Association between the Beighton Score and Stress Ultrasonographic Findings of the Anterior Talofibular Ligament in Healthy Young Women: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. The BS
2.3. Manual ADT
2.4. Stress US Evaluation of the ATFL
2.5. Statistical Analysis
3. Results
3.1. The Comparison of the ADT and US Findings between the Low- and High-BS Groups
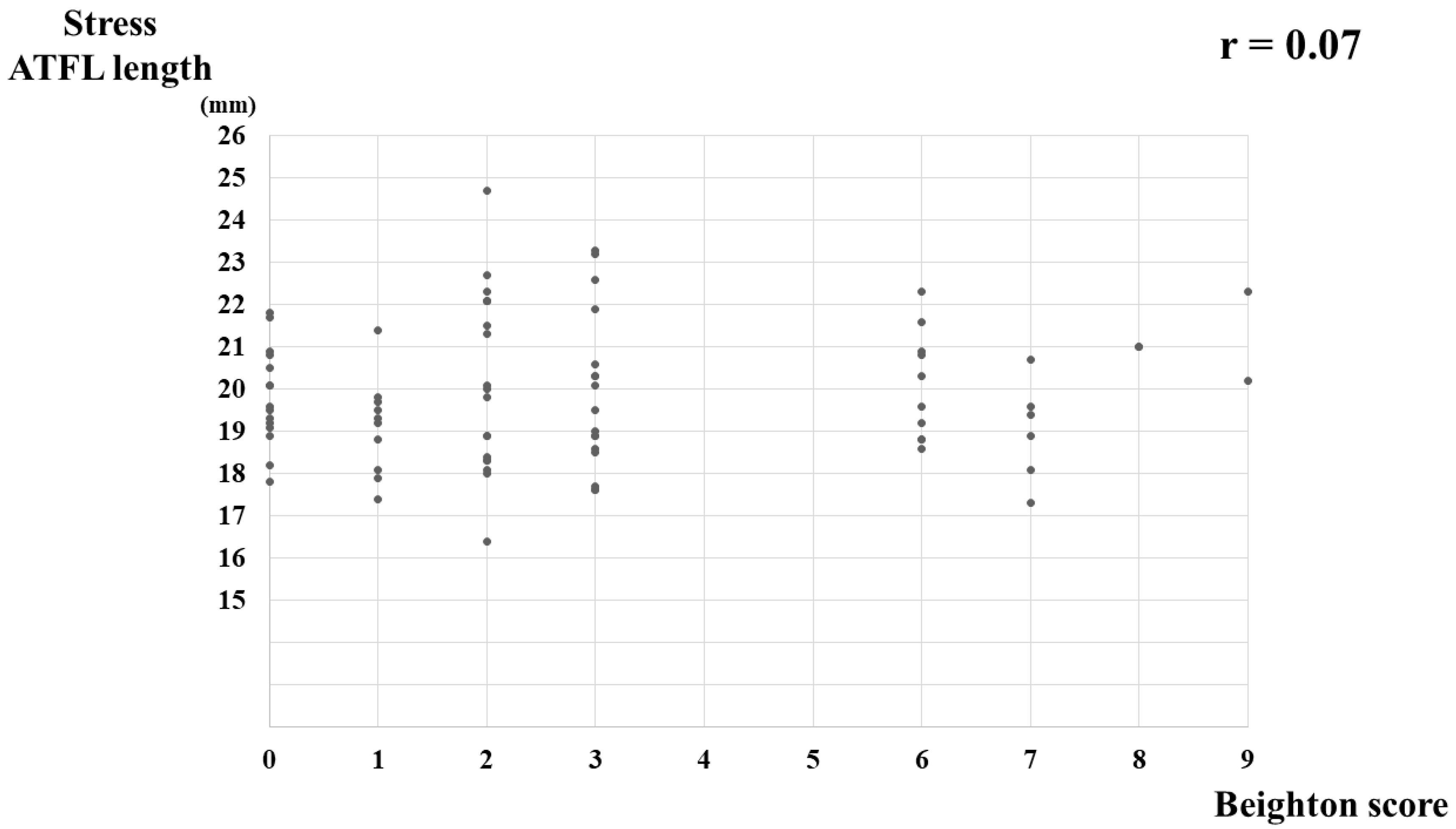
3.2. The Correlation between the BS and US Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remvig, L.; Jensen, D.V.; Ward, R.C. Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: Review of the literature. J. Rheumatol. 2007, 34, 804–809. [Google Scholar] [PubMed]
- Sacks, H.A.; Prabhakar, P.; Wessel, L.E.; Hettler, J.; Strickland, S.M.; Potter, H.G.; Fufa, D.T. Generalized Joint Laxity in Orthopaedic Patients: Clinical Manifestations, Radiographic Correlates, and Management. J. Bone Jt. Surg. Am. 2019, 101, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bulbena, A.; Duro, J.C.; Porta, M.; Faus, S.; Vallescar, R.; Martin-Santos, R. Clinical assessment of hypermobility of joints: Assembling criteria. J. Rheumatol. 1992, 19, 115–122. [Google Scholar] [PubMed]
- Beighton, P.; Horan, F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J. Bone Jt. Surg. Br. 1969, 51, 444–453. [Google Scholar] [CrossRef]
- Smits-Engelsman, B.; Klerks, M.; Kirby, A. Beighton score: A valid measure for generalized hypermobility in children. J. Pediatr. 2011, 158, 11–14. [Google Scholar] [CrossRef]
- Myer, G.D.; Ford, K.R.; Paterno, M.V.; Nick, T.G.; Hewett, T.E. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am. J. Sports Med. 2008, 36, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.R.; Lee, H.M.; Yoon, T.H.; Lee, H.M.; Chun, Y.M. Association Between Excessive Joint Laxity and a Wider Hill-Sachs Lesion in Anterior Shoulder Instability. Am. J. Sports Med. 2021, 49, 3981–3987. [Google Scholar] [CrossRef]
- Xu, H.X.; Lee, K.B. Modified Brostrom Procedure for Chronic Lateral Ankle Instability in Patients With Generalized Joint Laxity. Am. J. Sports Med. 2016, 44, 3152–3157. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, J.W.; Suh, J.W.; Shin, M.H.; Choi, W.J. Generalized Ligamentous Laxity Is an Independent Predictor of Poor Outcomes After the Modified Brostrom Procedure for Chronic Lateral Ankle Instability. Am. J. Sports Med. 2016, 44, 2975–2983. [Google Scholar] [CrossRef]
