A Novel Quantitative Parameter for Static Myocardial Computed Tomography: Myocardial Perfusion Ratio to the Aorta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dynamic Myocardial CTP Scan Protocol
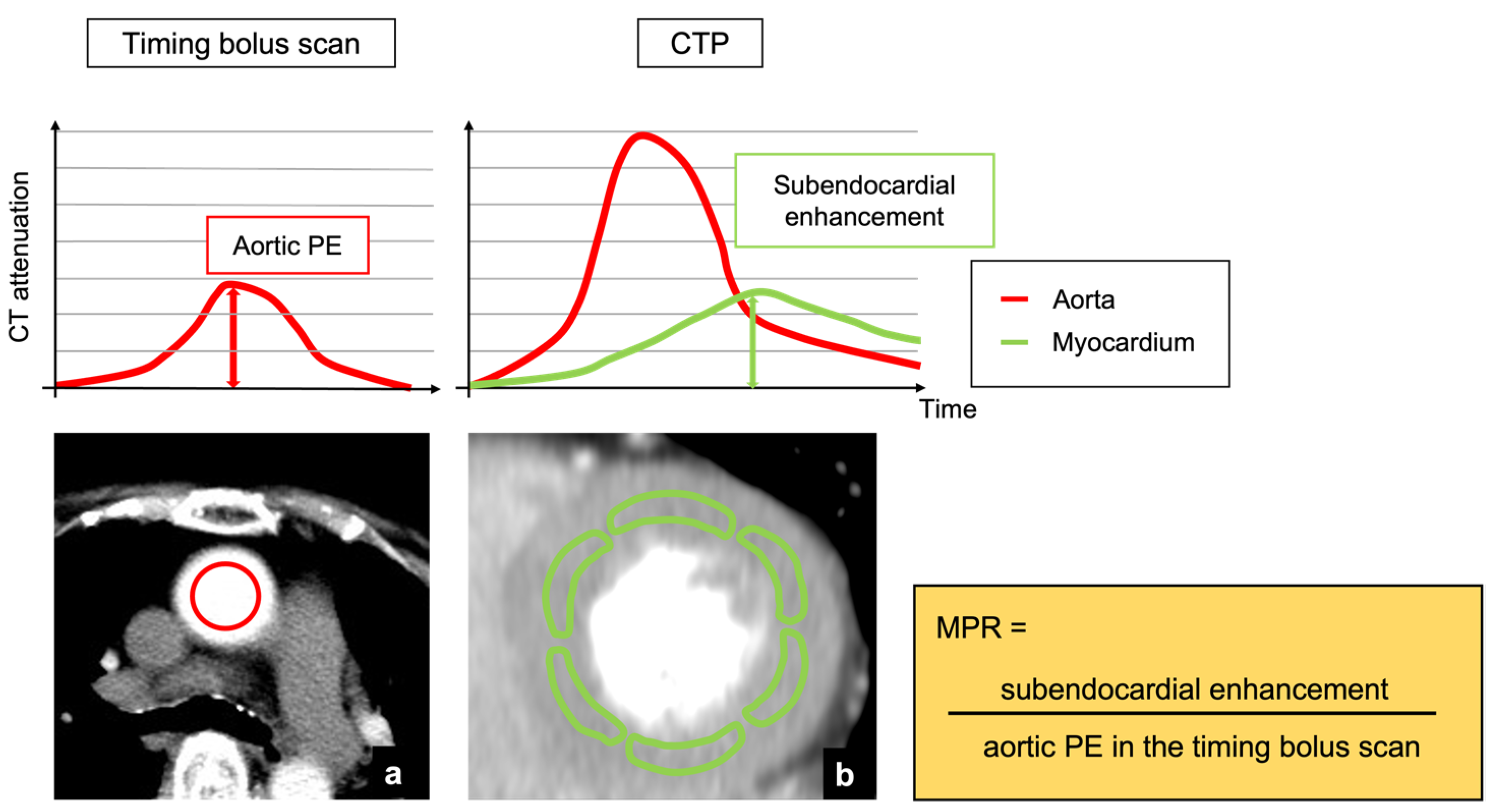
2.3. Analysis of Aortic Peak Enhancement in Timing Bolus and Dynamic CTP Scans
2.4. Post-Processing and Image Analysis of Myocardial CTP Imaging
2.5. SPECT-MPI Scan Protocol and Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of Myocardial Segments Assessed by SPECT-MPI
3.3. Aortic Peak Enhancement in Timing Bolus Scan and Dynamic CTP Scan
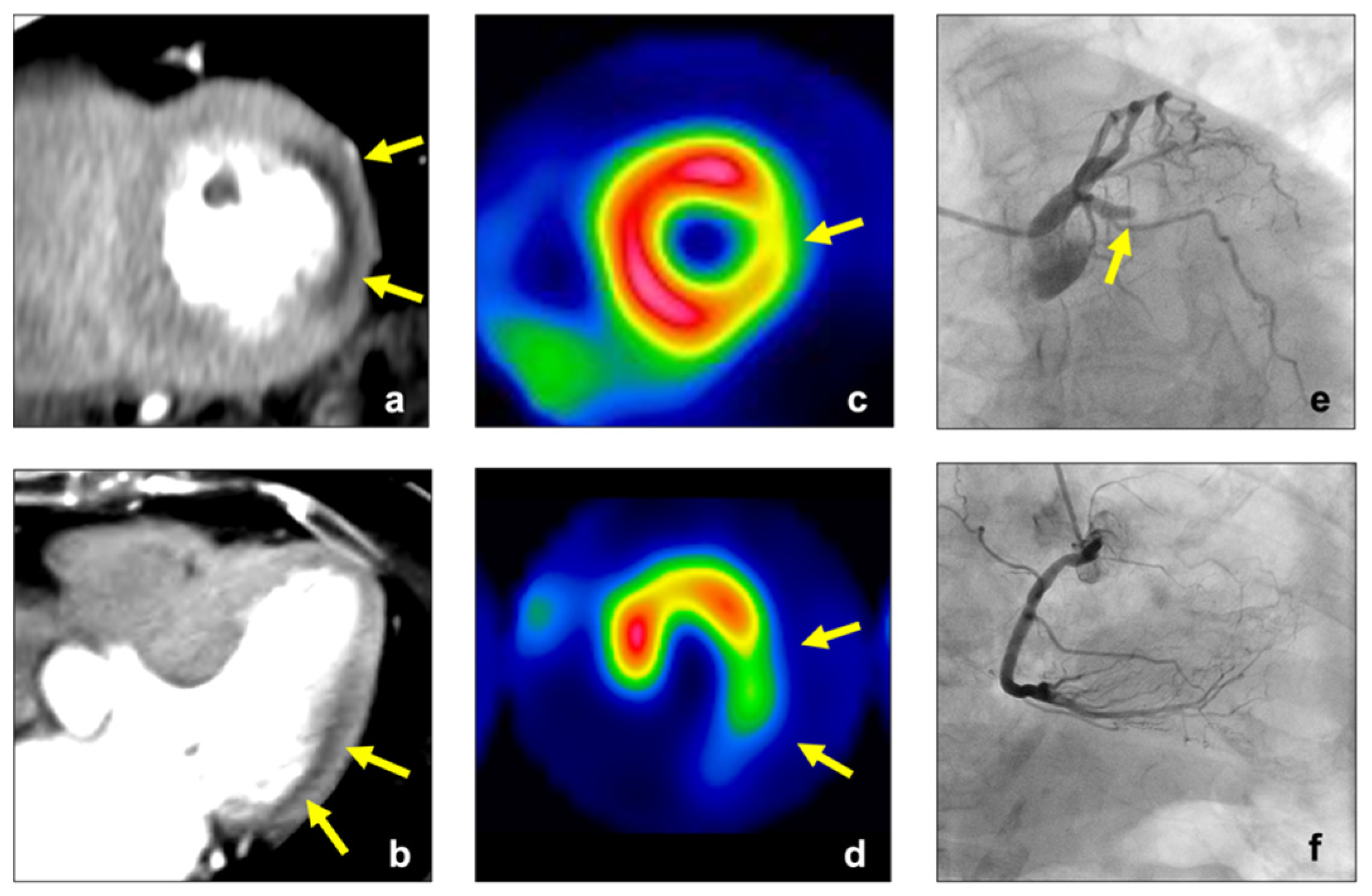
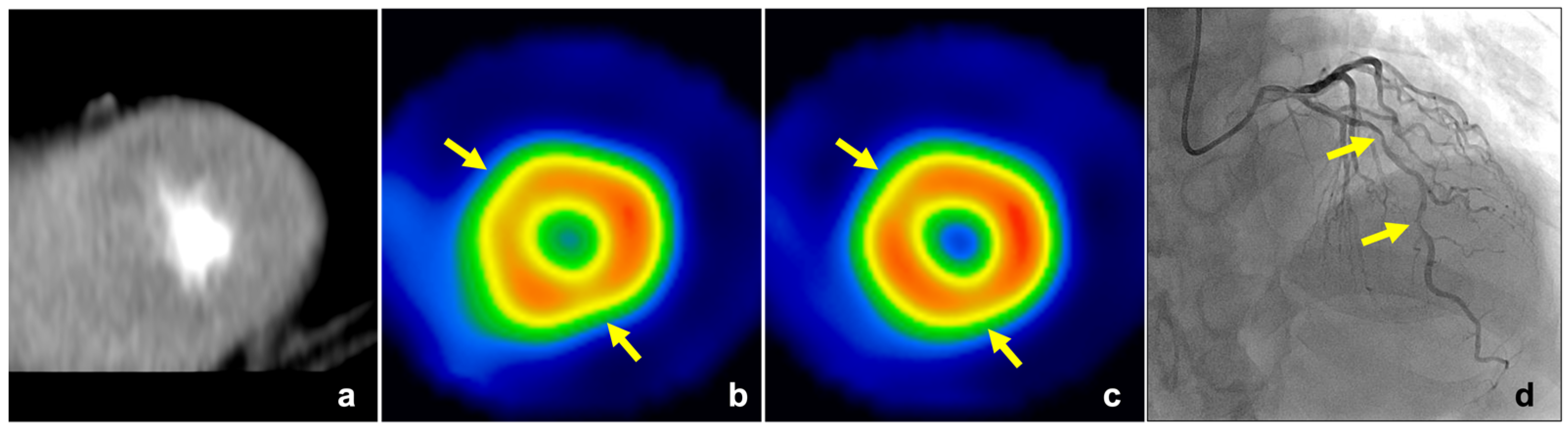
3.4. Comparisons in Endocardial CT Attenuation, TPR, and MPR between Normal and Abnormal Perfusion Segments
3.5. Diagnostic Accuracy of Visual Assessment, Endocardial CT Attenuation, TPR, and MPR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moroi, M.; Yamashina, A.; Tsukamoto, K.; Nishimura, T. The J-ACCESS Investigators. Coronary revascularization does not decrease cardiac events in patients with stable ischemic heart disease but might do in those who showed moderate to severe ischemia. Int. J. Cardiol. 2012, 158, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Nudi, F.; Neri, G.; Schillaci, O.; Pinto, A.; Procaccini, E.; Vetere, M.; Tomai, F.; Frati, G.; Biondi-Zoccai, G. Time to and risk of cardiac events after myocardial perfusion scintigraphy. J. Cardiol. 2015, 66, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the Short-Term Survival Benefit Associated With Revascularization Compared With Medical Therapy in Patients with No Prior Coronary Artery Disease Undergoing Stress Myocardial Perfusion Single Photon Emission Computed Tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.J.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’Rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal medical therapy with or without percutaneous coronary intervention to re- duce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.P.; Maredia, N.; Younger, J.