Analysis of Yes-Associated Protein-1 (YAP1) Target Gene Signature to Predict Progressive Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Breast Cancer Dataset Selection
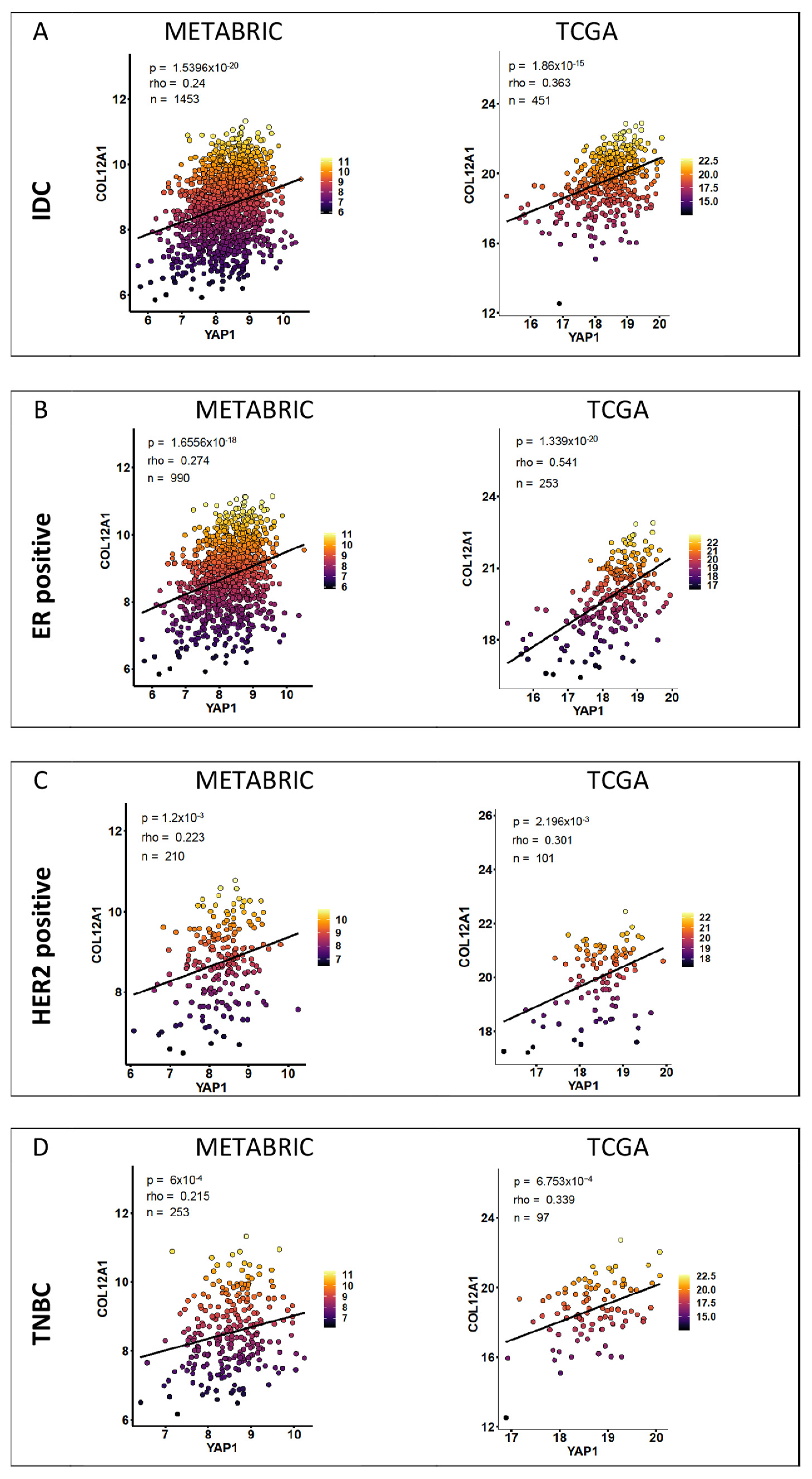
2.2. YAP Target Gene Expression Correlation
2.3. Correlation of YAP Target Genes and Clinical Parameters
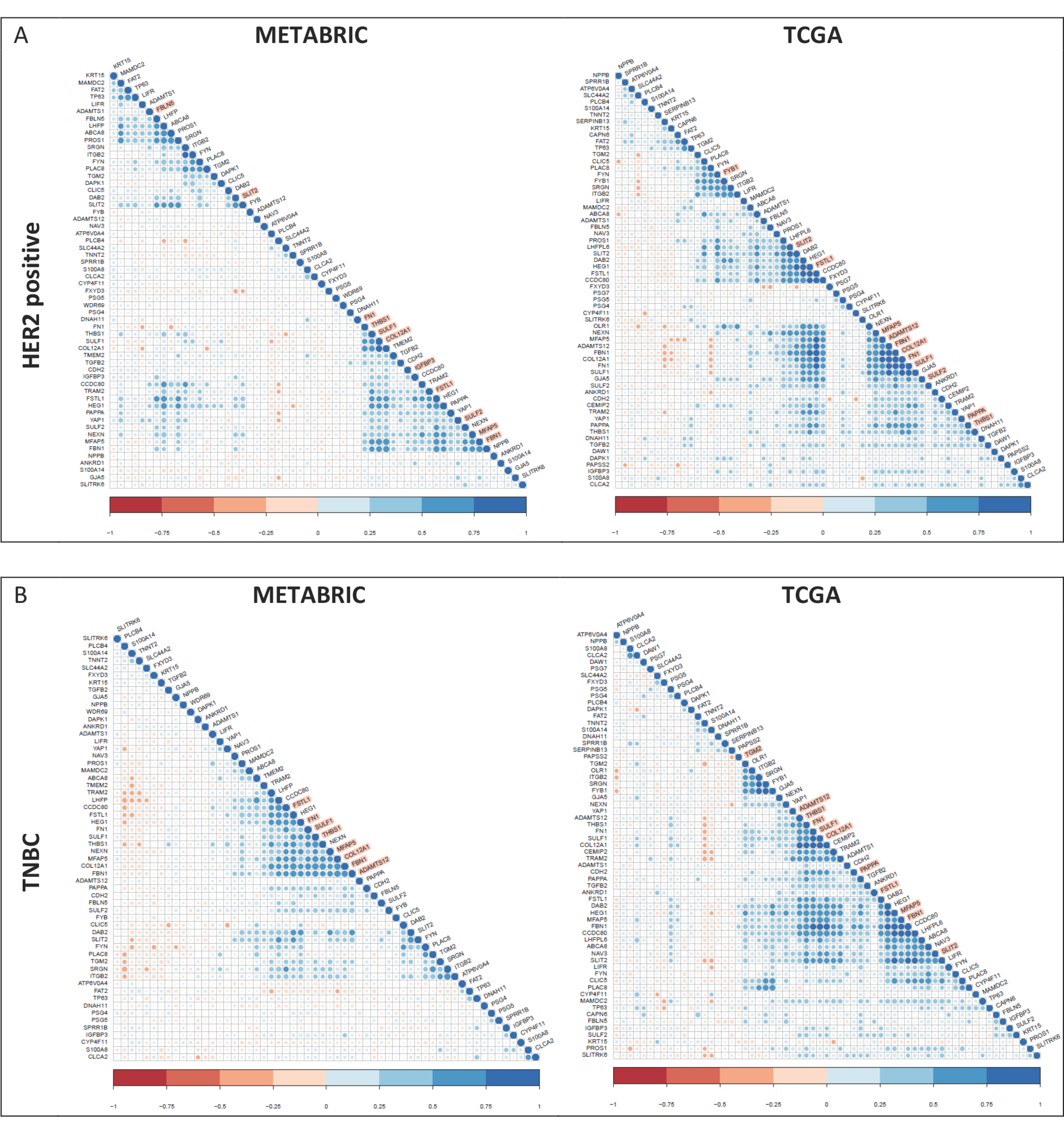
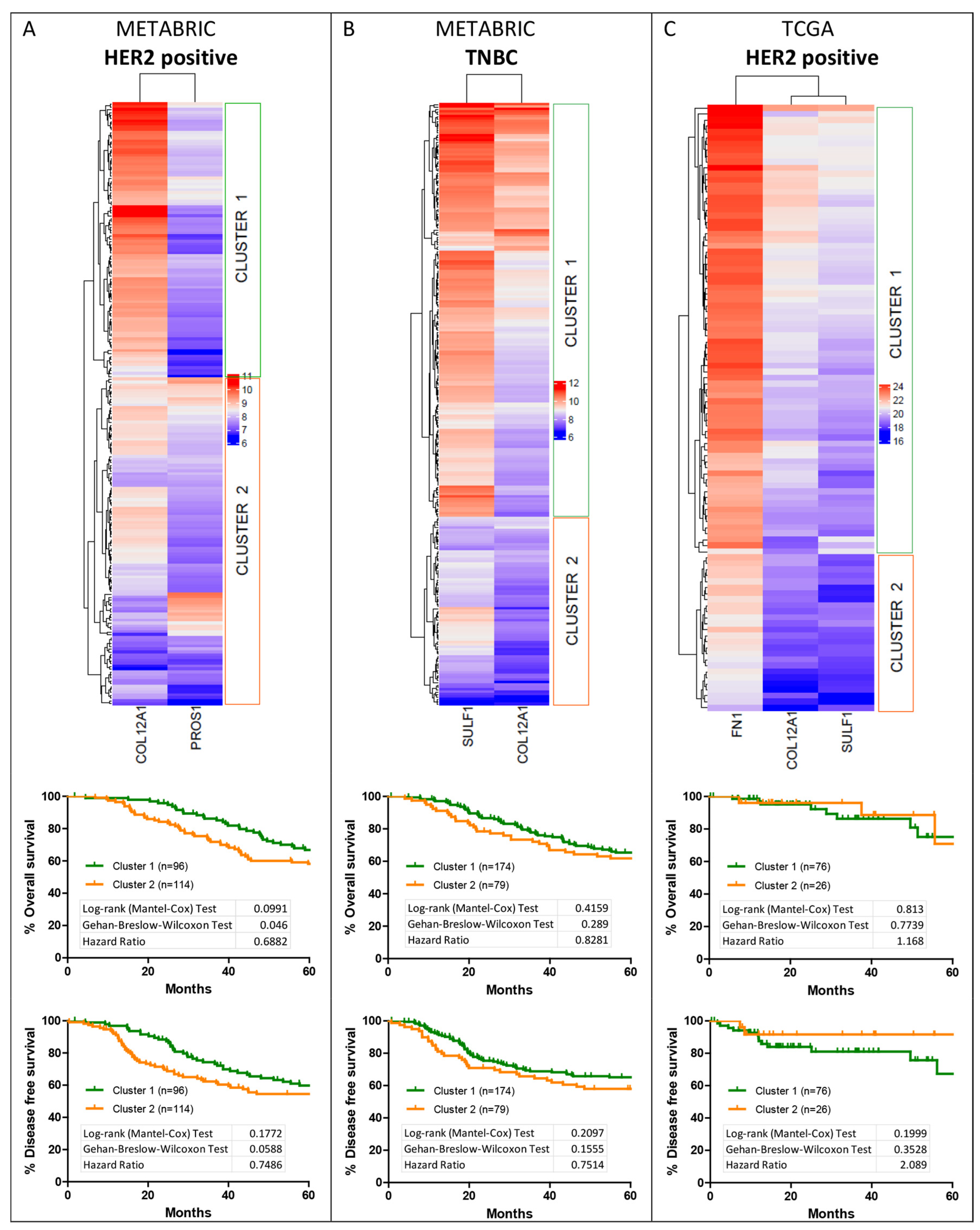
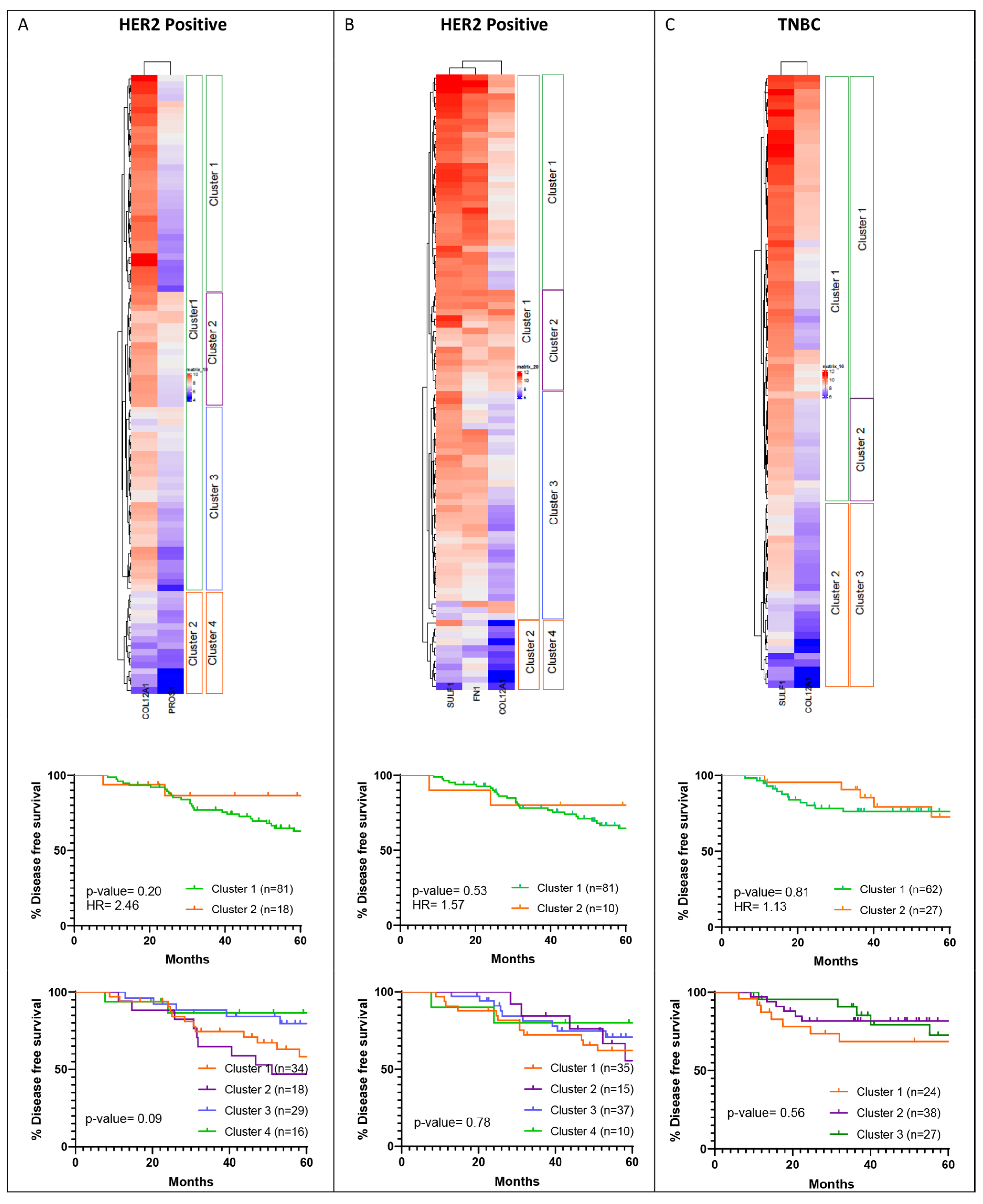
2.4. Cluster Analysis of Shortlisted YAP Signature Genes
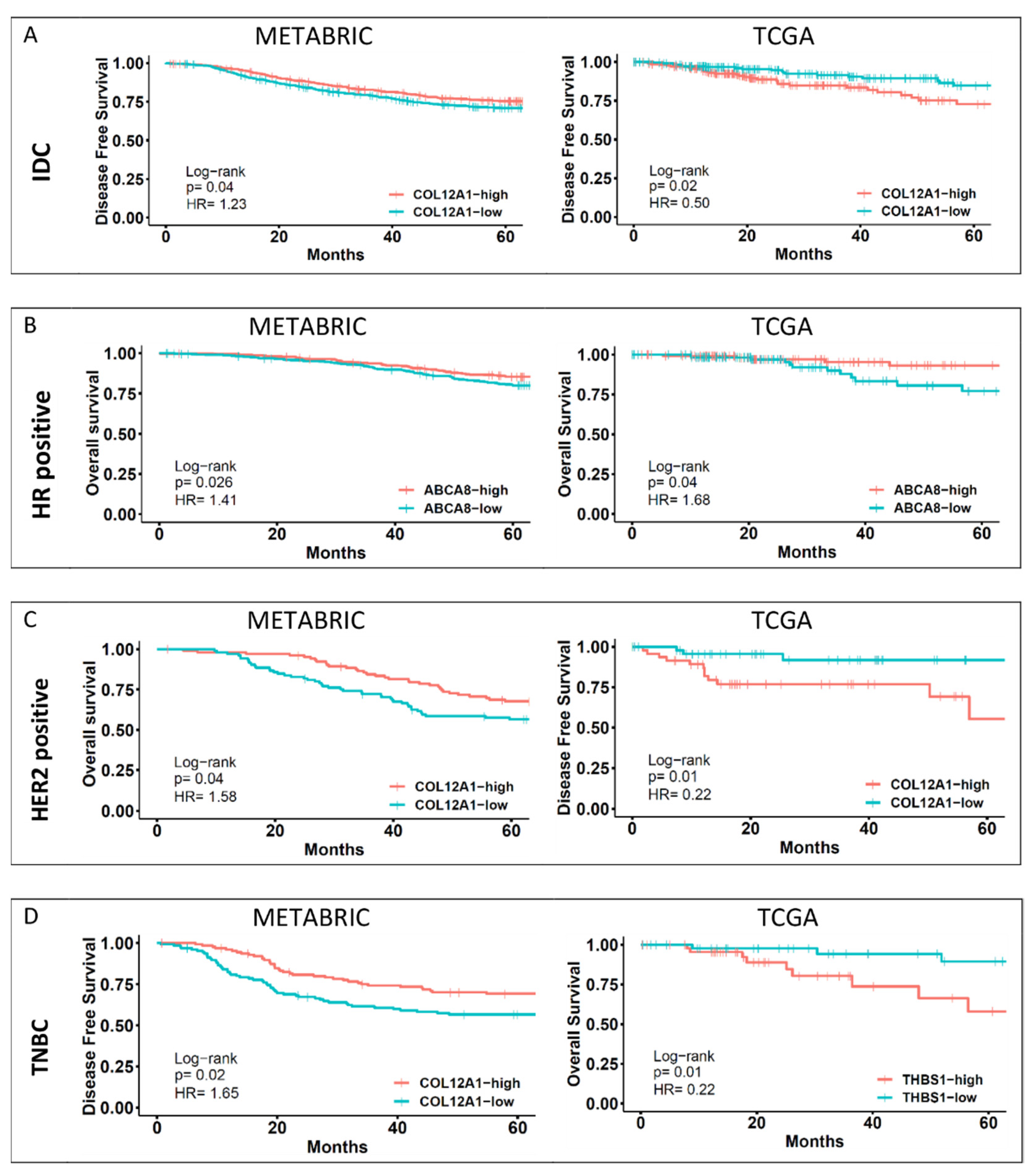
2.5. Survival Analysis
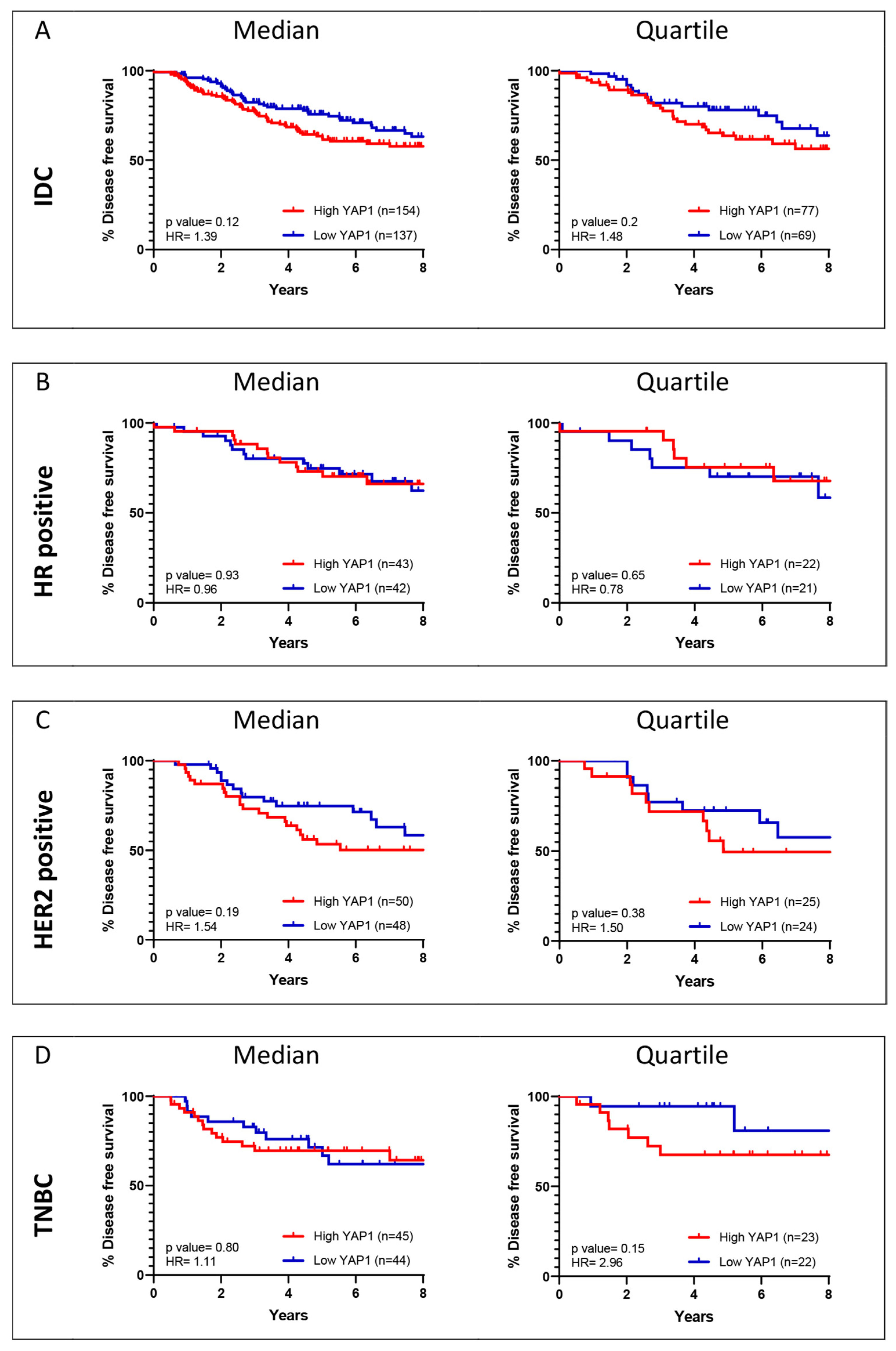
2.6. Survival Analysis for YAP1 Expression
2.7. Survival Analysis for YAP1 Target Gene Expression
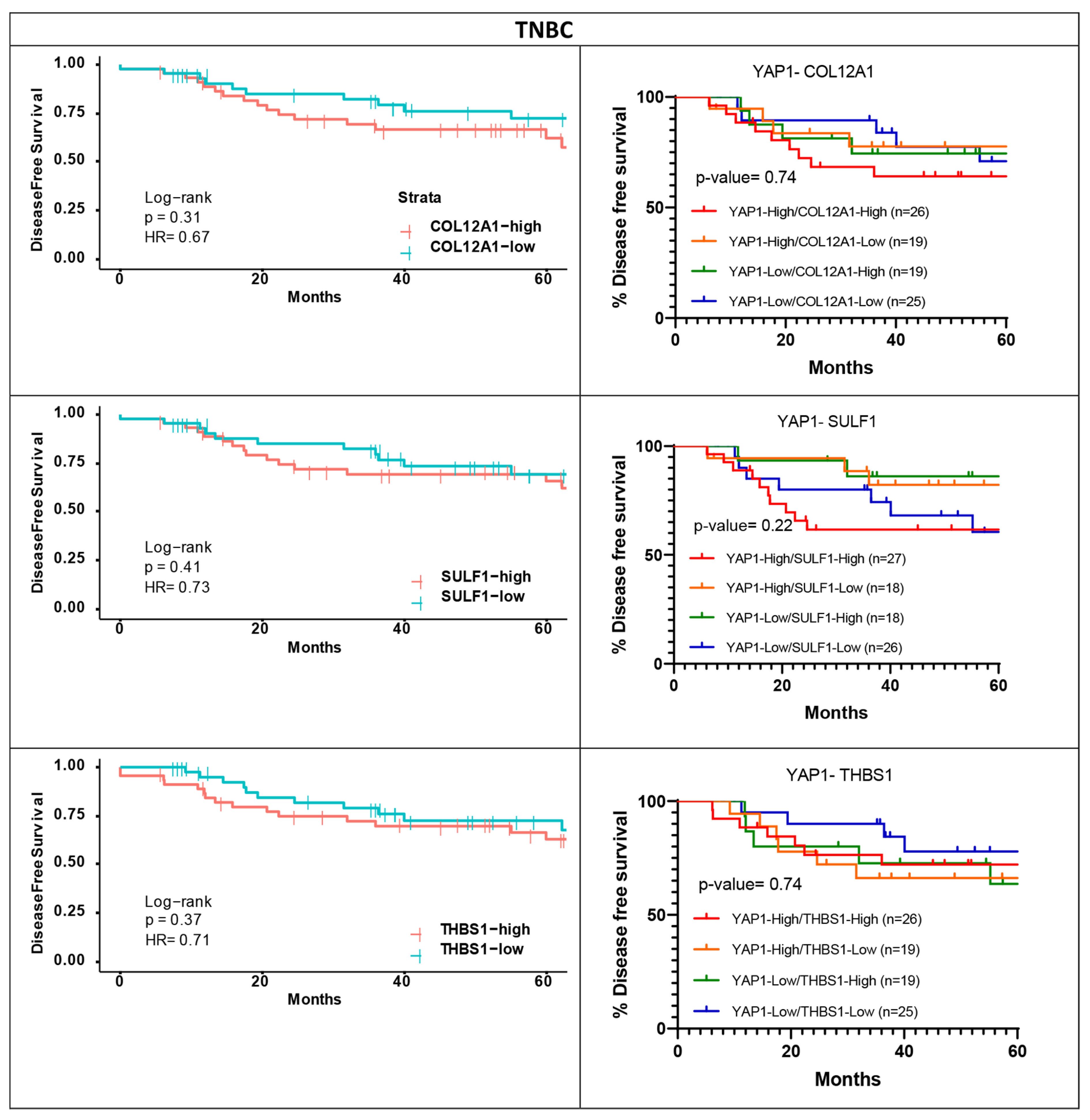
2.8. Survival Analysis for YAP1 and YAP1 Target Gene Expression
2.9. Survival Analysis for YAP Target Gene Cluster
2.10. Validation of Shortlisted Genes Using Independent Dataset
3. Results
3.1. Survival Analysis for YAP1 Gene Expression
3.2. YAP1 and YAP1 Target Gene Expression Correlation
3.3. YAP1 Target Genes and Association with Clinical Parameters
3.4. Survival Analysis
3.5. Hierarchical Clustering of Shortlisted Genes for Survival Analysis
3.6. Validation of the Shortlisted Genes in an Independent Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Vuong, D.; Simpson, P.; Green, B.; Cummings, M.; Lakhani, S.R. Molecular classification of breast cancer. Virchows Arch. 2014, 465, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Basso, S.M.M. Treatment of Estrogen Receptor-Positive Breast Cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for