- Yeo, E.D.; Park, J.Y.; Kim, J.H.; Lee, Y.K. Comparison of outcomes in patients with generalized ligamentous laxity and without generalized laxity in the arthroscopic modified Broström operation for chronic lateral ankle instability. Foot Ankle Int. 2017, 38, 1318–1323. [Google Scholar] [CrossRef]
- Whitehead, N.A.; Mohammed, K.D.; Fulcher, M.L. Does the Beighton Score Correlate with Specific Measures of Shoulder Joint Laxity? Orthop. J. Sports Med. 2018, 6, 2325967118770633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.H.; Kang, C.; Kim, N.S.; Yi, J.W.; Lee, G.S.; Jang, M.G.; Kim, T.H. Evaluation of the uninjured anterior talofibular ligament by ultrasound for assessing generalized joint hypermobility. Foot Ankle Surg. 2021, 27, 256–262. [Google Scholar] [CrossRef]
- Yokoe, T.; Tajima, T.; Kawagoe, S.; Yamaguchi, N.; Morita, Y.; Chosa, E. The Ratio of Stress to Nonstress Anterior Talofibular Ligament Length on Ultrasonography: Normative Values. Orthop. J. Sports Med. 2021, 9, 23259671211056305. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Yang, Y.; Chen, S.; Cai, Y. Ultrasound examination for the diagnosis of chronic anterior talofibular ligament injury. Ultrasound examination for the diagnosis of chronic anterior talofibular ligament injury. Acta Radiol. 2012, 53, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, D.H.; Song, H.K.; Bang, J.Y.; Lee, K.T.; Park, Y.U. Value of stress ultrasound for the diagnosis of chronic ankle instability compared to manual anterior drawer test, stress radiography, magnetic resonance imaging, and arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1022–1028. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Ma, X.; Wang, X.; Huang, J.; Zhang, C. Imaging diagnosis for chronic lateral ankle ligament injury: A systemic review with meta-analysis. J. Orthop. Surg. Res. 2018, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Tanen, L.; Docherty, C.L.; Van Der Pol, B.; Simon, J.; Schrader, J. Prevalence of chronic ankle instability in high school and division I athletes. Foot Ankle Spec. 2014, 7, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gustavsen, G.; Royer, T.; Wikstrom, E.A.; Glutting, J.; Kaminski, T.W. Increased ligament thickness in previously sprained ankles as measured by musculoskeletal ultrasound. J. Athl. Train. 2015, 50, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridges, A.J.; Smith, E.; Reid, J. Joint hypermobility in adults referred to rheumatology clinics. Ann. Rheum. Dis. 1992, 51, 793–796. [Google Scholar] [CrossRef]