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Jaarsma, C.; Leiner, T.; Bekkers, S.C.; Crijns, H.J.; Wildberger, J.E.; Nagel, E.; Nelemans, P.J.; Schalla, S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Techasith, T.; Cury, R.C. Stress Myocardial CT Perfusion: An Update and Future Perspective. JACC Cardiovasc. Imaging 2011, 4, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Merkus, D.; Klotz, E.; Mollet, N.; De Feyter, P.; Krestin, G. Stress Myocardial Perfusion: Imaging with Multidetector CT. Radiology 2014, 270, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Bamberg, F.; Becker, A.; Schwarz, F.; Marcus, R.P.; Greif, M.; Von Ziegler, F.; Blankstein, R.; Hoffmann, U.; Sommer, W.H.; Hoffmann, V.S.; et al. Detection of Hemodynamically Significant Coronary Artery Stenosis: Incremental Diagnostic Value of Dynamic CT-based Myocardial Perfusion Imaging. Radiology 2011, 260, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Huber, A.M.; Leber, V.; Gramer, B.M.; Muenzel, D.; Leber, A.; Rieber, J.; Schmidt, M.; Vembar, M.; Hoffmann, E.; Rummeny, E. Myocardium: Dynamic versus Single-Shot CT Perfusion Imaging. Radiology 2013, 269, 378–386. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Schulman-Marcus, J.; Min, J.K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Hear. J.-Cardiovasc. Imaging 2016, 17, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Kurata, A.; Kawaguchi, N.; Kido, T.; Inoue, K.; Suzuki, J.; Ogimoto, A.; Funada, J.-I.; Higaki, J.; Miyagawa, M.; Vembar, M.; et al. Qualitative and Quantitative Assessment of Adenosine Triphosphate Stress Whole-Heart Dynamic Myocardial Perfusion Imaging Using 256-Slice Computed Tomography. PLoS ONE 2013, 8, e83950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.H.; Kim, Y.-H.; Roh, J.-H.; Kang, J.-W.; Han, D.; Jung, J.; Kim, N.; Lee, J.B.; Ahn, J.-M.; Lee, J.