One Year of Recombinant Human Growth Hormone Treatment in Adults with Prader–Willi Syndrome Improves Body Composition, Motor Skills and Brain Functional Activity in the Cerebellum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.2.1. Anthropometric Methods
2.2.2. Body Composition Assessment
2.2.3. Metabolic Evaluation
2.2.4. Motor Function Assessment
2.2.5. Functional Magnetic Resonance Imaging (fMRI) Testing
2.2.6. Polysomnography
2.3. Statistical Analyses
3. Results
3.1. Comparison of Baseline Measurements in the PWS and Control Groups
3.2. Comparison between Parameters Measured at Baseline and 12 Months after the Initiation of rhGH Treatment in the PWS Group
| Baseline (n = 27) Median (IQR) | After 12 Months rhGH Treatment (n = 27) Median (IQR) | p-Value * | |
|---|---|---|---|
| Weight (kg) | 89.6 (70.5–105.5) | 87.7 (74.3–100.2) | 0.8091 |
| BMI (kg/m2) | 34.5 (31.1–41.3) | 34.0 (31.8–41.6) | 0.8615 |
| WC (cm) | 110 (101–124) | 112 (104–123.5) | 0.6103 |
| IGF-I (ng/mL) | 143 (95–188) | 217 (160–254) | <0.0001 |
| Glucose (mmol/L) | 5.00 (4.50–6.99) | 4.83 (4.33–5.49) | 0.1085 |
| HbA1c (%) | 5.60 (5.30–6.90) | 5.60 (5.30–6.20) | 0.2384 |
| Irisin (ng/mL) | 982.3 (519.4–1789.6) | 906.8 (583.5–1770.4) | 0.7614 |
| Myostatin (ng/mL) | 4.8 (2.95–7.72) | 5.57 (3.35–7.37) | 0.2176 |
| IL-6 (pg/mL) | 15.4(3.58–66.4) | 19.9 (3.44–75.0) | 0.1948 |
| Fat mass (%) | 56.3 (49.3–61.1) | 52.1 (49.9–59.2) | 0.0053 |
| Lean mass (%) | 43.7 (38.9–50.1) | 47.9 (40.8–50.1) | 0.0009 |
| ALM/weight | 0.19 (0.16–0.21) | 0.19 (0.17–0.21) | 0.0439 |
| ALM/BMI | 0.48 (0.38–0.55) | 0.49 (0.38–0.56) | 0.0262 |
| AHI (n/hour) | 15.2 (9.20–22.4) | 22.3 (7.45–29.4) | 0.0911 |
| Central apnea (n/TST) | 1.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.210 |
| Obstructive apnea (n/TST) | 7.5 (2.5–11.5) | 2.0 (1.0–10.25) | 0.450 |
| Hypoapnea (n/TST) | 75.0 (46.0–136.0) | 81.0 (28.75–150.25) | 0.100 |
| Baseline (n = 27) Median (IQR) | After 12 Months rhGH Treatment (n = 27) Median (IQR) | p-Value * | |
|---|---|---|---|
| Handgrip strength (kg) | 18 (13–22) | 16 (13–18) | 0.0750 |
| TUG (seconds) | 8.76 (7.56–11.9) | 8.40 (7.11–9.75) | 0.0167 |
| BBS (points) | 53 (49–54) | 55 (50–55) | 0.0208 |
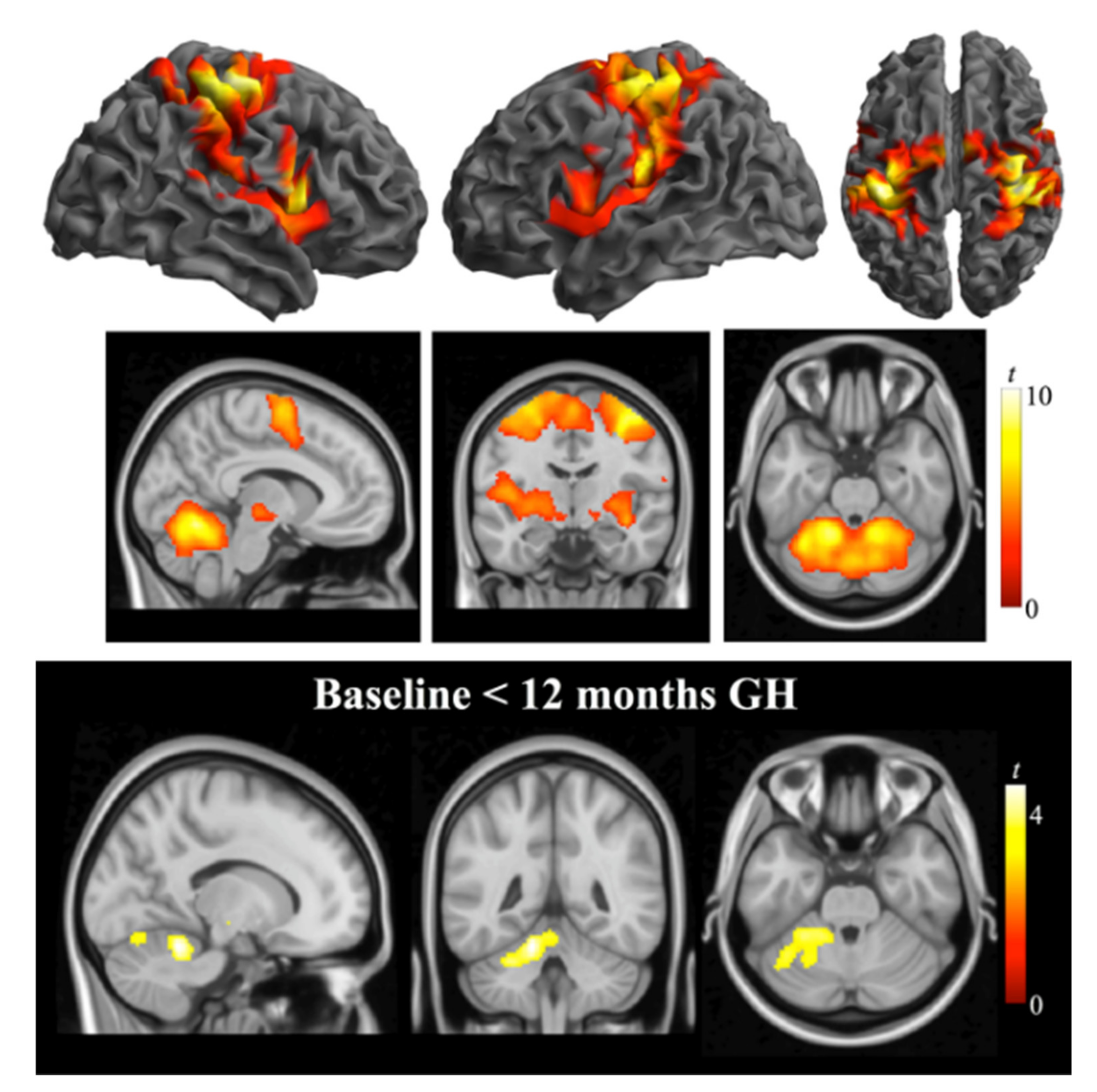
3.3. Correlations
4. Discussion
4.1. Body Composition
4.2. Motor Performance
4.3. fMRI
4.4. Myokines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B. Prader-Willi Syndrome. Curr. Probl. Pediatrics 1984, 14, 1–55. [Google Scholar] [CrossRef]
- Novell-Alsina, R.; Esteba-Castillo, S.; Caixàs, A.; Gabau, E.; Giménez-Palop, O.; Pujol, J.; Deus, J.; Torrents-Rodas, D. Compulsions in Prader-Willi Syndrome: Occurrence and Severity as a Function of Genetic Subtype. Actas Esp. Psiquiatr. 2019, 47, 79–87. [Google Scholar] [PubMed]
