Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas
Abstract
:1. Introduction
2. (Patho)Physiology of RDW Increase in Heart Failure
3. RDW Change in Heart Failure
3.1. RDW in Incident Heart Failure
3.2. RDW in Chronic HF
3.3. RDW in Acute HF
3.4. RDW in Advanced HF
4. RDW Longitudinal Changes in HF
4.1. Acute HF
4.2. Chronic HF
5. Systematic Reviews and Meta-Analyses
6. RDW and Prognostic Scores in HF
7. RDW and Congenital Heart Disease
8. RDW and Anemia
9. Future Perspectives
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bessman, J.D.; Gilmer, P.R.; Gardner, F.H., Jr. Improved classification of anemias by MCV and RDW. Am. J. Clin. Pathol. 1983, 80, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Buttarello, M.; Plebani, M. Automated blood cell counts: State of the art. Am. J. Clin. Pathol. 2008, 130, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyarel, H.; Isik, T.; Ayhan, E.; Ergelen, M. Red cell distrubition width (RDW): A novel risk factor for cardiovascular disease. Int. J. Cardiol. 2012, 154, 351–352. [Google Scholar] [CrossRef]
- Fava, C.; Cattazzo, F.; Hu, Z.D.; Lippi, G.; Montagnana, M. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: Useful or hype? Ann. Transl. Med. 2019, 7, 581. [Google Scholar] [CrossRef]
- Talarico, M.; Manicardi, M.; Vitolo, M.; Malavasi, V.L.; Valenti, A.C.; Sgreccia, D.; Rossi, R.; Boriani, G. Red cell distribution width and patient outcome in cardiovascular disease: A “real-world” analysis. J. Cardiovasc. Dev. Dis. 2021, 8, 120. [Google Scholar] [CrossRef]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M.; For the Cholesterol and Recurrent Events Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008, 117, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Abrahan, L.L.T.; Ramos, J.D.A.; Cunanan, E.L.; Tiongson, M.D.A.; Punzalan, F.E.R. Red cell distribution width and mortality in patients with acute coronary syndrome: A meta-analysis on prognosis. Cardiol. Res. 2018, 9, 144–152. [Google Scholar] [CrossRef]
- Sahin, O.; Akpek, M.; Sarli, B.; Baktir, A.O.; Savas, G.; Karadavut, S.; Elcik, D.; Saglam, H.; Kaya, M.G.; Arinc, H. Association of red blood cell distribution width levels with severity of coronary artery disease in patients with non-ST elevation myocardial infarction. Med. Princ. Pract. 2015, 24, 178–183. [Google Scholar] [CrossRef]
- Feng, G.H.; Li, H.P.; Li, Q.L.; Fu, Y.; Huang, R.B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc. Neurol. 2017, 2, 172–175. [Google Scholar] [CrossRef]
- Hattangadi, S.M.; Wong, P.; Zhang, L.; Flygare, J.; Lodish, H.F. From stem cell to red cell: Regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 2011, 118, 6258–6268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, V.; Diederich, L.; Stevenson Keller, T.C., IV; Kramer, C.M.; Luckstadt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red blood cell function and dysfunction: Redox regulation, nitric oxide metabolism, anemia. Antioxid. Redox. Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Turcato, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width in heart failure: A narrative review. World J. Cardiol. 2018, 10, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.; Ertoy, D.; Lin, H.; Sutliff, R.L.; Ezan, E.; Guyene, T.T.; Capecchi, M.; Corvol, P.; Bernstein, K.E. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J. Clin. Investig. 2000, 106, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Hullin, R.; Barras, N.; Abdurashidova, T.; Monney, P.; Regamey, J. Red cell distribution width and prognosis in acute heart failure: Ready for prime time! Intern. Emerg. Med. 2019, 14, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Forhecz, Z.; Gombos, T.; Borgulya, G.; Pozsonyi, Z.; Prohaszka, Z.; Janoskuti, L. Red cell distribution width in heart failure: Prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 2009, 158, 659–666. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Mehra, M.R.; Chiong, J.R.; Dunlap, S.H.; Ghali, J.K.; Lenihan, D.J.; Oren, R.M.; Wagoner, L.E.; Schwartz, T.A.; et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J. Card. Fail. 2010, 16, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Leitao, L.; Alves, C.J.; Sousa, D.M.; Neto, E.; Conceicao, F.; Lamghari, M. The alliance between nerve fibers and stem cell populations in bone marrow: Life partners in sickness and health. FASEB J. 2019, 33, 8697–8710. [Google Scholar] [CrossRef]