-Y.; et al. Stress Myocardial Perfusion CT in Patients Suspected of Having Coronary Artery Disease: Visual and Quantitative Analysis—Validation by Using Fractional Flow Reserve. Radiology 2015, 276, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Shrimpton, P.C.; Hillier, M.C.; A Lewis, M.; Dunn, M. National survey of doses from CT in the UK: 2003. Br. J. Radiol. 2006, 79, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Kurata, A.; Kido, T.; Nishiyama, Y.; Kido, T.; Miyagawa, M.; Ogimoto, A.; Mochizuki, T. Optimization of Coronary Attenuation in Coronary Computed Tomography Angiography Using Diluted Contrast Material. Circ. J. 2014, 78, 662–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, Y.; Kido, T.; Uetani, T.; Kurata, A.; Kono, T.; Ogimoto, A.; Miyagawa, M.; Soma, T.; Murase, K.; Iwaki, H.; et al. Differentiation of myocardial ischemia and infarction assessed by dynamic computed tomography perfusion imaging and comparison with cardiac magnetic resonance and single-photon emission computed tomography. Eur. Radiol. 2016, 26, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Kido, T.; Kurata, A.; Uetani, T.; Fukuyama, N.; Yokoi, T.; Nishiyama, H.; Kido, T.; Miyagawa, M.; Mochizuki, T. Optimal Scan Time for Single-Phase Myocardial Computed Tomography Perfusion to Detect Myocardial Ischemia—Derivation Cohort From Dynamic Myocardial Computed Tomography Perfusion–. Circ. J. 2016, 80, 2506–2512. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef] [Green Version]
- George, R.T.; Arbab-Zadeh, A.; Miller, J.M.; Kitagawa, K.; Chang, H.-J.; Bluemke, D.A.; Becker, L.; Yousuf, O.; Texter, J.; Lardo, A.C.; et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: A pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ. Cardiovasc. Imaging 2009, 2, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Miyagawa, M.; Kawaguchi, N.; Nakamura, M.; Kido, T.; Kurata, A.; Kido, T.; Ogimoto, A.; Higaki, J.; Mochizuki, T. Combined Supine and Prone Myocardial Perfusion Single-Photon Emission Computed Tomography With a