Clinical Characteristics of Anti-TIF-1γ Antibody-Positive Dermatomyositis Associated with Malignancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Tests
2.2. Evaluation of High-Resolution Computed Tomography (HRCT) Findings and Patterns
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Laboratory, Pulmonary Function Test, and Computed Tomography (CT) Findings
3.3. Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodgkinson, L.M.; Wu, T.T.; Fiorentino, D.F. Dermatomyositis Autoantibodies: How Can We Maximize Utility? Ann. Transl. Med. 2021, 9, 433. [Google Scholar] [CrossRef]
- Targoff, I.N.; Mamyrova, G.; Trieu, E.P.; Perurena, O.; Koneru, B.; O’Hanlon, T.P.; Miller, F.W.; Rider, L.G. A Novel to a 155-Kd Protein Is Associated with Dermatomyositis. Arthritis Rheum. 2006, 54, 3682–3689. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Watanabe, R.; Ishitsuka, Y.; Okiyama, N. Recent Advances in Dermatomyositis-Specific Autoantibodies. Curr. Opin. Rheumatol. 2016, 28, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Bonroy, C.; Piette, Y.; Allenbach, Y.; Bossuytf, X.; Damoiseaux, J. Positioning of myositis-specific and associated autoantibody (MSA/MAA) testing in disease criteria and routine diagnostic work-up. J. Transl. Autoimmun. 2022, 5, 100148. [Google Scholar] [CrossRef]
- Hozumi, H.; Fujisawa, T.; Nakashima, R.; Johkoh, T.; Sumikawa, H.; Murakami, A.; Enomoto, N.; Inui, N.; Nakamura, Y.; Hosono, Y.; et al. Comprehensive Assessment of Myositis-Specific Autoantibodies in Polymyositis/Dermatomyositis-Associated Interstitial Lung. Disease. Respir. Med. 2016, 121, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Vinik, O. Dermatomyositis and Malignancy. Can. Fam. Physician 2019, 65, 409–411. [Google Scholar] [PubMed]
- Bohan, A.; Peter, J.B. Polymyositis and Dermatomyositis (First of Two Parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Fujimoto, M.; Hasegawa, M.; Kondo, M.; Saito, Y.; Komura, K.; Matsushita, T.; Orito, H.; Hamaguchi, Y.; Yanaba, K.; et al. Identification of a Novel Autoantibody Reactive with 155 and 140-kDa Nuclear Proteins in Patients with Dermatomyositis: An Association with Malignancy. Rheumatology 2007, 46, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, S. TRIM Proteins and Cancer. Nat. Rev. Cancer. 2011, 11, 792–804. [Google Scholar] [CrossRef]
- Yu, C.; Ding, Z.; Liang, H.; Zhang, B.; Chen, X. The Roles of TIF1γ in Cancer. Front. Oncol. 