- Cosentino, M.; Marino, F.; Maestroni, G.J. Sympathoadrenergic modulation of hematopoiesis: A review of available evidence and of therapeutic perspectives. Front. Cell Neurosci. 2015, 9, 302. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Chow, A.; Merad, M.; Frenette, P.S. Circadian rhythms influence hematopoietic stem cells. Curr. Opin. Hematol. 2009, 16, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xanthopoulos, A.; Tryposkiadis, K.; Dimos, A.; Bourazana, A.; Zagouras, A.; Iakovis, N.; Papamichalis, M.; Giamouzis, G.; Vassilopoulos, G.; Skoularigis, J.; et al. Red blood cell distribution width in elderly hospitalized patients with cardiovascular disease. World J. Cardiol. 2021, 13, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Borne, Y.; Smith, J.G.; Melander, O.; Hedblad, B.; Engstrom, G. Red cell distribution width and risk for first hospitalization due to heart failure: A population-based cohort study. Eur. J. Heart Fail. 2011, 13, 1355–1361. [Google Scholar] [CrossRef]
- Emans, M.E.; Gaillard, C.A.; Pfister, R.; Tanck, M.W.; Boekholdt, S.M.; Wareham, N.J.; Khaw, K.T. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int. J. Cardiol. 2013, 168, 3550–3555. [Google Scholar] [CrossRef]
- Al-Najjar, Y.; Goode, K.M.; Zhang, J.; Cleland, J.G.; Clark, A.L. Red cell distribution width: An inexpensive and powerful prognostic marker in heart failure. Eur. J. Heart Fail. 2009, 11, 1155–1162. [Google Scholar] [CrossRef]
- Su, J.L.; Zhang, S.G.; Gao, R.J.; Han, Q.F.; Wang, L.H.; Zhou, Y.H.; Li, T.; Yao, H.C. Red cell distribution width is a predictor of mortality in patients with chronic heart failure. Int. J. Cardiol. 2016, 212, 79–81. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Pelle, A.J.; Beckers, P.J.; Possemiers, N.M.; Ramakers, C.; Vrints, C.J.; Van Hoof, V.; Denollet, J.; Conraads, V.M. Red cell distribution width as a marker of impaired exercise tolerance in patients with chronic heart failure. Eur. J. Heart Fail. 2012, 14, 54–60. [Google Scholar] [CrossRef]
- Hong, S.J.; Youn, J.C.; Oh, J.; Hong, N.; Lee, H.S.; Park, S.; Lee, S.H.; Choi, D.; Kang, S.M. Red cell distribution width as an independent predictor of exercise intolerance and ventilatory inefficiency in patients with chronic heart failure. Yonsei Med. J. 2014, 55, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Senthong, V.; Hudec, T.; Neale, S.; Wu, Y.; Hazen, S.L.; Tang, W.H. Relation of red cell distribution width to left ventricular end-diastolic pressure and mortality in patients with and without heart failure. Am. J. Cardiol. 2017, 119, 1421–1427. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, E.; Kilicgedik, A.; Kahveci, G.; Bakal, R.B.; Kirma, C. Red cell distribution width and its relationship with global longitudinal strain in patients with heart failure with reduced ejection fraction: A study using two-dimensional speckle tracking echocardiography. Kardiol. Pol. 2018, 76, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Zhang, Z.; Wang, Y.; Jiang, F.; Yang, K.; He, F.; Zhang, C. Predictive value of left ventricular myocardial strain by four-dimensional speckle tracking echocardiography combined with red cell distribution width in heart failure with preserved ejection fraction. Echocardiography 2019, 36, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Allen, L.A.; Pocock, S.J.; Shaw, L.K.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; et al. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM program and the Duke Databank. J. Am. Coll. Cardiol. 2007, 50, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolowiec, L.; Rogowicz, D.; Banach, J.; Buszko, K.; Surowiec, A.; Blazejewski, J.; Bujak, R.; Sinkiewicz, W. Prognostic significance of red cell distribution width and other red cell parameters in patients with chronic heart failure during two years of follow-up. Kardiol. Pol. 2016, 74, 657–664. [Google Scholar] [CrossRef]