- Guinovart, M.; Coronas, R.; Caixàs, A. Psychopathological Disorders in Prader-Willi Syndrome. Endocrinol. Diabetes Nutr. 2019, 66, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi Syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afifi, A.K.; Zellweger, H. Pathology of Muscular Hypotonia in the Prader-Willi Syndrome:Light and Electron Microscopic Study. JNS J. Neurol. Sci. 1969, 9, 49–61. [Google Scholar] [CrossRef]
- Reus, L.; Zwarts, M.J.; Vlimmeren, L.A.; van Willemsen, M.H.; Otten, B.J.; Nijhuis-van der Sanden, M.W. Motor problems in Prader-Willi syndrome: A systematic review on body composition and neuromuscular functioning. Neurosci. Biobehav. Rev. 2011, 35, 956–969. [Google Scholar] [CrossRef] [Green Version]
- Chiu, V.J.Y.; Tsai, L.P.; Wei, J.T.; Tzeng, I.S.; Wu, H.C. Motor Performance in Prader-Willi Syndrome Patients and Its Potential Influence on Caregiver’s Quality of Life. PeerJ 2017, 5, e4097. [Google Scholar] [CrossRef] [Green Version]
- Sode-Carlsen, R.; Farholt, S.; Rabben, K.F.; Bollerslev, J.; Sandahl Christiansen, J.; Höybye, C. Assessment of Physical Function in Adults with Prader-Willi Syndrome. Disabil. Rehabil. 2009, 31, 1780–1784. [Google Scholar] [CrossRef]
- Capodaglio, P.; Vismara, L.; Menegoni, F.; Baccalaro, G.; Galli, M.; Grugni, G. Strength Characterization of Knee Flexor and Extensor Muscles in Prader-Willi and Obese Patients. BMC Musculoskelet. Disord. 2009, 6, 6,10–47. [Google Scholar] [CrossRef] [Green Version]
- Grugni, G.; Crivellini, M.; Baccalaro, G.; Montesano, A.; Galli, M.; Romei, M.; Vismara, L. Clinical Implications of Gait Analysis in the Rehabilitation of Adult Patients with “Prader-Willi” Syndrome: A Cross-Sectional Comparative Study (“Prader-Willi” Syndrome vs Matched Obese Patients and Healthy Subjects). J. Neuroeng. Rehabil. 2007, 10, 4–14. [Google Scholar]
- Burman, P.; Ritzen, E.M.; Lindgren, A.C. Endocrine Dysfunction in Prader-Willi Syndrome: A Review with Special Reference to GH. Endocr. Rev. 2001, 22, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Eiholzer, U.; Whitmann, Y.B. A Comprehensive Team Approach to the Management of Patients with Prader-Willi Syndrome. J. Pediatric Endocrinol. Metab. 2004, 17, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F. PraderWilli syndrome and the hypothalamus. Acta Pediatr. Suppl. 1997, 86, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sone, S. Muscle Histochemistry in the Prader-Willi Syndrome. Brain Dev. 1994, 16, 183–188. [Google Scholar] [CrossRef]