- Malek, S.; Reinhold, E.J.; Pearce, G.S. The Beighton Score as a measure of generalised joint hypermobility. Rheumatol. Int. 2021, 41, 1707–1716. [Google Scholar] [CrossRef]
- Brasseur, J.L.; Luzzati, A.; Lazennec, J.Y.; Guerin-Surville, H.; Roger, B.; Grenier, P. Ultrasono-anatomy of the ankle ligaments. Surg. Radiol. Anat. 1994, 16, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Pinto, F.; Côrte-Real, N.; Gomes, T.M.; Consciência, J.G.; Glazebrook, M.; Oliva, X.M. Varus talar tilt combined with an internal rotation pivot stress assesses the supination instability vector in lateral ankle ligaments’ injury—Cadaver study. Foot Ankle Surg. 2020, 26, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Ogawa, M.; Masuda, Y.; Yamazaki, T.; Ozeki, S. 3D-CT stress test for the assessment of CFL insufficiency. J. Orthop. Sci. 2021, 26, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.; Marcelis, S.; Jager, T.; Shahabpour, M.; Van Roy, P.; Weaver, J.; Jacobson, J.A. Sonography of the normal ankle: A target approach using skeletal reference points. AJR Am. J. Roentgenol. 2009, 192, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Noguchi, H.; Sato, J.; Ishii h Toyabe, S. Mediolateral coronal laxity does not correlate with knee range of motion after total knee arthroplasty. Arch. Orthop. Trauma Surg. 2019, 139, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Pacey, V.; Nicholson, L.L.; Adams, R.D.; Munn, J.; Munns, C.F. Generalized joint hypermobility and risk of lower limb joint injury during sport: A systematic review with meta-analysis. Am. J. Sports Med. 2010, 38, 1487–1497. [Google Scholar] [CrossRef]
- Sueyoshi, T.; Emoto, G.; Yuasa, T. Generalized Joint Laxity and Ligament Injuries in High School-Aged Female Volleyball Players in Japan. Orthop. J. Sports Med. 2016, 4, 2325967116667690. [Google Scholar] [CrossRef] [Green Version]