- Bozorgi, A.; Mehrabi Nasab, E.; Khoshnevis, M.; Dogmehchi, E.; Hamze, G.; Goodarzynejad, H. Red Cell distribution width and severe left ventricular dysfunction in ischemic heart failure. Crit. Pathw. Cardiol. 2016, 15, 174–178. [Google Scholar] [CrossRef]
- Vizzardi, E.; Sciatti, E.; Bonadei, I.; Pezzali, N.L.; Lombardi, C.M.; Metra, M. Red cell distribution width and chronic heart failure: Prognostic role beyond echocardiographic parameters. Monaldi Arch. Chest Dis. 2016, 84, 59. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Kang, J.S.; Yu, J.X.; Yin, S.J.; Cong, X.F.; Chen, X. Differences in the predictive value of red cell distribution width for the mortality of patients with heart failure due to various heart diseases. J. Geriatr. Cardiol. 2015, 12, 647–654. [Google Scholar]
- Rahamim, E.; Zwas, D.R.; Keren, A.; Elbaz-Greener, G.; Ibrahimli, M.; Amir, O.; Gotsman, I. The ratio of hemoglobin to red cell distribution width: A strong predictor of clinical outcome in patients with heart failure. J. Clin. Med. 2022, 11, 886. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Santos, M.; Almeida, S.; Marques, I.; Bettencourt, P.; Carvalho, H. Tailoring diuretic therapy in acute heart failure: Insight into early diuretic response predictors. Clin. Res. Cardiol. 2013, 102, 745–753. [Google Scholar] [CrossRef]
- Oh, J.; Kang, S.M.; Hong, N.; Choi, J.W.; Lee, S.H.; Park, S.; Shin, M.J.; Jang, Y.; Chung, N. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J. Card Fail. 2009, 15, 517–522. [Google Scholar] [CrossRef]
- Hong, N.; Oh, J.; Kang, S.M.; Kim, S.Y.; Won, H.; Youn, J.C.; Park, S.; Jang, Y.; Chung, N. Red blood cell distribution width predicts early mortality in patients with acute dyspnea. Clin. Chim Acta 2012, 413, 992–997. [Google Scholar] [CrossRef]
- Turcato, G.; Cervellin, G.; Bonora, A.; Prati, D.; Zorzi, E.; Ricci, G.; Salvagno, G.L.; Maccagnani, A.; Lippi, G. red blood cell distribution width improves reclassification of patients admitted to the emergency department with acute decompensated heart failure. J. Med. Biochem. 2018, 37, 299–306. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Bonaque, J.C.; Redondo, B.; Caro, C.; Manzano-Fernandez, S.; Sanchez-Mas, J.; Garrido, I.P.; Valdes, M. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur. J. Heart Fail. 2009, 11, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.E.; Dalzell, J.R.; Bezlyak, V.; Tsorlalis, I.K.; Myles, R.C.; Spooner, R.; Ford, I.; Petrie, M.C.; Cobbe, S.M.; McMurray, J.J. Red cell distribution width has incremental prognostic value to B-type natriuretic peptide in acute heart failure. Eur. J. Heart Fail. 2009, 11, 1152–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalawadiya, S.K.; Zmily, H.; Farah, J.; Daifallah, S.; Ali, O.; Ghali, J.K. Red cell distribution width and mortality in predominantly African-American population with decompensated heart failure. J. Card. Fail. 2011, 17, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Konishi, H.; Takagi, A.; Miyauchi, K.; Daida, H. Red cell distribution width predicts short- and long-term outcomes of acute congestive heart failure more effectively than hemoglobin. Exp. Ther. Med. 2014, 8, 600–606. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Jia, J.; Chen, J.; Qin, S.; Tao, H.; Kong, Q.; Xue, Q.; Zhang, D. Comparison of prognostic value of red cell distribution width and NT-proBNP for short-term clinical outcomes in acute heart failure patients. Int. Heart J. 2014, 55, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Van Kimmenade, R.R.; Mohammed, A.A.; Uthamalingam, S.; Van Der Meer, P.; Felker, G.M.; Januzzi, J.L., Jr. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur. J. Heart Fail. 2010, 12, 129–136. [Google Scholar] [CrossRef]
- Salvatori, M.; Formiga, F.; Moreno-Gonzalez, R.; Chivite, D.; Migone De Amicis, M.; Cappellini, M.D.; Corbella, X. Red blood cell distribution width as a prognostic factor of mortality in elderly patients firstly hospitalized due to heart failure. Kardiol. Pol. 2019, 77, 632–638. [Google Scholar] [CrossRef]