her2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, A.; Kelkar, D.A.; Parikh, N.; Shashidhara, L.S.; Koppiker, C.B.; Kulkarni, M. Meta-Analysis of Prevalence of Triple-Negative Breast Cancer and Its Clinical Features at Incidence in Indian Patients With Breast Cancer. JCO Glob. Oncol. 2020, 6, 1052–1062. [Google Scholar] [CrossRef]
- Stringer-Reasor, E.M.; Elkhanany, A.; Khoury, K.; Simon, M.A.; Newman, L.A. Disparities in Breast Cancer Associated With African American Identity. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e29–e46. [Google Scholar] [CrossRef]
- Bhattacharyya, G.S.; Doval, D.C.; Desai, C.J.; Chaturvedi, H.; Sharma, S.; Somashekhar, S. Overview of Breast Cancer and Implications of Overtreatment of Early-Stage Breast Cancer: An Indian Perspective. JCO Glob. Oncol. 2020, 6, 789–798. [Google Scholar] [CrossRef]
- Steinhardt, A.A.; Gayyed, M.F.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589. [Google Scholar] [CrossRef] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, X.; Maglic, D.; Dill, M.T.; Mojumdar, K.; Ng, P.K.-S.; Jeong, K.J.; Tsang, Y.H.; Moreno, D.; Bhavana, V.H.; et al. Comprehensive Molecular Characterization of the Hippo Signaling Pathway in Cancer. Cell Rep. 2018, 25, 1304–1317.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, M.; Ham, K.; Hoque, M.O. A time for YAP1: Tumorigenesis, immunosuppression and targeted therapy. Int. J. Cancer 2018, 143, 2133–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015, 17, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Lamar, J.M.; Stern, P.; Liu, H.; Schindler, J.W.; Jiang, Z.