- Civardi, C.; Vicentini, R.; Grugni, G.; Cantello, R. Corticospinal Physiology in Patients With Prader-Willi Syndrome: A Transcranial Magnetic Stimulation Study. Arch. Neurol. 2004, 61, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Hinojo, L.; Casamitjana, L.; Pujol, J.; Martínez-Vilavella, G.; Esteba-Castillo, S.; Giménez-Palop, O.; Freijo, V.; Deus, J.; Caixàs, A. Cerebellar Dysfunction in Adults with Prader Willi Syndrome. J. Clin. Med. 2021, 10, 3320. [Google Scholar] [CrossRef]
- Caixàs, A.; Blanco-Hinojo, L.; Pujol, J.; Deus, J.; Giménez-Palop, O.; Torrents-Rodas, D.; Coronas, R.; Novell, R.; Esteba-Castillo, S. Altered Gesture Imitation and Brain Anatomy in Adult Prader-Willi Syndrome Patients. J. Int. Neuropsychol. Soc. 2021, 27, 1024–1036. [Google Scholar] [CrossRef]
- Lafortuna, C.L.; Minocci, A.; Capodaglio, P.; Gondoni, L.A.; Sartorio, A.; Vismara, L.; Rizzo, G.; Grugni, G. Skeletal Muscle Characteristics and Motor Performance After 2-Year Growth Hormone Treatment in Adults with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.E.; Whitman, B.Y.; Carrel, A.L.; Moerchen, V.; Bekx, M.T.; Allen, D.B. Two Years of Growth Hormone Therapy in Young Children with Prader-Willi Syndrome: Physical and Neurodevelopmental Benefits. Am. J. Med. Genet. A 2007, 143A, 443–448. [Google Scholar] [CrossRef]
- Sanchez-Ortiga, R.; Klibanski, A.; Tritos, N.A. Effects of Recombinant Human Growth Hormone Therapy in Adults with Prader-Willi Syndrome: A Meta-Analysis. CEN Clin. Endocrinol. 2012, 77, 86–93. [Google Scholar] [CrossRef]
- Sode-Carlsen, R.; Farholt, S.; Rabben, K.F.; Bollerslev, J.; Schreiner, T.; Jurik, A.G.; Frystyk, J.; Christiansen, J.S.; Höybye, C. Growth Hormone Treatment for Two Years Is Safe and Effective in Adults with Prader-Willi Syndrome. Growth Horm. IGF Res. 2011, 21, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sode-Carlsen, R.; Rabben, K.F.; Bollerslev, J.; Schreiner, T.; Jurik, A.G.; Christiansen, J.S.; Hoybye, C. Growth Hormone Treatment in Adults with Prader-Willi Syndrome: The Scandinavian Study. Endocr. Endocr. 2012, 41, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Gondoni, L.A.; Vismara, L.; Marzullo, P.; Vettor, R.; Liuzzi, A.; Grugni, G. Growth Hormone Therapy Improves Exercise Capacity in Adult Patients with Prader-Willi Syndrome. J. Endocrinol. Investig. 2008, 31, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Höybye, C.; Thoren, M.; Bohm, B. Cognitive, Emotional, Physical and Social Effects of Growth Hormone Treatment in Adults with Prader-Willi Syndrome. J. Intellect. Disabil. Res. 2005, 49, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Reus, L.; Van Vlimmeren, L.A.; Staal, J.; Bart, O.; Barto, J.; Nijhuis-van der Sanden, M.W. The Effect of Growth Hormone Treatment or Physical Training on Motor Performance in Prader-Willi Syndrome: A Systematic Review. NBR Neurosci. Biobehav. Rev. 2012, 36, 1817–1838. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Akerstrom, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of Myokines in Exercise and Metabolism. J. Appl. Physiol. 2007, 103, 1093. [Google Scholar] [CrossRef] [Green Version]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Yedrichowsky, M.P.; Korde, A.; Ye, L.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; Kajimura, S.; et al. A PGC1-a-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Castro-Gago, M.; Gómez-Lado, C.; Eiris-Puñal, J.; Carneiro, I.; Arce, V.M.; Devesa, J. Muscle Myostatin Expression in Children With Muscle Diseases. J. Child Neurol. 2007, 22, 38–40. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin Levels in Genetic and Essential Obesity: Clues for a Potential Dual Role. Sci. Rep. 2020, 10, 1020. [Google Scholar] [CrossRef] [Green Version]
- Faienza, M.F.; Brunnetti, G.; Grugni, G.; Fintini, D.; Convertino, A.; Pignataro, P.; Crinò, A.; Colucci, S.; Grano, M. The Genetic Background and Vitamin D Supplementation Can Affect Irisin Levels in Prader-Willi Syndrome. J. Endocrinol. Investig. 2021, 44, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.J.; Gross, I.; Pollak, Y.; Eldar-Geva, T.; Gross-Tsur, V. Irisin and the Metabolic Phenotype of Adults with Prader-Willi Syndrome. PLoS ONE 2015, 10, e0136864. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.J.; Tritos, N.A.; Samson, S.L.; Hoffman, A.R.; Katznelson, L. Amereican association of clinical endocrinologists and American Collage of Endocrinology disease state clinical review: Update on growth hormone stimulation testing and proposed revised cut point for the glucagon stimulation test in the diagnosis of adult growth hormone deficiency. Endocr. Pract. 2016, 22, 1235–1244. [Google Scholar] [PubMed]
- Schmidt, R.T.; Toews, J.V. Grip Strength as Measured by the Jamar Dynamometer. Arch. Phys. Med. Rehabil. 1970, 51, 321–327. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The Timed Up & Go: A Test of Basic Functional Mobility for Frail Elderly Persons. JGS J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Berg, K.; Wood-Dauphine, S. Measuring Balance in the Elderly: Preliminary Development of an Instrument. Physiother. Can. 1989, 6, 304–311. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Irizarry, K.A.; Miller, M.; Freemark, M.; Haqq, A.M. Prader Willi Syndrome: Genetics, metabolomics, hormonal function, and new approaches to therapy. Adv. Pediatrics 2016, 63, 47–77. [Google Scholar] [CrossRef] [Green Version]
- Sjöström, A.; Höybye, C. Twenty Years of GH Treatment in Adults with Prader-Willi Syndrome. JCM J. Clin. Med. 2021, 10, 2667. [Google Scholar] [CrossRef]