- Sotiropoulos, K.; Yerly, P.; Monney, P.; Garnier, A.; Regamey, J.; Hugli, O.; Martin, D.; Metrich, M.; Antonietti, J.P.; Hullin, R. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Fail. 2016, 3, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Imai, R.; Uemura, Y.; Okumura, T.; Takemoto, K.; Uchikawa, T.; Koyasu, M.; Ishikawa, S.; Iwamiya, S.; Ozaki, Y.; Shibata, R.; et al. Impact of red blood cell distribution width on non-cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J. Cardiol. 2017, 70, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xanthopoulos, A.; Giamouzis, G.; Melidonis, A.; Kitai, T.; Paraskevopoulou, E.; Paraskevopoulou, P.; Patsilinakos, S.; Triposkiadis, F.; Skoularigis, J. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc. Diabetol. 2017, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, L.; Szygula-Jurkiewicz, B.; Szczurek, W.; Pyka, L.; Niedziela, J.; Gasior, M. Mortality risk factors in patients with advanced heart failure and diabetes mellitus. Kardiol. Pol. 2019, 77, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Dabbah, S.; Chertin, L.; Khateeb, A.; Rosenfeld, I.; Suleiman, M.; Halabi, M. Red cell distribution width predicts death and appropriate therapy in patients with implantable cardioverter defibrillator: A simple measurement with prognostic value in a variety of diseases, may help in better selection of patients who will benefit the most from this device. Pacing Clin. Electrophysiol. 2017, 40, 1384–1388. [Google Scholar]
- Rickard, J.; Kumbhani, D.J.; Gorodeski, E.Z.; Martin, D.O.; Grimm, R.A.; Tchou, P.; Lindsay, B.D.; Tang, W.H.; Wilkoff, B.L. Elevated red cell distribution width is associated with impaired reverse ventricular remodeling and increased mortality in patients undergoing cardiac resynchronization therapy. Congest. Heart Fail. 2012, 18, 79–84. [Google Scholar] [CrossRef]
- Celikyurt, U.; Agacdiken, A.; Sahin, T.; Kozdag, G.; Vural, A.; Ural, D. Association between red blood cell distribution width and response to cardiac resynchronization therapy. J. Interv. Card. Electrophysiol. 2012, 35, 215–218. [Google Scholar] [CrossRef]
- Topaz, G.; Haim, M.; Kusniec, J.; Kazum, S.; Goldenberg, G.; Golovchiner, G.; Kornowski, R.; Strasberg, B.; Eisen, A. Association between red cell distribution width and mortality after cardiac resynchronization therapy. Isr. Med. Assoc. J. 2015, 17, 505–509. [Google Scholar]
- Miller, P.E.; Houston, B.A.; Schneider, A.L.; Bush, A.L.; Whitman, G.J.; Stevens, G.R.; Tedford, R.J.; Russell, S.D. Associations of preimplant red blood cell distribution width with clinical outcomes among individuals with left ventricular assist devices. ASAIO J. 2016, 62, 677–683. [Google Scholar] [CrossRef]
- Truby, L.K.; Sridharan, L.; Flores, R.J.; Garan, A.R.; Jennings, D.; Yuzefpolskaya, M.; Takeda, K.; Takayama, H.; Naka, Y.; Colombo, P.C.; et al. Red cell distribution width predicts 90 day mortality in continuous-flow left ventricular assist device patients. ASAIO J. 2019, 65, 233–240. [Google Scholar] [CrossRef]
- Ahmad, T.; Wang, T.; O’Brien, E.C.; Samsky, M.D.; Pura, J.A.; Lokhnygina, Y.; Rogers, J.G.; Hernandez, A.F.; Craig, D.; Bowles, D.E.; et al. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail. 2015, 3, 30–39. [Google Scholar] [CrossRef]
- Poglajen, G.; Sever, M.; Cernelc, P.; Haddad, F.; Vrtovec, B. Increased red cell distribution width is associated with poor stem cell mobilization in patients with advanced chronic heart failure. Biomarkers 2015, 20, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Szygula-Jurkiewicz, B.; Szczurek, W.; Skrzypek, M.; Nadziakiewicz, P.; Siedlecki, L.; Zakliczynski, M.; Gasior, M.; Zembala, M. Red blood cell distribution width in end-stage heart failure patients is independently associated with all-cause mortality after orthotopic heart transplantation. Transplant. Proc. 2018, 50, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B.; Lappe, D.L.; Anderson, J.L.; Muhlestein, J.B.; Budge, D.; May, H.T.; Bennett, S.T.; Bair, T.L.; Horne, B.D. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30-day outcomes. Int. J. Lab. Hematol. 2016, 38, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Zorzi, E.; Prati, D.; Ricci, G.; Bonora, A.; Zannoni, M.; Maccagnani, A.; Salvagno, G.L.; Sanchis-Gomar, F.; Cervellin, G.; et al. Early in-hospital variation of red blood cell distribution width predicts mortality in patients with acute heart failure. Int. J. Cardiol. 2017, 243, 306–310. [Google Scholar] [CrossRef]