2019, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, Y.; Tonomura, K.; Fujimoto, M. Transcriptional Intermediary factor 1 (TIF1) and Anti-TIF1γ Antibody-Positive Dermatomyositis. Immunol. Med. 2021, 44, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Venturini, L.; You, J.; Stadler, M.; Galien, R.; Lallemand, V.; Koken, M.H.; Mattei, M.G.; Ganser, A.; Chambon, P.; Losson, R.; et al. TIF1gamma, a Novel Member of the Transcriptional Intermediary factor 1 Family. Oncogene 1999, 18, 1209–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.L.; Zhang, Y.; Sigurgeirsson, B.; Pukkala, E.; Mellemkjaer, L.; Airio, A.; Evans, S.R.; Felson, D.T. Frequency of Specific Cancer Types in Dermatomyositis and Polymyositis: A Population-Based Study. Lancet 2001, 357, 96–100. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Wu, H.; Zhao, N.; Tang, Y.; Li, X.; Liang, Y. Characteristics and Predictors of Malignancy in Dermatomyositis: Analysis of 239 Patients from Northern China. Oncol. Lett. 2018, 16, 5960–5968. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.L.; Chen, Y.J.; Lin, M.W.; Wu, C.Y.; Liu, P.C.; Chen, T.J.; Chen, Y.C.; Jih, J.S.; Chen, C.C.; Lee, D.D.; et al. Malignancies Associated with Dermatomyositis and Polymyositis in Taiwan: A Nationwide Population-Based Study. Br. J. Dermatol. 2009, 161, 854–860. [Google Scholar] [CrossRef]
- Antiochos, B.B.; Brown, L.A.; Li, Z.; Tosteson, T.D.; Wortmann, R.L.; Rigby, W.F.C. Malignancy Is Associated with Dermatomyositis but Not Polymyositis in Northern New England, USA. J. Rheumatol. 2009, 36, 2704–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dankó, K.; Ponyi, A.; Molnar, A.P.; András, C.; Constantin, T. Paraneoplastic Myopathy. Curr. Opin. Rheumatol. 2009, 21, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, J.; Ghoreschi, K.; Volc, S. Characteristics of Dermatomyositis Patients with and without Associated Malignancy. J. Dtsch. Dermatol. Ges. 2021, 19, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Muro, Y.; Sugiura, K.; Akiyama, M. Cutaneous Manifestations in Dermatomyositis: Key Clinical and Serological Features—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 293–302. [Google Scholar] [CrossRef]
- Daly, M.L.; Gordon, P.A.; Creamer, D. Cutaneous Features of Dermatomyositis Associated with Myositis-Specific Antibodies. Br. J. Dermatol. 2017, 176, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Al Awqati, M.Z.; Sluzevich, J.C.; Berianu, F. Ulcerative Paraneoplastic Dermatomyositis in the Setting of Positive Transcriptional Intermediary factor 1-γ Antibody. J. Rheumatol. 2021, 48, 1340. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Kuo, K.; Chung, L.; Zaba, L.; Li, S.; Casciola-Rosen, L. Distinctive Cutaneous and Systemic Features Associated with Antitranscriptional Intermediary factor-1γ Antibodies in Adults with Dermatomyositis. J. Am. Acad. Dermatol. 