Clinical Outcomes with Targeted Temperature Management (TTM) in Comatose Out-of-Hospital Cardiac Arrest Patients—A Retrospective Cohort Study
Abstract
:1. Background
2. Materials and Methods
2.1. Study Sample, Setting, and Design
2.2. Baseline Characteristics and Clinical Outcomes
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Descriptive Statistics: Baseline Characteristics
3.3. Primary Outcome: 90-Day Mortality
3.4. Secondary Outcome: CPC Score and ICU Length of Stay
3.5. Subgroup Analysis
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, J.P.; Deakin, C.D.; Soar, J.; Bottiger, B.W.; Smith, G.; European Resuscitation Council. European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation 2005, 67 (Suppl. 1), S39–S86. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Monsieurs, K.G.; Nolan, J.P.; Bossaert, L.L.; Greif, R.; Maconochie, I.K.; Nikolaou, N.I.; Perkins, G.D.; Soar, J.; Truhlář, A.; Wyllie, J.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation 2015, 95, 1–80. [Google Scholar] [CrossRef]
- Bray, J.E.; Stub, D.; Bloom, J.E.; Segan, L.; Mitra, B.; Smith, K.; Finn, J.; Bernard, S. Changing target temperature from 33 °C to 36 °C in the ICU management of out-of-hospital cardiac arrest: A before and after study. Resuscitation 2017, 113, 39–43. [Google Scholar] [CrossRef]
- Salter, R.; Bailey, M.; Bellomo, R.; Eastwood, G.; Goodwin, A.; Nielsen, N.; Pilcher, D.; Nichol, A.; Saxena, M.; Shehabi, Y.; et al. Changes in Temperature Management of Cardiac Arrest Patients Following Publication of the Target Temperature Management Trial. Crit. Care Med. 2018, 46, 1722–1730. [Google Scholar] [CrossRef]
- Garfield, B.; Abdoolraheem, M.Y.; Dixon, A.; Aswani, A.; Paul, R.; Sherren, P.; Glover, G. Temporal Changes in Targeted Temperature Management for Out-of-Hospital Cardiac Arrest-Examining the Effect of the Targeted Temperature Management Trial: A Retrospective Cohort Study. Ther. Hypothermia Temp. Manag. 2021, 11, 230–237. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Schafer, A.; Bauersachs, J.; Akin, M. Therapeutic Hypothermia following Cardiac Arrest after the TTM2 trial—More Questions Raised than Answered. Curr. Probl. Cardiol. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Van Wees, C.; Rietdijk, W.; Mandigers, L.; van der Graaf, M.; Scholte, N.T.B.; Adriaansens, K.O.; van den Berg, R.C.M.; den Uil, C.A. Do Women Have a Higher Mortality Risk Than Men following ICU Admission after Out-of-Hospital Cardiac Arrest? A Retrospective Cohort Analysis. J. Clin. Med. 2021, 10, 4286. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Bro-Jeppesen, J.; Kjaergaard, J.; Wanscher, M.; Nielsen, N.; Friberg, H.; Bjerre, M.; Hassager, C. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33 °C or 36 °C. Resuscitation 2014, 85, 1480–1487. [Google Scholar] [CrossRef]
- Annborn, M.; Bro-Jeppesen, J.; Nielsen, N.; Ullén, S.; Kjaergaard, J.; Hassager, C.; Wanscher, M.; Hovdenes, J.; Pellis, T.; Pelosi, P.; et al. The association of targeted temperature management at 33 and 36 °C with outcome in patients with moderate shock on admission after out-of-hospital cardiac arrest: A post hoc analysis of the Target Temperature Management trial. Intensive Care Med. 2014, 40, 1210–1219. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Annborn, M.; Hassager, C.; Wise, M.P.; Pelosi, P.; Nielsen, N.; Erlinge, D.; Wanscher, M.; Friberg, H.; Friberg, H.; et al. Hemodynamics and vasopressor support during targeted temperature management at 33 °C Versus 36 °C after out-of-hospital cardiac arrest: A post hoc study of the target temperature management trial. Crit. Care Med. 2015, 43, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.J.; Danielson, K.R.; Counts, C.R.; Ruark, K.; Scruggs, S.; Hough, C.L.; Maynard, C.; Sayre, M.R.; Carlbom, D.J. Targeted Temperature Management at 33 Versus 36 Degrees: A Retrospective Cohort Study. Crit. Care Med. 2020, 48, 362–369. [Google Scholar] [CrossRef]
- Abazi, L.; Awad, A.; Nordberg, P.; Jonsson, M.; Taccone, F.S.; Wickerts, C.J.; Svensson, L.; Hollenberg, J.; Ringh, M.; Forsberg, S. Long-term survival in out-of-hospital cardiac arrest patients treated with targeted temperature control at 33 degrees C or 36 degrees C: A national registry study. Resuscitation 2019, 143, 142–147. [Google Scholar] [CrossRef]
- Minini, A.; Annoni, F.; Peluso, L.; Bogossian, E.G.; Creteur, J.; Taccone, F.S. Which Target Temperature for Post-Anoxic Brain Injury? A Systematic Review from “Real Life”. Studies. Brain Sci. 2021, 11, 186. [Google Scholar] [CrossRef]
- Lascarrou, J.-B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.-F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef]