-G.; Hynes, R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. USA 2012, 109, E2441–E2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-Y.; Li, Y.-H.; Lin, H.-X.; Liao, Y.-J.; Mai, S.-J.; Liu, Z.-W.; Zhang, Z.-L.; Jiang, L.-J.; Zhang, J.-X.; Kung, H.-F.; et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer 2013, 13, 349. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-X.; Bai, E.; Jin, G.-H.; He, C.-C.; Guo, X.-J.; Wang, L.-J.; Li, M.; Ying, X.; Zhu, Q. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J. Immunol. Res. 2014, 2014, 261365. [Google Scholar] [CrossRef]
- Lee, K.-W.; Lee, S.S.; Kim, S.-B.; Sohn, B.H.; Lee, H.-S.; Jang, H.-J.; Park, Y.-Y.; Kopetz, S.; Kim, S.S.; Oh, S.C.; et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin. Cancer Res. 2014, 21, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Cheong, J.-H.; Kim, H.; Noh, S.H.; Kim, H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012, 32, 3827–3834. [Google Scholar]
- Wang, Y.; Dong, Q.; Zhang, Q.; Li, Z.; Wang, E.; Qiu, X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010, 101, 1279–1285. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Luo, J.; Zheng, J.; Shao, G. YAP1 overexpression is associated with poor prognosis of breast cancer patients and induces breast cancer cell growth by inhibiting PTEN. FEBS Open Bio 2019, 9, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, M.; Tan, T.Z.; Sulaiman, N.B.S.; Lamar, J.M.; Bansal, P.; Cui, J.; Qiao, Y.; Ito, Y. RUNX1 and RUNX3 protect against YAP-mediated EMT, stemness and shorter survival outcomes in breast cancer. Oncotarget 2018, 9, 14175–14192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Lim, D.