2015, 72, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hida, A.; Yamashita, T.; Hosono, Y.; Inoue, M.; Kaida, K.; Kadoya, M.; Miwa, Y.; Yajima, N.; Maezawa, R.; Arai, S.; et al. Anti-TIF1-γ Antibody and Cancer-Associated Myositis: A Clinicohistopathologic Study. Neurology 2016, 87, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Masiak, A.; Kulczycka, J.; Czuszyńska, Z.; Zdrojewski, Z. Clinical Characteristics of Patients with Anti-TIF1-γ Antibodies. Reumatologia 2016, 54, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Ihn, H.; Yamane, K.; Kikuchi, K.; Yazawa, N.; Soma, Y.; Tamaki, K. Serum KL-6 in adult patients with polymyositis and dermatomyositis. Rheumatology 2000, 39, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, A. The Value of Intravenous Immunoglobulin Therapy in Idiopathic Inflammatory Myositis in the Current Transformed Era of Biologics. Cureus 2020, 12, e7049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorizzo, L.J., III; Jorizzo, J.L. The Treatment and Prognosis of Dermatomyositis: An Updated Review. J. Am. Acad. Dermatol. 2008, 59, 99–112. [Google Scholar] [CrossRef]
- Kovacs, S.O.; Kovacs, S.C. Dermatomyositis. J. Am. Acad. Dermatol. 1998, 39, 899–922. [Google Scholar] [CrossRef]


| ARS (47) | MDA-5 (24) | TIF-1γ (14) | p-Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (years) | 59.7 ± 10.2 | 53.9 ± 11.9 | 68.6 ± 10.7 | 0.001 * |
| PM/DM/CADM | 9/29/9 | 0/13/11 | 0/13/1 | 0.001 * |
| Sex (male/female) | 10/37 | 8/16 | 5/9 | 0.435 |
| Duration from onset to first visit (days) | 226.6 ± 422.18 | 92 ± 182.06 | 119.36 ± 155.87 | 0.535 |
| Duration from first visit to treatment initiation (days) | 131.45 ± 609.84 | 9.08 ± 10.75 | 121.5 ± 437.31 | 0.736 |
| Outcome (death/alive) | 45/2 | 14/10 | 12/2 | 0.812 |
| Malignacy | 5 (11%) | 0 (0%) | 12 (86%) | <0.001 * |
| Clinical Symptoms at the onset | ||||
| Dyspnea on effort | 26 (55%) | 13 (52%) | 0 (0%) | <0.001 * |
| Fever | 14 (30%) | 13 (52%) | 2 (14%) | 0.068 |
| Myalgia | 19 (40%) | 11 (46%) | 3 (21%) | 0.130 |
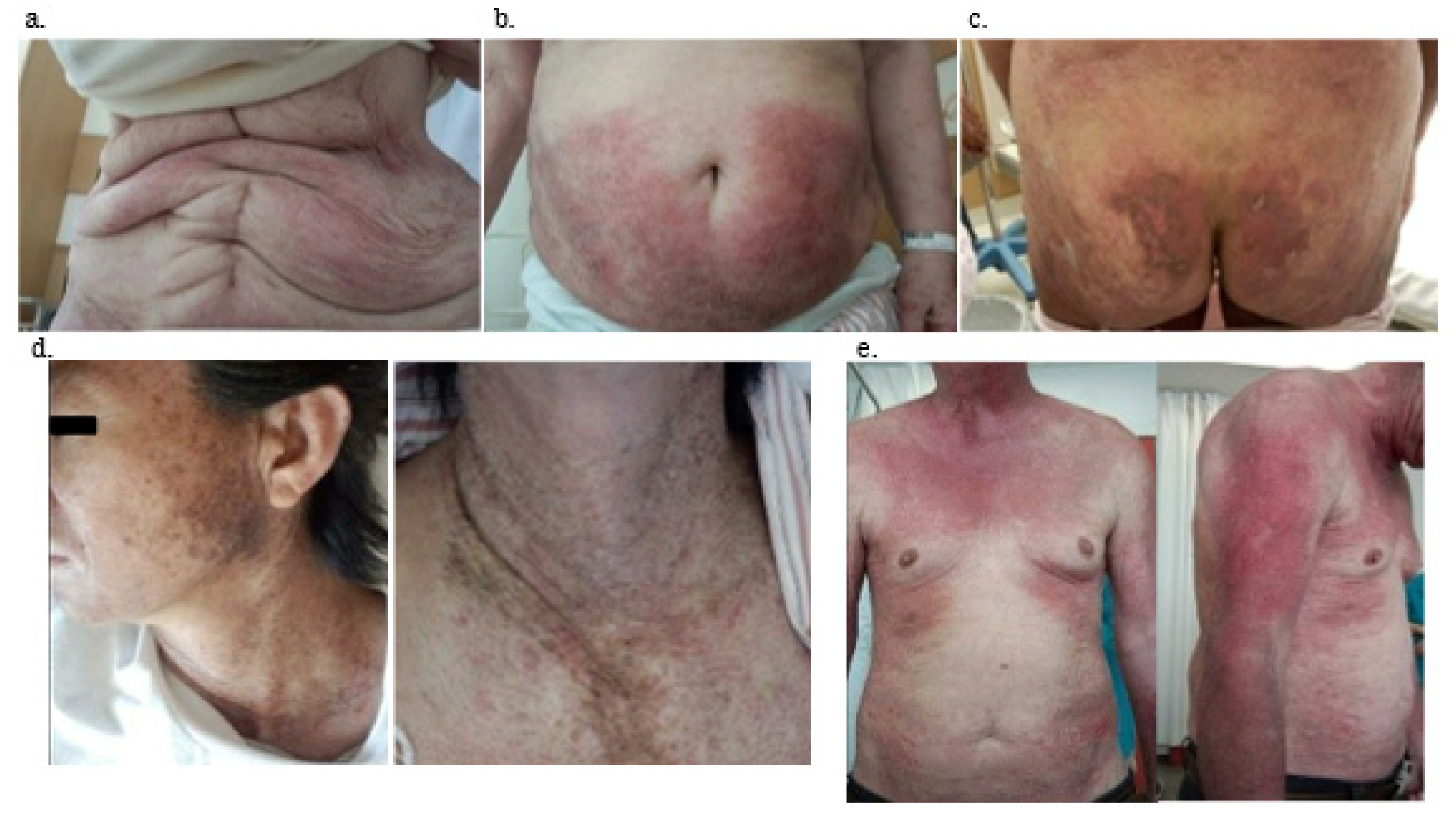
| Skin manifestation | 10 (21%) | 14 (58%) | 14 (100%) | <0.001 |
| Dysphagia | 0 (0%) | 0 (0%) | 8 (71%) | <0.001 * |
| ARS (47) | MDA-5 (24) | TIF-1γ (14) | p-Value | |
| Physical examination | ||||
| V-neck sign (shawl sign) | 1 (2%) | 5 (21%) | 8 (57%) | <0.001 * |
| Raynaud phenomenon | 7 (15%) | 2 (8%) | 0 (0%) | 0.063 |
| Gottron’s sign | 20 (43%) | 24 (100%) | 6 (43%) | 0.223 |
| Reverse Gottron’s sign | 2 (4%) | 17 (71%) | 21(3%) | 0.752 |
| Arthralgia | 20 (43%) | 16 (67%) | 0 (0%) | 0.080 |
| Erythema | 3 (6%) | 2 (8%) | 9 (64%) | <0.001 * |
| Mechanics hand | 21 (45%) | 15 (62%) | 4 (29%) | 0.083 |
| Heliotrope | 3 (6%) | 20 (83%) | 9 (64%) | 0.020 |
| Nailfold bleeding | 12 (26%) | 16 (67%) | 14 (100%) | <0.001 * |
| Muscle weakness | 16 (34%) | 18 (75%) | 9 (64%) | 0.301 |
| Serological examination | ||||
| CRP (mg/dL) | 2.37 ± 5.63 | 0.64 ± 0.84 | 0.5 ± 0.6 | 0.176 |