- Polderman, K.H.; Herold, I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: Practical considerations, side effects, and cooling methods. Crit. Care Med. 2009, 37, 1101–1120. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Karakike, E.; Ekmektzoglou, K.; Kyprianou, M.; Gkolfakis, P.; Chalkias, A.; Kouskouni, E.; Xanthos, T. Sinus Bradycardia During Targeted Temperature Management: A Systematic Review and Meta-Analysis. Ther. Hypothermia Temp. Manag. 2020, 10, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bro-Jeppesen, J.; Hassager, C.; Wanscher, M.; Østergaard, M.; Nielsen, N.; Erlinge, D.; Friberg, H.; Køber, L.; Kjaergaard, J. Targeted Temperature Management at 33 °C Versus 36 °C and Impact on Systemic Vascular Resistance and Myocardial Function After Out-of-Hospital Cardiac Arrest. Circ. Cardiovasc. Interv. 2014, 7, 663–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chocron, R.; Fahrenbruch, C.; Yin, L.; Guan, S.; Drucker, C.; Shin, J.; Eisenberg, M.; Chatterjee, N.A.; Kudenchuk, P.J.; Rea, T. Association between functional status at hospital discharge and long-term survival after out-of-hospital-cardiac-arrest. Resuscitation 2021, 164, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, J.; Wahlström, J.; Dankiewicz, J.; Annborn, M.; Agarwal, S.; Dupont, A.; Forsberg, S.; Friberg, H.; Hand, R.; Hirsch, K.G.; et al. Functional outcomes associated with varying levels of targeted temperature management after out-of-hospital cardiac arrest—An INTCAR2 registry analysis. Resuscitation 2020, 146, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | All TTM Patients (n = 798) | TTM33 Group (n = 583) | TTM36 Group (n = 215) | p Value | Missing (n) |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, years (IQR) | 63.8 (53.8–72.2) | 63.4 (53.0–71.5) | 64.9 (52.9–74.4) | 0.30 | 0 |
| Female, n (%) | 191 (23.9) | 137 (23.5) | 54 (25.1) | 0.66 | 0 |
| Medical history, n (%) | |||||
| Hypertension | 283 (37.9) | 203 (37.9) | 80 (38.1) | 0.96 | 52 |
| Hypercholesterolemia | 198 (26.6) | 157 (29.3) | 41 (19.5) | 0.006 | 53 |
| Diabetes Mellitus | 151 (19.8) | 104 (18.8) | 47 (22.4) | 0.27 | 36 |
| Family history of CVD | 115 (18.8) | 94 (21.4) | 21 (12.2) | 0.01 | 186 |
| Smoking | 215 (32.7) | 167 (35.0) | 48 (26.5) | 0.04 | 140 |
| Peripheral vascular disease | 66 (8.7) | 50 (9.1) | 16 (7.7) | 0.54 | 38 |
| Previous myocardial infarction | 175 (22.0) | 130 (22.3) | 45 (21.0) | 0.69 | 2 |
| Chronic heart failure | 89 (11.2) | 63 (10.8) | 26 (12.1) | 0.60 | 3 |
| Previous PCI | 116 (14.6) | 78 (13.4) | 38 (17.8) | 13 | 3 |