-S. The SRF-YAP-IL6 axis promotes breast cancer stemness. Cell Cycle 2016, 15, 1311–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri-Saccà, M.; Barba, M.; Pizzuti, L.; Vici, P.; Di Lauro, L.; Dattilo, R.; Vitale, I.; Bartucci, M.; Mottolese, M.; De Maria, R. The Hippo transducers TAZ and YAP in breast cancer: Oncogenic activities and clinical implications. Exp. Rev. Mol. Med. 2015, 17, e14. [Google Scholar] [CrossRef]
- Andrade, D.; Mehta, M.; Griffith, J.; Panneerselvam, J.; Srivastava, A.; Kim, T.-D.; Janknecht, R.; Herman, T.; Ramesh, R.; Munshi, A. YAP1 inhibition radiosensitizes triple negative breast cancer cells by targeting the DNA damage response and cell survival pathways. Oncotarget 2017, 8, 98495–98508. [Google Scholar] [CrossRef] [Green Version]
- Sebio, A.; Lenz, H.-J. Molecular pathways: Hippo signaling, a critical tumor suppressor. Clin. Cancer Res. 2015, 21, 5002–5007. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Feng, J.; Chen, C. Hippo pathway in mammary gland development and breast cancer. Acta Biochim. Biophys. Sin. 2014, 47, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Cao, B.; Wang, Y.; Ma, C.; Zeng, Z.; Liu, L.; Li, X.; Tao, D.; Gong, J.; Xie, D. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 175. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, M.; Xu, X.; Zhang, L.; Huang, Y.; Xu, Z.; He, K.; Wang, H.; Wang, H.; Teng, L. COL12A1, a novel potential prognostic factor and therapeutic target in gastric cancer. Mol. Med. Rep. 2019, 20, 3103–3112. [Google Scholar] [CrossRef] [Green Version]