- Michels, F.; Pereira, H.; Calder, J.; Matricali, G.; Glazebrook, M.; Guillo, S.; Karlsson, J.; Acevedo, J.; Batista, J.; Bauer, T.; et al. Searching for consensus in the approach to patients with chronic lateral ankle instability: Ask the expert. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2095–2102. [Google Scholar] [CrossRef]
- de Asla, R.J.; Kozanek, M.; Wan, L.; Rubash, H.E.; Li, G. Function of anterior talofibular and calcaneofibular ligaments during in-vivo motion of the ankle joint complex. J. Orthop. Surg. Res. 2009, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; McKay, M.; Baldwin, J.; Nicholson, L.; Chan, C.; Burns, J.; Hiller, C.E. Beighton scores and cut-offs across the lifespan: Cross-sectional study of an Australian population. Rheumatology 2017, 56, 1857–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, C.; Wilkinson, J. Persistent joint laxity and congenital dislocation of the hip. J. Bone Jt. Surg. Br. 1964, 46, 40–45. [Google Scholar] [CrossRef]
- Jansson, A.; Saartok, T.; Werner, S.; Renstrom, P. General joint laxity in 1845 Swedish school children of different ages: Age- and gender-specific distributions. Acta Paediatr. 2004, 93, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Clinch, J.; Deere, K.; Sayers, A.; Palmer, S.; Riddoch, C.; Tobias, J.H.; Clark, E.M. Epidemiology of generalized joint laxity (hypermobility) in fourteen-year-old children from the UK: A populationbased evaluation. Arthritis Rheum. 2011, 63, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, J.; Parslow, C.; Lim, E.; Hayward, A. Joint hypermobility: The use of a new assessment tool to measure lower limb hypermobility. Clin. Exp. Rheumatol. 2005, 23, 413–420. [Google Scholar] [PubMed]
- Meyer, K.J.; Chan, C.; Hopper, L.; Nicholson, L.L. Identifying lower limb specific and generalised joint hypermobility in adults: Validation of the Lower Limb Assessment Score. BMC Musculoskelet. Disord. 2017, 18, 514. [Google Scholar] [CrossRef] [Green Version]
- Martin, A. An acquired or heritable connective tissue disorder? A review of hypermobile Ehlers Danlos Syndrome. Eur. J. Med. Genet. 2019, 62, 103672. [Google Scholar] [CrossRef]
- Kumar, B.; Lenert, P. Joint hypermobility syndrome: Recognizing a commonly overlooked cause of chronic pain. Am. J. Med. 2017, 130, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Attenborough, A.S.; Sinclair, P.J.; Smith, R.M.; Hiller, C.E. The effect of exercise on ligament laxity during inversion/eversion rotations at the ankle joint. J. Foot Ankle Res. 2014, 7 (Suppl. 1), A5. [Google Scholar] [CrossRef] [Green Version]