| Previous CABG | 54 (6.8) | 41 (7.1) | 13 (6.1) | 0.63 | 3 |
| Previous ICD implantation | 21 (2.6) | 9 (1.5) | 12 (5.6) | 0.004 | 2 |
| Previous pacemaker implantation | 19 (2.4) | 12 (2.1) | 7 (3.3) | 0.33 | 2 |
| Previous TIA or stroke | 64 (8.0) | 46 (7.9) | 18 (8.4) | 0.83 | 1 |
| Pulmonary embolism | 11 (1.4) | 7 (1.2) | 4 (1.9) | 0.48 | 2 |
| Arrest characteristics, n (%) | |||||
| Location of arrest | 0.38 | 0 | |||
| Home | 440 (55.1) | 316 (54.2) | 124 (57.7) | ||
| Public | 358 (44.9) | 267 (45.8) | 91 (42.3) | ||
| Witnessed arrest | 592 (76.4) | 448 (78.3) | 144 (70.9) | 0.03 | 23 |
| Bystander CPR | 504 (65.2) | 363 (64.0) | 141 (68.4) | 0.25 | 25 |
| Estimated time to CPR | 0.30 | 127 | |||
| 0–5 min | 515 (76.8) | 380 (75.1) | 135 (81.8) | ||
| 6–10 min | 125 (18.6) | 100 (19.8) | 25 (15.2) | ||
| 11–20 min | 26 (3.9) | 21 (4.2) | 5 (3.0) | ||
| >20 min | 5 (0.7) | 5 (1.0) | 0 (0) | ||
| AED defibrillation | 320 (40.3) | 232 (39.8) | 88 (41.5) | 0.66 | 3 |
| Shockable initial rhythm | 596 (78.1) | 435 (77.7) | 161 (79.3) | 0.63 | 35 |
| Defibrillation by EMS | 523 (65.6) | 382 (65.5) | 141 (65.9) | 0.92 | 1 |
| Time to ROSC | 0.07 | 149 | |||
| 0–5 min | 33 (5.1) | 28 (5.8) | 5 (3.0) | ||
| 6–10 min | 116 (17.9) | 92 (19.0) | 24 (14.5) | ||
| 11–20 min | 297 (45.8) | 224 (46.4) | 73 (44.0) | ||
| >20 min | 203 (31.3) | 139 (28.8) | 64 (38.6) | ||
| Pre-hospital intubation | 509 (63.8) | 364 (62.4) | 145 (67.4) | 0.19 | 0 |
| Primary cardiac cause | 693 (92.0) | 508 (91.7) | 185 (93.0) | 0.57 | 45 |
| Characteristics | All TTM Patients (n = 798) | TTM33 Group (n = 583) | TTM36 Group (n = 215) | p-Value | Missing (n) |
|---|---|---|---|---|---|
| Post arrest care characteristics | |||||
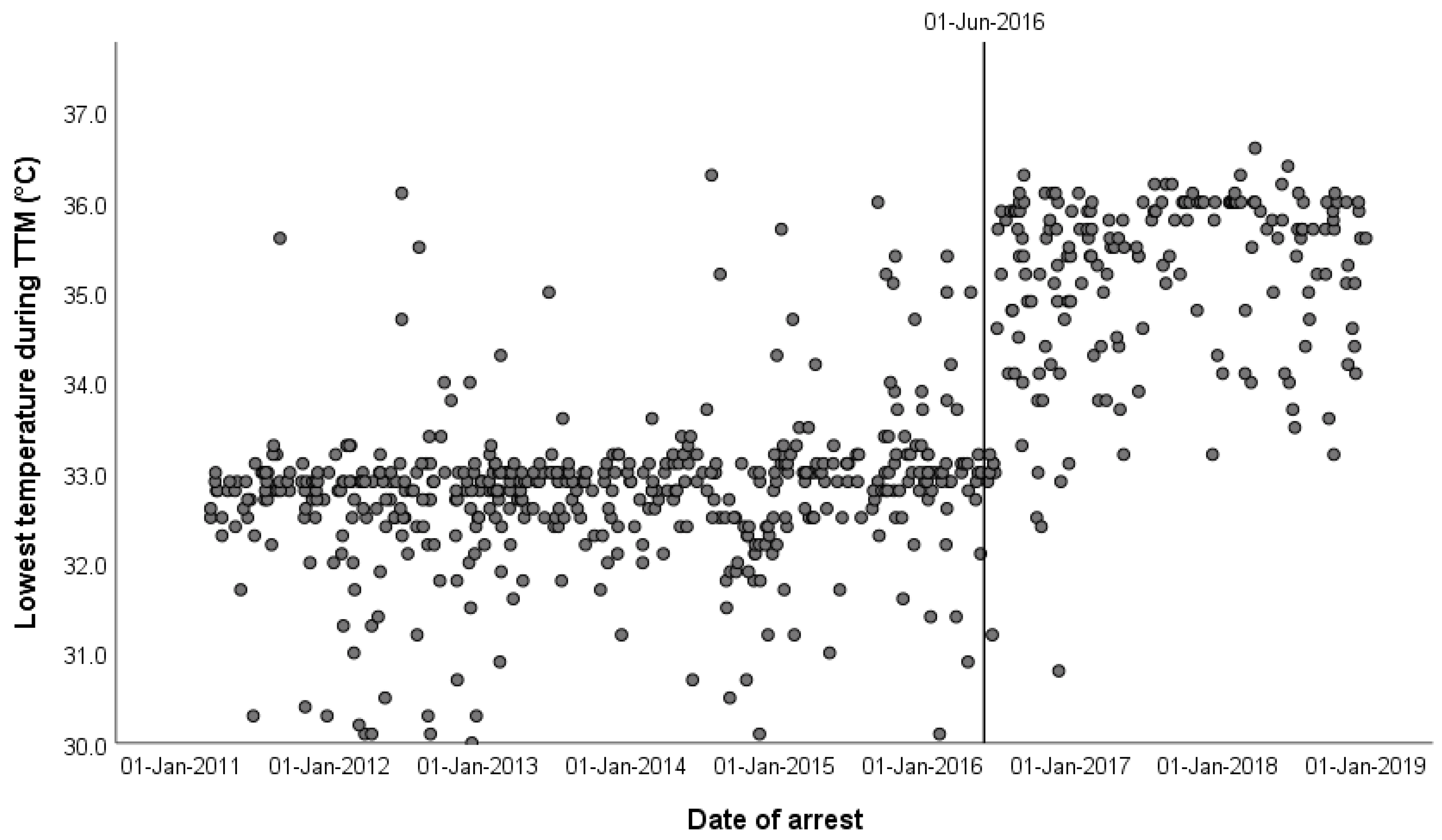
| Lowest temperature during TTM, °C (IQR) | 33.0 (32.7–34.2) | 32.9 (32.5–33.0) | 35.6 (34.7–36.0) | <0.001 | 117 |
| Bradycardia during TTM, n (%) | 389 (55.5) | 327 (60.2) | 62 (39.2) | <0.001 | 97 |
| Lowest MAP during TTM, mmHg (IQR) | 57 (52–61) | 57 (54–63) | 59 (54–63) | <0.001 | 51 |
| Inotropics/vasoactive drugs, n (%) | 737 (98.4) | 567 (98.1) | 170 (99.4) | 0.23 | 49 |
| Mechanical circulatory support, n (%) | 0.003 | 0 | |||
| IABP | 94 (11.6) | 82 (13.9) | 12 (5.5) | ||
| Impella | 1 (0.1) | 0 (0.0) | 1 (0.5) | ||
| ECMO | 7 (0.9) | 4 (0.7) | 3 (1.4) | ||
| CVVH, n (%) | 37 (5.0) | 29 (5.0) | 8 (4.7) | 0.88 | 51 |
| Clinical outcomes | |||||
