completion | <1 month | 6 (54.5%) |
| 1–2 months | 3 (27.3%) | |
| 1–3 months | 1 (9.1%) | |
| 3 months | 1 (9.1%) | |
| Follow-up procedures after RT | Repeat PET | 1 (9.1%) |
| Follow up by CT | 8 (72.7%) | |
| Both PET and CT | 2 (18.2%) | |
| Discussion with multidisciplinary team after CRT | Always | 3 (27.3%) |
| >75% of patients | 3 (27.3%) | |
| <50% of patients | 2 (18.1%) | |
| Never | 3 (27.3%) | |
| Is surgery (after CRT) considered? | Yes | 1 (9.1%) |
| In case of downstaging | 3 (27.3%) | |
| No | 7 (63.6%) | |
| Factors influencing surgical decisions * | Response to CRT | 6 (85.7%) |
| Tumor size/invasion | 2 (28.6%) |
| Variables | Belgium | Croatia | Greece | Israel | Norway | Poland | Portugal | Romania | Slovenia | Switzerland | The Netherlands |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Durvamulab registered | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Durvamulab reimbursed | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reimbursement date | May 2020 | November 2020 | 2017 | January 2019 | October 2019 | January 2021 | October 2019 | January 2021 | August 2019 | 2018 | April 2019 |
| Durvamulab reimbursement/local approval | -- | -- | NHSC | NHSC | NHSC | Therapeutic Program | Pharmaceutic. Committee | Different authorities (a) | Prescribing physician | NHSC | Pharmaceutic. Committee |
| Optimal time to start Durvamulab after CRT | <6 weeks | -- | <6 weeks | <2 weeks | <2 weeks | <6 weeks | 4–6 weeks | <6 weeks | <2 weeks | <6 weeks | <6 weeks |
| Barriers to implement Durvamulab in practice | Eligibility criteria (b) | Evaluation response (c) | Eligibility criteria (b) | Evaluation response (c) | None | Eligibility criteria (b) | Price, Eligibility criteria (b) | Adverse events | None | None | None |
| Treatment with Durvamulab for up to 1 year | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Variables | Belgium | Croatia | Greece | Israel | Norway | Poland | Portugal | Romania | Slovenia | Switzerland | The Netherlands |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AEs risk management plan with Durvamulab | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes |
| Durvamulab main AEs: Pneumonitis | Yes | Yes | Yes | -- | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Durvamulab main AEs: Endocrine events | Yes | No | Yes | -- | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Durvamulab main AEs: Others | -- | -- | Skin-related | -- | -- | GI tract | -- | Colitis | -- | Skin-related | Skin-relatedColitis |
| Variables | Belgium | Croatia | Greece | Israel | Norway | Poland | Portugal | Romania | Slovenia | Switzerland | Netherlands |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment of choice in brain—oligoprogression | LAT Surgery | LAT | LAT | LAT | LAT Surgery | LAT Surgery | LAT Surgery | LAT Surgery | LAT Surgery | LAT | LAT |
| Treatment of choice if a patient progresses after the completion of 12 months treatment with Durvamulab (f) | ICI/ICI-based combination, if more than 6 months has passed from the treatment completion | Other | ICI/ICI-based combination, if more than 12 months passed from the treatment completion | As per 1st line treatment * independently of time from treatment completion | ICI/ICI-based combination, if more than 6 months has passed from the treatment completion | ICI/ICI-based combination, if more than 6 months has passed from the treatment completion | Other (a) | ICI/ICI-based combination, if more than 12 months passed from the treatment completion | As per 1st line treatment* independently of time from treatment completion | As per 1st line treatment * independently of time from treatment completion | Other (b) |
| Treatment of choice if a patient progresses during treatment with Durvamulab | As per 1st line treatment * independently of time from treatment completion | ICI would not be considered an option | ICI would not be considered an option | ICI/ICI-based combination, if more than 3 months has passed from the treatment completion | Other (c) | ICI would not be considered an option | Other (d) | ICI would not be considered an option | ICI would not be considered an option (d) | ICI would not be considered an option | ICI would not be considered an option |
| Time to define immune-sensitive disease (e) | Uncertain | 12 months | 12 months | 6 months | Uncertain | Uncertain | Uncertain | 12 months | Uncertain | 3 months | uncertain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbarya, A.; Shalata, W.; Addeo, A.; Charpidou, A.; Cuppens, K.; Brustugun, O.T.; Rajer, M.; Jakopovic, M.; Marinca, M.V.; Pluzanski, A.; et al. Real-World Journey of Unresectable Stage III NSCLC Patients: Current Dilemmas for Disease Staging and Treatment. J. Clin. Med. 2022, 11, 1738. https://doi.org/10.3390/jcm11061738
Agbarya A, Shalata W, Addeo A, Charpidou A, Cuppens K, Brustugun OT, Rajer M, Jakopovic M, Marinca MV, Pluzanski A, et al. Real-World Journey of Unresectable Stage III NSCLC Patients: Current Dilemmas for Disease Staging and Treatment. Journal of Clinical Medicine. 2022; 11(6):1738. https://doi.org/10.3390/jcm11061738
Chicago/Turabian StyleAgbarya, Abed, Walid Shalata, Alfredo Addeo, Andriani Charpidou, Kristof Cuppens, Odd Terje Brustugun, Mirjana Rajer, Marco Jakopovic, Mihai V. Marinca, Adam Pluzanski, and et al. 2022. "Real-World Journey of Unresectable Stage III NSCLC Patients: Current Dilemmas for Disease Staging and Treatment" Journal of Clinical Medicine 11, no. 6: 1738. https://doi.org/10.3390/jcm11061738
APA StyleAgbarya, A., Shalata, W., Addeo, A., Charpidou, A., Cuppens, K., Brustugun, O. T., Rajer, M., Jakopovic, M., Marinca, M. V., Pluzanski, A., Hiltermann, J., & Araújo, A. (2022). Real-World Journey of Unresectable Stage III NSCLC Patients: Current Dilemmas for Disease Staging and Treatment. Journal of Clinical Medicine, 11(6), 1738. https://doi.org/10.3390/jcm11061738