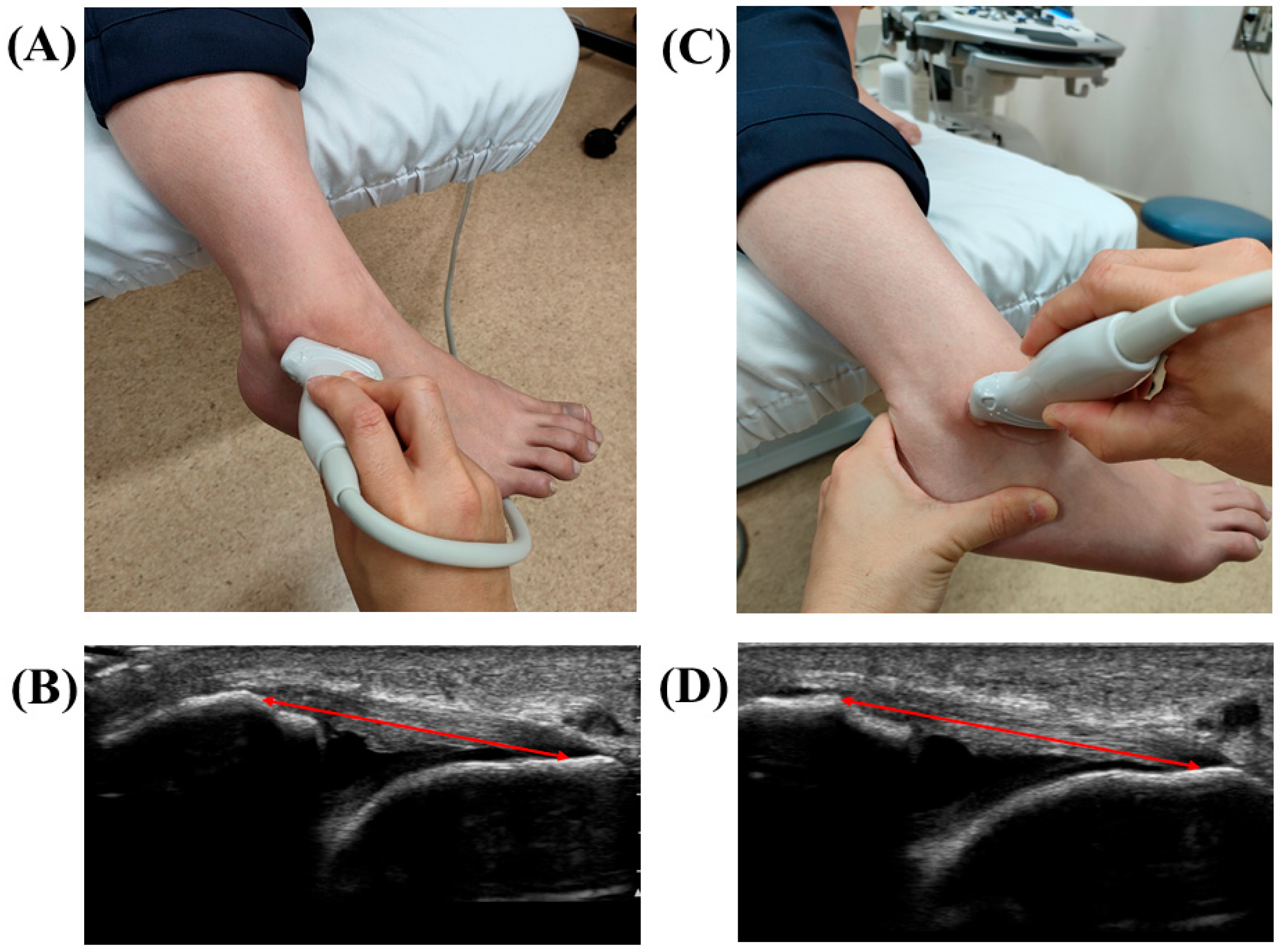


| Variables | Low-BS Group (n = 61) | High-BS Group (n = 20) | p Value |
|---|---|---|---|
| Age, year | 23.7 ± 2.1 (20–33) | 24.3 ± 1.8 (21–27) | 0.14 |
| Height, cm | 157.5 ± 5.7 (147.4–166.1) | 159.5 ± 5.2 (147.0–167.2) | 0.21 |
| Weight, kg | 50.3 ± 4.6 (41.0–60.4) | 52.4 ± 4.8 (44.4–63.8) | 0.09 |
| BMI | 20.3 ± 1.4 (18.4–25.2) | 20.8 ± 1.5 (18.0–23.4) | 0.13 |
| Foot size, cm | 23.0 ± 1.1 (21.0–25.3) | 23.3 ± 1.1 (20.5–25.2) | 0.22 |
| Side of the ankle, n (%) | 0.25 | ||
| right | 45 (73.8) | 12 (60.0) | |
| left | 16 (26.2) | 8 (40.0) | |
| Beighton score | 1.6 ± 1.1 (0–3) | 6.8 ± 1.0 (6–9) | <0.001 |
| Variables | Low BS Group (n = 61) | High BS Group (n = 20) | p Value | Effect Size |
|---|---|---|---|---|
| ADT | 0.03 | 0.60 | ||
| Grade 1, n (%) | 44 (72.1) | 9 (45.0) | ||
| Grade 2, n (%) | 17 (27.9) | 11 (55.0) | ||
| Ultrasonographic findings | ||||
| nonstress ATFL length, mm | 18.2 ± 1.5 (17.8–18.6) | 18.1 ± 1.1 (17.6–18.6) | 0.99 | 0.08 |
| stress ATFL length, mm | 19.9 ± 1.7 (19.4–20.3) | 20.0 ± 1.4 (19.3–20.6) | 0.58 | 0.06 |
| ATFL ratio | 1.09 ± 0.05 (1.08–1.10) | 1.10 ± 0.05 (1.08–1.13) | 0.19 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoe, T.; Tajima, T.; Yamaguchi, N.; Morita, Y.; Chosa, E. Association between the Beighton Score and Stress Ultrasonographic Findings of the Anterior Talofibular Ligament in Healthy Young Women: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 1759. https://doi.org/10.3390/jcm11071759
Yokoe T, Tajima T, Yamaguchi N, Morita Y, Chosa E. Association between the Beighton Score and Stress Ultrasonographic Findings of the Anterior Talofibular Ligament in Healthy Young Women: A Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(7):1759. https://doi.org/10.3390/jcm11071759
Chicago/Turabian StyleYokoe, Takuji, Takuya Tajima, Nami Yamaguchi, Yudai Morita, and Etsuo Chosa. 2022. "Association between the Beighton Score and Stress Ultrasonographic Findings of the Anterior Talofibular Ligament in Healthy Young Women: A Cross-Sectional Study" Journal of Clinical Medicine 11, no. 7: 1759. https://doi.org/10.3390/jcm11071759