- Verghese, E.T.; Drury, R.; Green, C.A.; Holliday, D.L.; Lu, X.; Nash, C.; Speirs, V.; Thorne, J.L.; Thygesen, H.H.; Zougman, A.; et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J. Pathol. 2013, 231, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Mercatelli, D.; Ray, F.; Giorgi, F.M. Pan-cancer and single-cell modelling of genomic alterations through gene expression. Front. Genet. 2019, 10, 671. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lichtenberg, T.M.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416. [Google Scholar] [CrossRef] [Green Version]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Therneau, T.M., Ed.; Springer: New York, NY, USA, 2000. [Google Scholar]
- Leong, H.S.; Yates, T.; Wilson, C.; Miller, C.J. ADAPT: A database of affymetrix probesets and transcripts. Bioinformatics 2005, 21, 2552–2553. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef] [Green Version]
- McVeigh, T.P.; Kerin, M. Clinical use of the oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer Targets Ther. 2017, 9, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Slodkowska, E.A.; Ross, J.S. MammaPrintTM 70-gene signature: Another milestone in personalized medical care for breast cancer patients. Expert Rev. Mol. Diagn. 2009, 9, 417–422. [Google Scholar] [CrossRef]
- Cardozo, J.L.; Drukker, C.; Schmidt, M.; Veer, L.V.; Glas, A.; Witteveen, A.; Cardoso, F.; Piccart-Gebhart, M.J.; Poncet, C.; Rutgers, E.J. Outcome of patients with an ultralow risk 70-gene signature in the MINDACT trial. J. Clin. Oncol. 2021, 39, 500. [Google Scholar] [CrossRef]