- Makhoul, B.F.; Khourieh, A.; Kaplan, M.; Bahouth, F.; Aronson, D.; Azzam, Z.S. Relation between changes in red cell distribution width and clinical outcomes in acute decompensated heart failure. Int. J. Cardiol. 2013, 167, 1412–1416. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Takemoto, K.; Uchikawa, T.; Koyasu, M.; Watanabe, H.; Mitsuda, T.; Miura, A.; Imai, R.; Watarai, M.; et al. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. J. Cardiol. 2016, 67, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Melchio, R.; Rinaldi, G.; Testa, E.; Giraudo, A.; Serraino, C.; Bracco, C.; Spadafora, L.; Falcetta, A.; Leccardi, S.; Silvestri, A.; et al. Red cell distribution width predicts mid-term prognosis in patients hospitalized with acute heart failure: The RDW in Acute Heart Failure (RE-AHF) study. Intern. Emerg. Med. 2019, 14, 239–247. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Girerd, N.; Arrigo, M.; Medeiros, P.B.; Ricardo, M.B.; Almeida, T.; Rola, A.; Tolppanen, H.; Laribi, S.; Gayat, E.; et al. Enlarging red blood cell distribution width during hospitalization identifies a very high-risk subset of acutely decompensated heart failure patients and adds valuable prognostic information on top of hemoconcentration. Medicine 2016, 95, e3307. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Kang, S.M.; Won, H.; Hong, N.; Kim, S.Y.; Park, S.; Lee, S.H.; Jang, Y.; Chung, N. Prognostic value of change in red cell distribution width 1 month after discharge in acute decompensated heart failure patients. Circ. J. 2012, 76, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Nunez, J.; Nunez, E.; Rizopoulos, D.; Minana, G.; Bodi, V.; Bondanza, L.; Husser, O.; Merlos, P.; Santas, E.; Pascual-Figal, D.; et al. Red blood cell distribution width is longitudinally associated with mortality and anemia in heart failure patients. Circ. J. 2014, 78, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Cauthen, C.A.; Tong, W.; Jain, A.; Tang, W.H. Progressive rise in red cell distribution width is associated with disease progression in ambulatory patients with chronic heart failure. J. Card. Fail. 2012, 18, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Aung, N.; Ling, H.Z.; Cheng, A.S.; Aggarwal, S.; Flint, J.; Mendonca, M.; Rashid, M.; Kang, S.; Weissert, S.; Coats, C.J.; et al. Expansion of the red cell distribution width and evolving iron deficiency as predictors of poor outcome in chronic heart failure. Int. J. Cardiol. 2013, 168, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Papamichalis, M.; Zajichek, A.; Milinovich, A.; Kattan, M.W.; Skoularigis, J.; Starling, R.C.; Triposkiadis, F. In-hospital red blood cell distribution width change in patients with heart failure. Eur. J. Heart Fail. 2019, 21, 1659–1661. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, M.; Sadoune, M.; Launay, J.M.; Baudet, M.; Cohen-Solal, A.; Logeart, D. Inflammation versus mechanical stretch biomarkers over time in acutely decompensated heart failure with reduced ejection fraction. Int. J. Cardiol. 2017, 226, 53–59. [Google Scholar] [CrossRef]
- Huang, Y.L.; Hu, Z.D.; Liu, S.J.; Sun, Y.; Qin, Q.; Qin, B.D.; Zhang, W.W.; Zhang, J.R.; Zhong, R.Q.; Deng, A.M. Prognostic value of red blood cell distribution width for patients with heart failure: A systematic review and meta-analysis of cohort studies. PLoS ONE 2014, 9, e104861. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Li, L.; Li, G.; Liu, T. Prognostic value of red blood cell distribution width in heart failure patients: A meta-analysis. Int. J. Cardiol. 2015, 179, 495–499. [Google Scholar] [CrossRef]
- Hou, H.; Sun, T.; Li, C.; Li, Y.; Guo, Z.; Wang, W.; Li, D. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci. Rep. 2017, 7, 43420. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodward, M.; Patel, A.; McMurray, J.; MacMahon, S. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef]
- Passantino, A.; Monitillo, F.; Iacoviello, M.; Scrutinio, D. Predicting mortality in patients with acute heart failure: Role of risk scores. World J. Cardiol. 2015, 7, 902–911. [Google Scholar] [CrossRef]