- Frixou, M.; Vlek, D.; Lucas-Herald, A.K.; Keir, L.; Kyriakou, A.; Shaikh, M.G. The Use of Growth Hormone Therapy in Adults with PraderWilli Syndrome: A Systematic Review. Clin. Endocrinol. 2021, 94, 645–655. [Google Scholar] [CrossRef]
- Butler, M.G.; Smith, B.K.; Lee, J.; Gibson, C.; Schmoll, C.; Moore, W.V.; Donnelly, J.E. Effects of Growth Hormone Treatment in Adults with Prader-Willi Syndrome. YGHIR Growth Horm. IGF Res. 2013, 23, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Mac Dermid, J.C.; Fehr, L.B.; Lindsay, K.C. The Effect of Physical Factors on Grip Strength and Dexterity. Br. J. Hand Ther. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Hsu, W.L.; Chiu, V.J.Y.; Chang, W.H.; Lin, M.C.; Wei, J.T.; Tzeng, I.S. Hand Strength and Dexterity in Patients with Prader-Willi Syndrome: A Pilot Intervention Study. J. Int. Med. Res. 2018, 46, 4669–4677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykens, E.M. Maladaptive and Compulsive Behavior in Prader-Willi Syndrome: New Insights From Older Adults. Am. J. Ment. Retard. 2004, 109, 142. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Capodaglio, P.; Menegoni, F.; Vismara, L.; Cimolin, V.; Grugni, G.; Galli, M. Characterisation of Balance Capacity in Prader Willi Patients. Res. Dev. Disabil. 2011, 32, 81–86. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85. [Google Scholar] [CrossRef]
- Bennie, S.; Bruner, K.; Dizon, A.; Fritz, H.; Goodman, B.; Peterson, S. Measurements of Balance: Comparison of the Timed “Up and Go” Test and Functional Reach Test with the Berg Balance Scale. J. Phys. Ther. Sci. 2003, 15, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.; Wood-Dauphinée, S.; Williams, J.I.; Maki, B. Measuring Balance in the Elderly: Validation of an Instrument. Can. J. Public Health Rev. Can. De Sante Publique 1992, 83, S7–S11. [Google Scholar]
- Titomanlio, L.; De Brasi, D.; Romano, A.; Genesio, R.; Diano, A.; Giudice, E. Partial cerebellar hypoplasia in a patient with Prader-Willi syndrome. Acta Paediatr. 2006, 95, 861–863. [Google Scholar] [CrossRef]
- Miller, J.; Couch, J.; Schwenk, K.; Long, M.; Towler, S.; Theriaque, D.; He, G.; Liu, Y.; Driscoll, D.; Leonard, C. Early Childhood Obesity Is Associated With Compromised Cerebellar Development. Dev. Neuropsychol. 2009, 34, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Watanabe, M.; Suzuki, K.; Suzuki, Y. Cerebellar Volumes Associate with Behavioral Phenotypes in Prader-Willi Syndrome. Cerebellum 2020, 19, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Jin, D.K.; Cho, S.S.; Kim, J.H.; Hong, S.D.; Paik, K.H.; Oh, Y.J.; Kim, A.H.; Kwon, E.K.; Choe, Y.H. Regional Cerebral Glucose Metabolic Abnormality in Prader-Willi Syndrome: A 18F-FDG PET Study under Sedation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006, 47, 1088–1092. [Google Scholar]