| LDH (IU/L) | 375.0 ± 107.4 | 393.0 ± 134.0 | 362 ± 108 | 0.476 |
| CK (mg/dL) | 45 ± 485 | 338 ± 540 | 754 ± 822 | 0.804 |
| KL-6 (U/mL) | 1327.2 ± 493.1 | 686.0 ± 489.6 | 209 ±56 | 0.110 |
| Ferritin (ng/mL) | 306.7 ± 114.9 | 938.9 ± 915 | 3875 ± 2646 | <0.001 * |
| Radiological findings | ||||
| ILA on HRCT | 47 (100%) | 24 (100%) | 0 (0%) | <0.001 * |
| Sex | Age | Skin Lesion | Cancer Lesion | Treatment | Primary Lesion | Overall Outcome | |
|---|---|---|---|---|---|---|---|
| 1 | F | 71 | improved | PD | PSL | uterus | worse |
| 2 | F | 54 | improved | CR | PSL→PSL + IVIG | uterus | worse |
| 3 | M | 62 | unknown | no treatment | none | unknown | unknown |
| 4 | F | 74 | deteriorated | PD | PSL + Tac | lung | death |
| 5 | M | 79 | unknown | no treatment | PSL | stomach | death |
| 6 | F | 60 | improved | CR | PSL→PSL + Tac→PSL + AZP→PSL + IVIG | breast | unknown |
| 7 | F | 64 | deteriorated | no treatment | PSL + Tac | unknown | unknown |
| 8 | F | 87 | improved | unknown | PSL | ovary | unknown |
| 9 | M | 54 | deteriorated | CR | PSL | colon | unknown |
| 10 | F | 70 | improved | PD | unknown | breast | better |
| 11 | F | 88 | deteriorated | PD | PSL→PSL + Tac | lung | death |
| 12 | F | 70 | improved | PD | PSL→PSL + Tac | colon | unchanged |
| 13 | M | 61 | improved | PR | PSL | lung | better |
| 14 | M | 67 | improved | PR | PSL + AZP | lymphoma | better |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, Y.; Tominaga, M.; Iitoh, E.; Kaieda, S.; Koga, T.; Fujimoto, K.; Chikasue, T.; Obara, H.; Kakuma, T.; Ida, H.; et al. Clinical Characteristics of Anti-TIF-1γ Antibody-Positive Dermatomyositis Associated with Malignancy. J. Clin. Med. 2022, 11, 1925. https://doi.org/10.3390/jcm11071925
Harada Y, Tominaga M, Iitoh E, Kaieda S, Koga T, Fujimoto K, Chikasue T, Obara H, Kakuma T, Ida H, et al. Clinical Characteristics of Anti-TIF-1γ Antibody-Positive Dermatomyositis Associated with Malignancy. Journal of Clinical Medicine. 2022; 11(7):1925. https://doi.org/10.3390/jcm11071925
Chicago/Turabian StyleHarada, Yumi, Masaki Tominaga, Eriko Iitoh, Shinjiro Kaieda, Takuma Koga, Kiminori Fujimoto, Tomonori Chikasue, Hitoshi Obara, Tatsuyuki Kakuma, Hiroaki Ida, and et al. 2022. "Clinical Characteristics of Anti-TIF-1γ Antibody-Positive Dermatomyositis Associated with Malignancy" Journal of Clinical Medicine 11, no. 7: 1925. https://doi.org/10.3390/jcm11071925
APA StyleHarada, Y., Tominaga, M., Iitoh, E., Kaieda, S., Koga, T., Fujimoto, K., Chikasue, T., Obara, H., Kakuma, T., Ida, H., Kawayama, T., & Hoshino, T. (2022). Clinical Characteristics of Anti-TIF-1γ Antibody-Positive Dermatomyositis Associated with Malignancy. Journal of Clinical Medicine, 11(7), 1925. https://doi.org/10.3390/jcm11071925