- Schaafsma, E.; Zhang, B.; Schaafsma, M.; Tong, C.-Y.; Zhang, L.; Cheng, C. Impact of Oncotype DX testing on ER+ breast cancer treatment and survival in the first decade of use. Breast Cancer Res. 2021, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, P.; Romero-Pérez, L.; Amaral, A.T.; Puerto-Camacho, P.; Jordán, C.; Marcilla, D.; Grünewald, T.G.P.; Alonso, J.; de Alava, E.; Díaz-Martín, J. Hippo pathway effectors YAP1/TAZ induce an EWS–FLI1-opposing gene signature and associate with disease progression in Ewing sarcoma. J. Pathol. 2019, 250, 374–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiemer, S.E.; Zhang, L.; Kartha, V.; Packer, T.S.; Almershed, M.; Noonan, V.; Kukuruzinska, M.; Bais, M.; Monti, S.; Varelas, X. A YAP/TAZ-regulated molecular signature is associated with Oral squamous cell carcinoma. Mol. Cancer Res. 2015, 13, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Craven, K.E.; Gökmen-Polar, Y.; Badve, S.S. CIBERSORT analysis of TCGA and METABRIC identifies subgroups with better outcomes in triple negative breast cancer. Sci. Rep. 2021, 11, 4691. [Google Scholar] [CrossRef]
- Jaramillo-Rodríguez, Y.; Cerda-Flores, R.M.; Ruiz-Ramos, R.; López-Márquez, F.C.; Calderón-Garcidueñas, A.L. YAP Expression in Normal and Neoplastic Breast Tissue: AnImmunohistochemical Study. Arch. Med Res. 2014, 45, 223–228. [Google Scholar] [CrossRef]
- Yuan, M.; Tomlinson, V.A.L.; Lara, R.; Holliday, D.L.; Chelala, C.; Harada, T.; Gangeswaran, R.; Manson-Bishop, C.; Smith, P.J.S.; Danovi, S.A.; et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008, 15, 1752–1759. [Google Scholar] [CrossRef]