- Ferrero, P.; Iacovoni, A.; D’Elia, E.; Vaduganathan, M.; Gavazzi, A.; Senni, M. Prognostic scores in heart failure—Critical appraisal and practical use. Int. J. Cardiol. 2015, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Giamouzis, G.; Tryposkiadis, K.; Paraskevopoulou, E.; Paraskevopoulou, P.; Karagiannis, G.; Patsilinakos, S.; Parissis, J.; Farmakis, D.; Butler, J.; et al. A simple score for early risk stratification in acute heart failure. Int. J. Cardiol. 2017, 230, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Tryposkiadis, K.; Giamouzis, G.; Konstantinou, D.; Giannakoulas, G.; Karvounis, H.; Kattan, M.W.; Skoularigis, J.; Parissis, J.; Starling, R.C.; et al. Larissa Heart Failure Risk score: A proposed simple score for risk stratification in chronic heart failure. Eur. J. Heart Fail. 2018, 20, 614–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitai, T.; Xanthopoulos, A.; Tang, W.H.W.; Kaji, S.; Furukawa, Y.; Oishi, S.; Akiyama, E.; Suzuki, S.; Yamamoto, M.; Kida, K.; et al. Validation of the Larissa Heart Failure Risk score for risk stratification in acute heart failure. Int. J. Cardiol. 2020, 307, 119–124. [Google Scholar] [CrossRef]
- Adler, E.D.; Voors, A.A.; Klein, L.; Macheret, F.; Braun, O.O.; Urey, M.A.; Zhu, W.; Sama, I.; Tadel, M.; Campagnari, C.; et al. Improving risk prediction in heart failure using machine learning. Eur. J. Heart Fail. 2020, 22, 139–147. [Google Scholar] [CrossRef]
- Horne, B.D.; Budge, D.; Masica, A.L.; Savitz, L.A.; Benuzillo, J.; Cantu, G.; Bradshaw, A.; McCubrey, R.O.; Bair, T.L.; Roberts, C.A.; et al. Early inpatient calculation of laboratory-based 30-day readmission risk scores empowers clinical risk modification during index hospitalization. Am. Heart J. 2017, 185, 101–109. [Google Scholar] [CrossRef]
- Massin, M.M. Relation between red cell distribution width and clinical outcome after surgery for congenital heart disease in children. Pediatr. Cardiol. 2012, 33, 1021–1025. [Google Scholar] [CrossRef]
- Mawlana, W.; Donia, A.; Elamrousy, D. Relation between red cell distribution width and left ventricular function in children with heart failure. ISRN Pediatr. 2014, 2014, 234835. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sudhakar, A.; Mohan, M.; Balachandran, R.; Raj, B.; Sumangala, S.G.; Kumar, R.K. Elevated red cell distribution width is associated with delayed postoperative recovery after correction of Tetralogy of Fallot. Ann. Pediatr. Cardiol. 2013, 6, 121–125. [Google Scholar] [CrossRef]
- Kojima, T.; Yasuhara, J.; Kumamoto, T.; Shimizu, H.; Yoshiba, S.; Kobayashi, T.; Sumitomo, N. Usefulness of the red blood cell distribution width to predict heart failure in patients with a fontan circulation. Am. J. Cardiol. 2015, 116, 965–968. [Google Scholar] [CrossRef]
- Miyamoto, K.; Inai, K.; Takeuchi, D.; Shinohara, T.; Nakanishi, T. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ. J. 2015, 79, 1100–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomkiewicz-Pajak, L.; Plazak, W.; Kolcz, J.; Pajak, J.; Kopec, G.; Dluzniewska, N.; Olszowska, M.; Moryl-Bujakowska, A.; Podolec, P. Iron deficiency and hematological changes in adult patients after Fontan operation. J. Cardiol. 2014, 64, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggen, V.J.M.; Van Den Bosch, A.E.; Van Kimmenade, R.R.; Eindhoven, J.A.; Witsenburg, M.; Cuypers, J.; Leebeek, F.W.G.; Boersma, E.; Roos-Hesselink, J.W. Red cell distribution width in adults with congenital heart disease: A worldwide available and low-cost predictor of cardiovascular events. Int. J. Cardiol. 2018, 260, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Sun, Y.J.; Xiong, C.M.; Zeng, W.J.; Ni, X.H.; Zhao, Z.H.; Liu, Z.H.; Gu, Q.; He, J.G. Red blood cell distribution width predicts survival in patients with Eisenmenger syndrome. Clin. Chem. Lab. Med. 2014, 52, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quintana, E.; Estupinan-Leon, H.; Riano-Ruiz, M.; Rodriguez-Gonzalez, F.; Tugores, A. Red blood cell distribution width in addition to N-terminal prohormone of B-type natriuretic peptide concentration improves assessment of risk of cardiovascular events in adult patients with congenital heart disease. Arch. Cardiovasc. Dis. 2020, 113, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Bonaque, J.C.; Pascual-Figal, D.A.; Manzano-Fernandez, S.; Gonzalez-Canovas, C.; Vidal, A.; Munoz-Esparza, C.; Garrido, I.P.; Pastor-Perez, F.; Valdes, M. Red blood cell distribution width adds prognostic value for outpatients with chronic heart failure. Rev. Esp. Cardiol. 