- Stoodley, C.J.; Schmahmann, J.D. Functional Topography of the Human Cerebellum. Handb. Clin. Neurol. 2018, 154, 59–70. [Google Scholar] [PubMed]
- Martínez-Moreno, C.G.; Calderón-Vallejo, D.; Harvey, S.; Aramburo, C.; Quintanar, J.L. Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? Int. J. Mol. Sci. 2018, 19, 375. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, X.; Chen, X.; Chen, T.; Ye, P.; Jiang, L.; Fu, Y.; Xie, X.; Shan, X.; Yan, Z. Differences in the Functional Connectivity Density of the Brain between Individuals with Growth Hormone Deficiency and Idiopathic Short Stature. PNEC Psychoneuroendocrinol. 2019, 103, 67–75. [Google Scholar] [CrossRef]
- Annerén, G.; Tuvemo, T.; Gustafsson, J. Growth Hormone Therapy in Young Children with Down Syndrome and a Clinical Comparison of Down and Prader-Willi Syndromes. Growth Horm. IGF Res. 2000, 10 (Suppl. B), S87–S91. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Gross-Tsur, V.; Sabag, Y.; Nice, S.; Genstil, L.; Benarroch, F.; Constantini, N. Myokine Levels after Resistance Exercise in Young Adults with Prader-Willi Syndrome (PWS). Am. J. Med. Genet. Part A 2020, 182, 115–121. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metab. Clin. Exp. 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Roca-Rivada, A.; Castelao, C.; Landrove, M.O.; Pardo, M.; Senin, L.L.; Seoane, L.M.; Crujeiras, A.B.; Casanueva, F.F.; Baltar, J. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caixàs, A.; Giménez-Palop, O.; Broch, M.; Vilardell, C.; Megía, A.; Simón, I.; Jiménez-Pérez, G.; Mauricio, D.; Vendrell, J.; Richard, C.; et al. Adult Subjects with Prader-Willi Syndrome Show More Low-Grade Systemic Inflammation than Matched Obese Subjects. J. Endocrinol. Investig. 2008, 31, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.; Gabarrós, A.; Deus, J.; Juncadella, M.; Acebes, J.J.; Torres, A.; Pujol, J. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience 2011, 179, 131–142. [Google Scholar] [CrossRef]
- Pujol, J.; Conesa, G.; Deus, J.; López-Obarrio, L.; Isamat, F.; Capdevila, A. Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. J. Neurosurg. 1998, 88, 863–869. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Pujol, J.; Macià, D.; Blanco-Hinojo, L.; Martínez-Vilavella, G.; Sunyer, J.; de la Torre, R.; Caixàs, A.; Martín-Santos, R.; Deus, J.; Harrison, B.J. Does motion-related brain functional connectivity reflect both artifacts and genuine neural activity? Neuroimage 2014, 101, 87–95. [Google Scholar] [CrossRef]
| Control Group (n = 22) Median (IQR) | PWS Group (n = 27) Median (IQR) | p-Value * | |
|---|---|---|---|
| Weight (kg) | 65.9 (58.8–71.3) | 89.6 (70.5–105.5) | <0.0001 |