- Real, S.A.S.; Parveen, F.; Rehman, A.U.; Khan, M.A.; Deo, S.V.S.; Shukla, N.K.; Husain, S.A. Aberrant Promoter Methylation of YAP Gene and its Subsequent Downregulation in Indian Breast Cancer Patients. BMC Cancer 2018, 18, 711. [Google Scholar] [CrossRef]
- Cha, Y.J.; Bae, S.J.; Kim, D.; Ahn, S.G.; Jeong, J.; Koo, J.S.; Yoo, T.-K.; Park, W.-C.; Lee, A.; Yoon, C.I. High Nuclear Expression of Yes-Associated Protein 1 Correlates With Metastasis in Patients With Breast Cancer. Front. Oncol. 2021, 11, 82. [Google Scholar] [CrossRef]
- Kim, S.K.; Jung, W.H.; Koo, J.S. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 3224–3234. [Google Scholar] [PubMed]
- Lehn, S.; Tobin, N.P.; Sims, A.H.; Stål, O.; Jirström, K.; Axelson, H.; Landberg, G. Decreased expression of Yes-associated protein is associated with outcome in the luminal A breast cancer subgroup and with an impaired tamoxifen response. BMC Cancer 2014, 14, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Z.; Wei, B.; Qin, M.; Liang, Z. Bioinformatics analysis identified MMP14 and COL12A1 as immune-related biomarkers associated with pancreatic adenocarcinoma prognosis. Math. Biosci. Eng. 2021, 18, 5921–5942. [Google Scholar] [CrossRef] [PubMed]






| METABRIC | TCGA | |
|---|---|---|
| IDC | 23 | 27 |
| HR-Positive | 26 | 34 |
| HER2-Positive | 24 | 21 |
| TNBC | 13 | 18 |
| METABRIC | |
|---|---|
| IDC | 9 |
| HR-Positive | 5 |
| HER2-Positive | 3 |
| TNBC | 1 |
| Genes Shortlisted Based on Survival Analysis | |||||||
|---|---|---|---|---|---|---|---|
| METABRIC | TCGA | ||||||