2012, 65, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Tseliou, E.; Terrovitis, J.V.; Kaldara, E.E.; Ntalianis, A.S.; Repasos, E.; Katsaros, L.; Margari, Z.J.; Matsouka, C.; Toumanidis, S.; Nanas, S.N.; et al. Red blood cell distribution width is a significant prognostic marker in advanced heart failure, independent of hemoglobin levels. Hellenic J. Cardiol. 2014, 55, 457–461. [Google Scholar]
- Yaegashi, D.; Oikawa, M.; Yokokawa, T.; Misaka, T.; Kobayashi, A.; Kaneshiro, T.; Yoshihisa, A.; Nakazato, K.; Ishida, T.; Takeishi, Y. Red blood cell distribution width is a predictive factor of anthracycline-induced cardiotoxicity. Front. Cardiovasc. Med. 2020, 7, 594685. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Zwinderman, A.H.; Ng, L.L.; Demissei, B.; Hillege, H.L.; Zannad, F.; Van Veldhuisen, D.J.; Samani, N.J.; Ponikowski, P.; Metra, M.; et al. Biomarker-guided versus guideline-based treatment of patients with heart failure: Results from BIOSTAT-CHF. J. Am. Coll. Cardiol. 2018, 71, 386–398. [Google Scholar] [CrossRef]
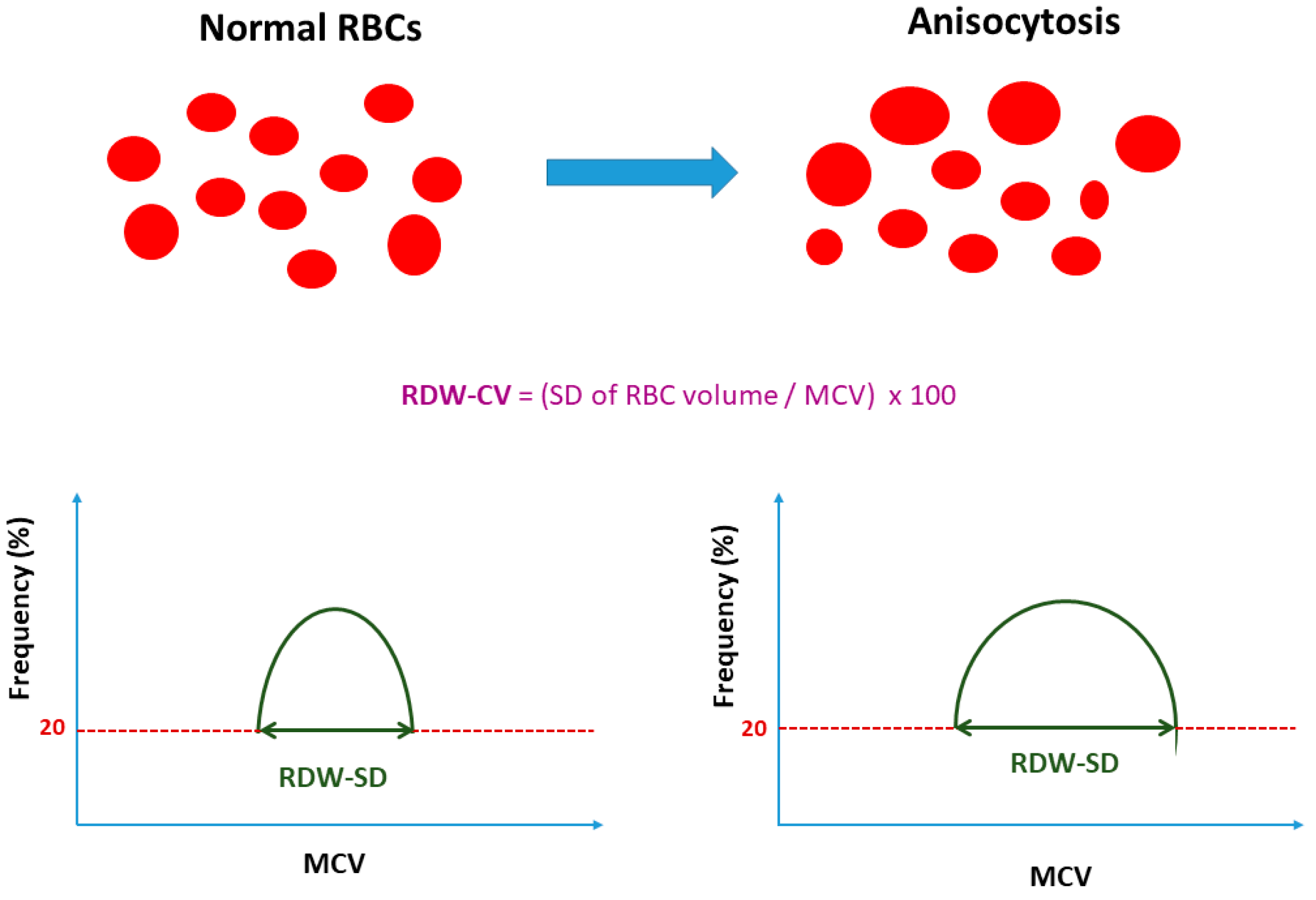
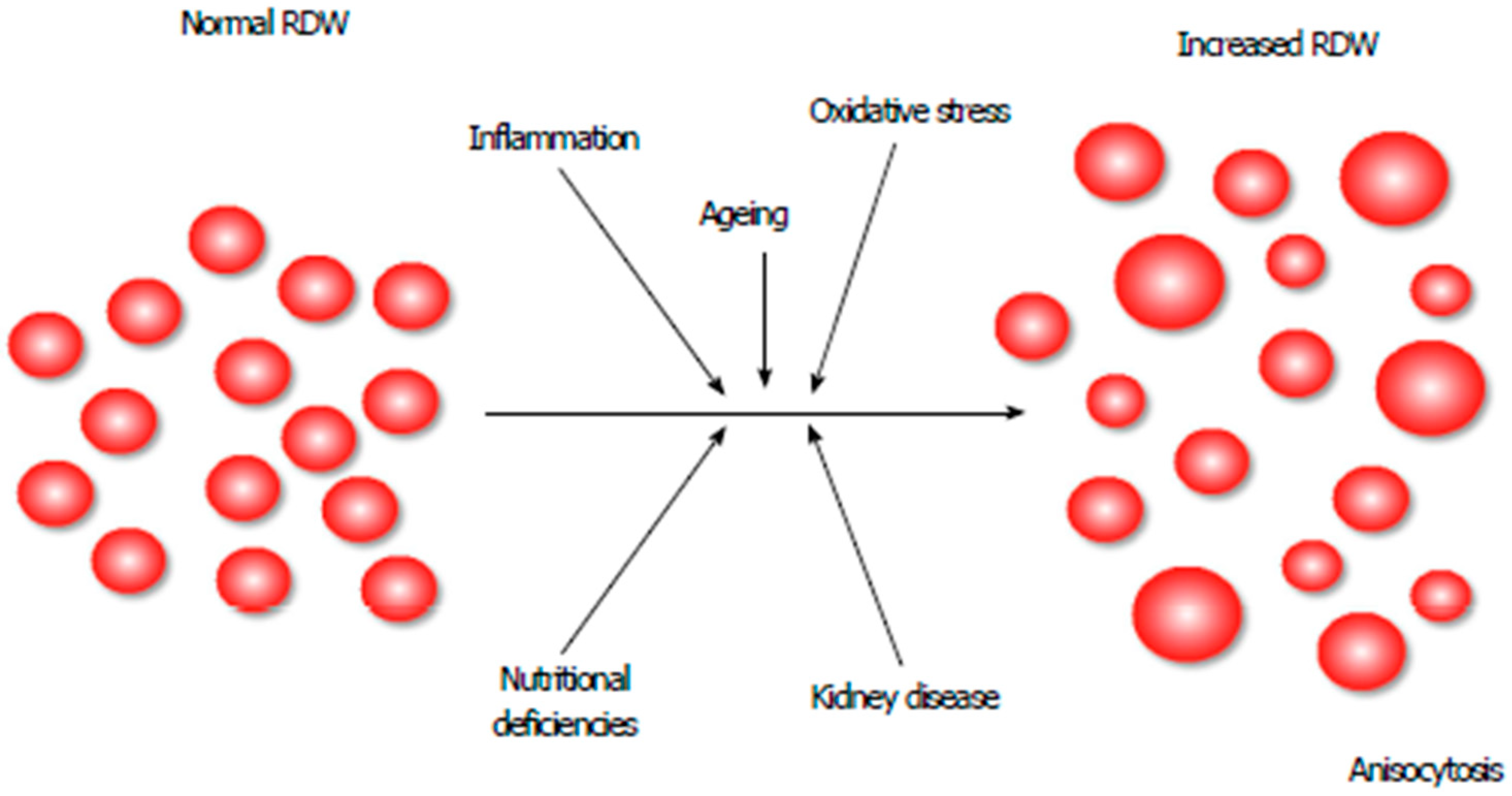
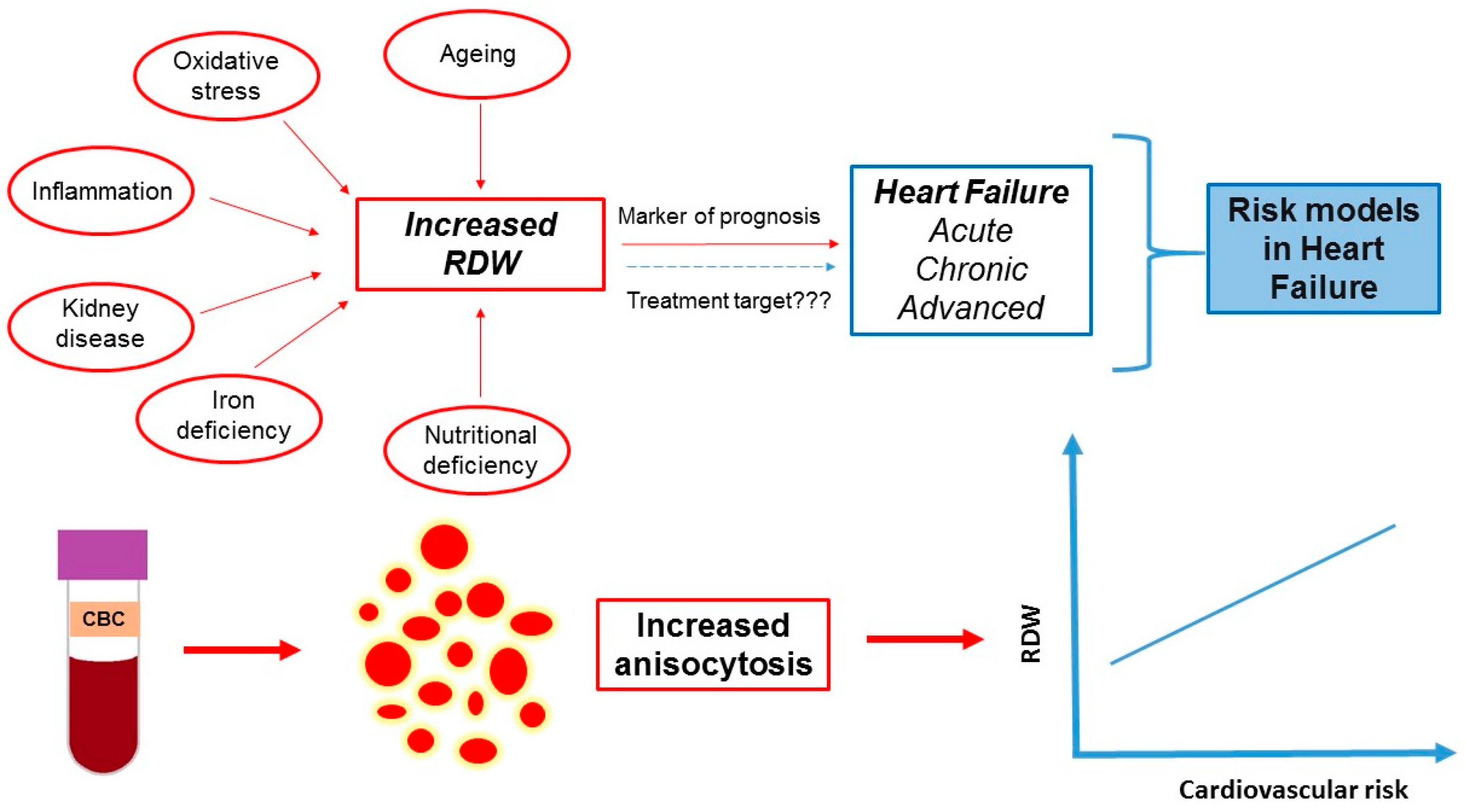
| Facts | Open Issues/Controversies |
|---|---|
| RDW is an integral marker of the complete blood count and can be calculated by automatic hematology analyzers. | No universal consensus on the recommended method of RDW calculation (standard deviation or coefficient of variation) currently exists. |
| The typical reference range value of RDW-CV is 11.5–15% and of RDW-SD is 39–46 fL. | No universal reference range values exist. Those often vary depending on the method of RDW calculation and the available hematological analyzers used. |
| RDW is an established simple prognostic marker in heart failure (acute, chronic and advanced). | There are limited data on the role of RDW in cardio-oncology. |
| Several pathophysiological mechanisms of the RDW increase in heart failure have been proposed (inflammation, adrenergic stimulation, undernutrition, etc.). | The exact pathophysiological mechanism of RDW increase in heart failure remains unknown. |
| RDW values at hospital admission and discharge have been associated with prognosis in heart failure patients. | There is a debate on the prognostic value of in-hospital RDW changes (ΔRDW). |
| The current RDW indications include the classification of several types of anemia and the estimation of patients’ risk in cardiovascular diseases (including heart failure) | RDW may be used in the future to guide therapy in heart failure. |
| Reference | Number of Subjects | Study Design | Outcome | Results | Conclusion |
|---|---|---|---|---|---|
| (a) Population-Based Cohort | |||||
| [25] | 17,533 | Retrospective (Mean follow up 11.2 years) | Incident HF | Adj. HR 1.44, (95% CI 1.15–1.80) | RDW is associated with HF events in an apparently healthy middle-aged population. |
| [24] | 26, 784 | Retrospective (Mean follow up 15 years) | Risk of hospitalization due to HF | Adj. HR 1.47, (95% CI 1.14–1.89) | Red cell distribution width was associated with long-term incidence of first hospitalization due to HF among middle-aged subjects. |
| (b) Chronic Heart Failure | |||||
| [38] | 6888 | Retrospective (Follow up 24 months) | All-cause mortality and cardiovascular hospitalization | A lower Hb/RDW ratio was a predictor of mortality (Q1 vs. Q6: Adj HR 1.84 (1.63–2.08) | Hb/RDW ratio is a prognostic tool for predicting HF mortality and cardiovascular hospitalizations. |
| [32] | 169 HFpEF vs. 50 controls | Prospective | Predictive value of deformation imaging combined with RDW | The associations of clinical and echocardiographic parameters with HFpEF were improved by adding RDW (p < 0.01) | RDW has an independent incremental predictive value for HFpEF. |