Cadmium Zinc Telluride Camera for Detection of Coronary Artery Disease. Circ. J. 2014, 78, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qin, L.; Shi, X.; Zeng, Y.; Jing, H.; Schoepf, U.J.; Jin, Z. Adenosine-Stress Dynamic Myocardial Perfusion Imaging With Second-Generation Dual-Source CT: Comparison With Conventional Catheter Coronary Angiography and SPECT Nuclear Myocardial Perfusion Imaging. Am. J. Roentgenol. 2012, 198, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Raijmakers, P.G.; Appelman, Y.E.; Harms, H.J.; de Haan, S.; Oever, M.L.P.V.D.; van Kuijk, C.; Allaart, C.P.; Hoekstra, O.S.; Lammertsma, A.A.; et al. Coronary risk factors and myocardial blood flow in patients evaluated for coronary artery disease: A quantitative [15O]H2O PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2011, 39, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, Y.; Kido, T.; Kurata, A.; Yokoi, T.; Fukuyama, N.; Uetani, T.; Nishiyama, H.; Kawaguchi, N.; Tahir, E.; Miyagawa, M.; et al. Peak enhancement ratio of myocardium to aorta for identification of myocardial ischemia using dynamic myocardial computed tomography perfusion imaging. J. Cardiol. 2017, 70, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, B.S.; Cameron, J.D.; Leung, M.; Meredith, I.T.; Leong, D.P.; Antonis, P.R.; Crossett, M.; Troupis, J.; Harper, R.; Malaiapan, Y.; et al. Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: A comparison with fractional flow reserve. JACC Cardiovasc. Imaging 2012, 5, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, X.; Lu, Z.; Shen, C.; Zhang, J. Diagnostic performance of quantitative, semi-quantitative, and visual analysis of dynamic CT myocardial perfusion imaging: A validation study with invasive fractional flow reserve. Eur. Radiol. 2021, 31, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; I Guaricci, A.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Fazzari, F.; Berzovini, C.; et al. Quantitative vs. qualitative evaluation of static stress computed tomography perfusion to detect haemodynamically significant coronary artery disease. Eur. Hear. J.-Cardiovasc. Imaging 2018, 19, 1244–1252. [Google Scholar] [CrossRef]