| BMI (kg/m2) | 22.3 (21.2–22.6) | 34.5 (31.1–41.3) | <0.0001 |
| WC (cm) | 80 (72–83) | 110 (101–124) | <0.0001 |
| IGF-I (ng/mL) | 226 (193–304) | 143 (95–188) | <0.0001 |
| Glucose (mmol/L) | 4.36 (4.16–4.88) | 5.0 (4.55–6.99) | 0.0039 |
| HbA1c (%) | 5.1 (4.9–5.3) | 5.6 (5.3–6.9) | 0.0002 |
| Irisin (ng/mL) | 89.8 (41.8–219.4) | 982.3 (519.4–1789.6) | <0.0001 |
| Myostatin (ng/mL) | 5.44 (3.05–8.1) | 4.8 (2.95–7.72) | 0.6714 |
| IL-6 (pg/mL) | 11.5 (1.69–44.7) | 15.4(3.58–66.4) | 0.6191 |
| Fat mass (%) | 30.8 (28.4–39.0) | 56.3 (49.3–61.1) | <0.0001 |
| Lean mass (%) | 69.2 (61.0–71.6) | 43.7 (38.9–50.1) | <0.0001 |
| ALM/weight | 0.30 (0.26–0.32) | 0.19 (0.16–0.21) | <0.0001 |
| ALM/BMI | 0.80 (0.70–1.02) | 0.48 (0.38–0.55) | <0.0001 |
| Handgrip strength (kg) | 31 (28–39) | 18 (13–22) | <0.0001 |
| BBS (points) | 56 (55–56) | 53 (49–54) | <0.0001 |
| TUG (seconds) | 5.16 (4.74–5.45) | 8.76 (7.56–11.9) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casamitjana, L.; Blanco-Hinojo, L.; Giménez-Palop, O.; Pujol, J.; Martínez-Vilavella, G.; Esteba-Castillo, S.; Pareja, R.; Freijo, V.; Vigil, L.; Deus, J.; et al. One Year of Recombinant Human Growth Hormone Treatment in Adults with Prader–Willi Syndrome Improves Body Composition, Motor Skills and Brain Functional Activity in the Cerebellum. J. Clin. Med. 2022, 11, 1831. https://doi.org/10.3390/jcm11071831
Casamitjana L, Blanco-Hinojo L, Giménez-Palop O, Pujol J, Martínez-Vilavella G, Esteba-Castillo S, Pareja R, Freijo V, Vigil L, Deus J, et al. One Year of Recombinant Human Growth Hormone Treatment in Adults with Prader–Willi Syndrome Improves Body Composition, Motor Skills and Brain Functional Activity in the Cerebellum. Journal of Clinical Medicine. 2022; 11(7):1831. https://doi.org/10.3390/jcm11071831
Chicago/Turabian StyleCasamitjana, Laia, Laura Blanco-Hinojo, Olga Giménez-Palop, Jesús Pujol, Gerard Martínez-Vilavella, Susanna Esteba-Castillo, Rocío Pareja, Valentín Freijo, Laura Vigil, Joan Deus, and et al. 2022. "One Year of Recombinant Human Growth Hormone Treatment in Adults with Prader–Willi Syndrome Improves Body Composition, Motor Skills and Brain Functional Activity in the Cerebellum" Journal of Clinical Medicine 11, no. 7: 1831. https://doi.org/10.3390/jcm11071831
APA StyleCasamitjana, L., Blanco-Hinojo, L., Giménez-Palop, O., Pujol, J., Martínez-Vilavella, G., Esteba-Castillo, S., Pareja, R., Freijo, V., Vigil, L., Deus, J., & Caixàs, A. (2022). One Year of Recombinant Human Growth Hormone Treatment in Adults with Prader–Willi Syndrome Improves Body Composition, Motor Skills and Brain Functional Activity in the Cerebellum. Journal of Clinical Medicine, 11(7), 1831. https://doi.org/10.3390/jcm11071831