| Mortality at 90 days, n (%) | 322 (40.4) | 229 (39.3) | 93 (43.3) | 0.31 | 0 |
| Neurologic status at ICU discharge, n (%) | 0.13 | 26 | |||
| Favorable neurologic outcome, CPC 1–2 | 450 (58.3) | 342 (59.2) | 108 (53.7) | ||
| Poor neurologic outcome, CPC 3–5 | 322 (41.7) | 229 (40.1) | 93 (46.3) | ||
| Neurologic status at ICU discharge, n (%) | 0.002 | 26 | |||
| CPC 1—Full neurologic recovery | 269 (34.8) | 194 (34.0) | 75 (37.3) | ||
| CPC 2—Mildly impaired | 181 (23.4) | 148 (25.9) | 33 (16.4) | ||
| CPC 3—Awake with severly impaired neurologic status | 29 (3.8) | 22 (3.9) | 7 (3.5) | ||
| CPC 4—Comatose, unresponsive | 23 (3.0) | 10 (1.8) | 13 (6.5) | ||
| CPC 5—Dead | 270 (35.0) | 197 (34.5) | 73 (34.5) | ||
| ICU length of stay, days (IQR) | 4.0 (3.0–7.0) | 5.0 (3.0–7.0) | 4.0 (2.0–6.0) | 0.001 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholte, N.T.B.; van Wees, C.; Rietdijk, W.J.R.; van der Graaf, M.; Jewbali, L.S.D.; van der Jagt, M.; van den Berg, R.C.M.; Lenzen, M.J.; den Uil, C.A. Clinical Outcomes with Targeted Temperature Management (TTM) in Comatose Out-of-Hospital Cardiac Arrest Patients—A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 1786. https://doi.org/10.3390/jcm11071786
Scholte NTB, van Wees C, Rietdijk WJR, van der Graaf M, Jewbali LSD, van der Jagt M, van den Berg RCM, Lenzen MJ, den Uil CA. Clinical Outcomes with Targeted Temperature Management (TTM) in Comatose Out-of-Hospital Cardiac Arrest Patients—A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(7):1786. https://doi.org/10.3390/jcm11071786
Chicago/Turabian StyleScholte, Niels T. B., Christiaan van Wees, Wim J. R. Rietdijk, Marisa van der Graaf, Lucia S. D. Jewbali, Mathieu van der Jagt, Remco C. M. van den Berg, Mattie J. Lenzen, and Corstiaan A. den Uil. 2022. "Clinical Outcomes with Targeted Temperature Management (TTM) in Comatose Out-of-Hospital Cardiac Arrest Patients—A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 7: 1786. https://doi.org/10.3390/jcm11071786
APA StyleScholte, N. T. B., van Wees, C., Rietdijk, W. J. R., van der Graaf, M., Jewbali, L. S. D., van der Jagt, M., van den Berg, R. C. M., Lenzen, M. J., & den Uil, C. A. (2022). Clinical Outcomes with Targeted Temperature Management (TTM) in Comatose Out-of-Hospital Cardiac Arrest Patients—A Retrospective Cohort Study. Journal of Clinical Medicine, 11(7), 1786. https://doi.org/10.3390/jcm11071786