- Schwarz, F.; Hinkel, R.; Baloch, E.; Marcus, R.P.; Hildebrandt, K.; Sandner, T.A.; Kupatt, C.; Hoffmann, V.; Wintersperger, B.J.; Reiser, M.F.; et al. Myocardial CT perfusion imaging in a large animal model: Comparison of dynamic versus single-phase acquisitions. JACC Cardiovasc. Imaging 2013, 6, 1229–1238. [Google Scholar] [CrossRef] [Green Version]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography With Fractional Flow Reserve Meta-Analysis. Circ. Cardiovasc. Imaging 2015, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Rochitte, C.E.; George, R.T.; Chen, M.Y.; Arbab-Zadeh, A.; Dewey, M.; Miller, J.M.; Niinuma, H.; Yoshioka, K.; Kitagawa, K.; Nakamori, S.; et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: The CORE320 study. Eur. Hear. J. 2014, 35, 1120–1130. [Google Scholar] [CrossRef]
- Meyer, M.; Nance, J.W.; Schoepf, U.J.; Moscariello, A.; Weininger, M.; Rowe, G.W.; Ruzsics, B.; Kang, D.K.; Chiaramida, S.A.; Schoenberg, S.O.; et al. Cost-effectiveness of substituting dual-energy CT for SPECT in the assessment of myocardial perfusion for the workup of coronary artery disease. Eur. J. Radiol. 2012, 81, 3719–3725. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Karagiannidis, E.; Moysidis, D.V.; Sofidis, G.; Bompoti, A.; Stalikas, N.; Panteris, E.; Arvanitidis, C.; Herrmann, M.D.; Michaelson, J.S.; et al. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hell. J. Cardiol. 2021, 62, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 15, 211780. [Google Scholar] [CrossRef] [PubMed]


| Age (Years) | 70.5 (9.5) |
| Men (%) | 19 (76%) |
| Body mass index (kg/m2) | 24.1 (3.1) |
| Coronary risk factors (number [%]) | |
| Hypertension | 18 (72%) |
| Dyslipidemia | 12 (48%) |
| Diabetes mellitus | 8 (32%) |
| Positive smoking history | 16 (64%) |