| IDC (n = 7) | HR-Positive (n = 5) | HER2-Positive (n = 2) | TNBC (n = 2) | IDC (n = 8) | HR-Positive (n = 10) | HER2-Positive (n = 3) | TNBC (n = 1) |
| ABCA8 | ABCA8 | COL12A1 | COL12A1 | CAPN6 | ABCA8 | COL12A1 | THBS1 |
| ADAMTS1 | FBLN5 | PROS1 | SULF1 | COL12A1 | CAPN6 | FN1 | |
| COL12A1 | MAMDC2 | DAPK1 | COL12A1 | SULF1 | |||
| FN1 | SULF1 | FBN1 | DAB2 | ||||
| IGFBP3 | THBS1 | FN1 | FBN1 | ||||
| LIFR | FSTL1 | FN1 | |||||
| MAMDC2 | MFAP5 | LIFR | |||||
| THBS1 | MFAP5 | ||||||
| PROS1 | |||||||
| SULF1 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatasubramanian, G.; Kelkar, D.A.; Mandal, S.; Jolly, M.K.; Kulkarni, M. Analysis of Yes-Associated Protein-1 (YAP1) Target Gene Signature to Predict Progressive Breast Cancer. J. Clin. Med. 2022, 11, 1947. https://doi.org/10.3390/jcm11071947
Venkatasubramanian G, Kelkar DA, Mandal S, Jolly MK, Kulkarni M. Analysis of Yes-Associated Protein-1 (YAP1) Target Gene Signature to Predict Progressive Breast Cancer. Journal of Clinical Medicine. 2022; 11(7):1947. https://doi.org/10.3390/jcm11071947
Chicago/Turabian StyleVenkatasubramanian, Gomathi, Devaki A. Kelkar, Susmita Mandal, Mohit Kumar Jolly, and Madhura Kulkarni. 2022. "Analysis of Yes-Associated Protein-1 (YAP1) Target Gene Signature to Predict Progressive Breast Cancer" Journal of Clinical Medicine 11, no. 7: 1947. https://doi.org/10.3390/jcm11071947
APA StyleVenkatasubramanian, G., Kelkar, D. A., Mandal, S., Jolly, M. K., & Kulkarni, M. (2022). Analysis of Yes-Associated Protein-1 (YAP1) Target Gene Signature to Predict Progressive Breast Cancer. Journal of Clinical Medicine, 11(7), 1947. https://doi.org/10.3390/jcm11071947