| Family history of coronary artery disease | 10 (40%) |
| HR (bpm) | |
| Baseline | 65.4 (10.2) |
| Stress | 80.0 (8.0) |
| Time periods between CT and SPECT (days) | 27 (13–43) |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| MPR | 78 (66–86) | 84 (80–88) | 48 (39–58) | 95 (92–97) |
| TPR | 63 (51–74) | 84 (80–88) | 43 (33–53) | 92 (89–95) |
| CT attenuation | 51 (39–63) | 86 (82–89) | 41 (30–52) | 90 (87–93) |
| (HU) | ||||
| Visual | 67 (54–77) | 90 (86–92) | 55 (43–65) | 93 (90–96) |
| assessment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouchi, T.; Tanabe, Y.; Takemoto, T.; Yoshida, K.; Yamamoto, Y.; Miyazaki, S.; Fukuyama, N.; Nishiyama, H.; Inaba, S.; Kawaguchi, N.; et al. A Novel Quantitative Parameter for Static Myocardial Computed Tomography: Myocardial Perfusion Ratio to the Aorta. J. Clin. Med. 2022, 11, 1816. https://doi.org/10.3390/jcm11071816
Kouchi T, Tanabe Y, Takemoto T, Yoshida K, Yamamoto Y, Miyazaki S, Fukuyama N, Nishiyama H, Inaba S, Kawaguchi N, et al. A Novel Quantitative Parameter for Static Myocardial Computed Tomography: Myocardial Perfusion Ratio to the Aorta. Journal of Clinical Medicine. 2022; 11(7):1816. https://doi.org/10.3390/jcm11071816
Chicago/Turabian StyleKouchi, Takanori, Yuki Tanabe, Takumasa Takemoto, Kazuki Yoshida, Yuta Yamamoto, Shigehiro Miyazaki, Naoki Fukuyama, Hikaru Nishiyama, Shinji Inaba, Naoto Kawaguchi, and et al. 2022. "A Novel Quantitative Parameter for Static Myocardial Computed Tomography: Myocardial Perfusion Ratio to the Aorta" Journal of Clinical Medicine 11, no. 7: 1816. https://doi.org/10.3390/jcm11071816
APA StyleKouchi, T., Tanabe, Y., Takemoto, T., Yoshida, K., Yamamoto, Y., Miyazaki, S., Fukuyama, N., Nishiyama, H., Inaba, S., Kawaguchi, N., Kido, T., Yamaguchi, O., & Kido, T. (2022). A Novel Quantitative Parameter for Static Myocardial Computed Tomography: Myocardial Perfusion Ratio to the Aorta. Journal of Clinical Medicine, 11(7), 1816. https://doi.org/10.3390/jcm11071816