| [31] | 59 HFrEF vs. 40 controls | Prospective | LV global longitudinal strain | RDW showed negative correlations with LV global longitudinal strain (r = −0.41, p = 0.001) | Elevated RDW is associated with poorer LV deformation assessed by speckle tracking echocardiography in HF patients with similar EF. |
| [30] | 1084 | Prospective | LVEDP, mortality | RDW was independently associated with high LVEDP (Adj. OR per unit change 1.14, 95% CI 1.0 to 1.29) and 5 year-mortality (Adj. HR 4.11, 95% CI 2.12 to 7.96) | RDW was independently associated with high LVEDP and with mortality. |
| [36] | 232 | Prospective (Follow up 12 months) | Cardiovascular death and/or HF hospitalization | RDW > 14.45%, Adj. OR:3.894, (95%CI 1.042–14.55) | RDW is a better predictor of adverse outcome than several echocardiographic parameters. |
| [27] | 215 | Prospective (Mean follow up 24.2 months) | All-cause mortality | Adj. OR: 2.963 (95% CI 1.066–6.809) | RDW may be an indicator in the risk stratification. |
| [35] | 350 | Retrospective (Follow up 12 months) | All-cause mortality and HF hospitalization | Higher mortality and HF re-admission in patients with RDW > 14.5 (vs. RDW ≤ 14.5) (p < 0.001 and p = 0.004, respectively). Levels of RDW were associated with the presence of severe LV dysfunction (LVEF < 30%) * | Elevated RDW may be used as a prognostic tool among HF patients with the documented myocardial infarction. |
| [34] | 165 | Prospective (Follow up 24 months) | All-cause mortality | Adj. HR 1.19 (95% CI 1.004–1.411) at 12 months | RDW is an independent predictor of mortality at 12 months, but it loses its significance during longer-term follow up. |
| [37] | 1021 (CHD vs. DCM vs. VHD) | Retrospective (Mean follow up 21 months) | All-cause mortality | The AUC of RDW for predicting mortality due to CHD and DCM was 0.704 (95% CI 0.609–0.799) and 0.753 (95% CI 0.647–0.860), respectively. The AUC of the RDW for predicting mortality from VHD was 0.593 | RDW is a prognostic indicator for patients with HF caused by CHD and DCM. |
| [29] | 85 HF vs. 107 controls | Prospective | Peak VO2, VE/VCO2 slope | RDW is an independent predictor for peak VO2 (β = −0.247, p = 0.035) and VE/VCO2 slope (β = 0.366, p = 0.004) | Higher RDW is independently related to peak VO2 and VE/VCO2 slope. |
| [28] | 118 | Prospective | Exercise capacity | Log[RDW] is associated with VO2peak (β = –0.277, p = 0.003) | Higher RDW is independently related to impaired exercise capacity. |
| [96] | 698 | Prospective (Median follow up 2.5 years) | All-cause mortality HF hospitalization | All-cause mortality HR (for RDW > 15.4%) 2.63, (95% CI 2.01–3.45) HF hospitalization HR (for RDW > 15.4%) 2.37, (95% CI, 1.80–3.13) | RDW value is a risk marker for the occurrence of both death and hospitalization for HF in outpatients with chronic HF, independent of anemia. |
| [26] | 1087 | Retrospective (Median follow up 52 months) | All-cause mortality | Adj. HR 1.12, (95% CI 1.05–1.16) | RDW has similar independent prognostic power to NT-proBNP. |
| [33] | 2679 | Retrospective (Median follow up 34 months) | Morbidity and mortality | Adj. HR 1.17 per 1-SD increase, p < 0.001 | RDW is an independent predictor of morbidity and mortality. |
| (c) Acute Heart Failure | |||||
| [49] | 897 (≥65 years) | Retrospective | All-cause mortality at 1 year | Adj. HR 1.41 (95% CI, 1.05–1.90) | A higher baseline RDW was associated with increased risk for 1-year all-cause mortality. |
| [42] | 2278 ED visits | Retrospective (Follow up 4 years) | All-cause mortality at 30 days | AUC 0.723, (95% CI 0.693–0.763) | The prognostic assessment of acute HF patients in the ED can be improved by combining RDW with other laboratory tests. |
| [52] | 218 patients (71 diabetics) | Prospective (Follow up 1 year) | All-cause mortality or rehospitalization for HF at 1 year | Diabetics: Adj HR: 1.349, (95% CI 1.120–1.624) Non-diabetics: Adj HR: 1.142, (95% CI 1.011–1.291 (βinteraction = −0.002; SE = 0.001; p = 0.042) between DM and RDW longitudinal changes | RDW has similar prognostic significance (diabetic and non-diabetic) in HF patients. RDW longitudinal changes show significant difference in diabetic and non-diabetic patients. |
| [51] | 278 HFpEF patients | Retrospective (Follow up 3 years) | Non cardiac mortality | Adj. HR 1.169, (95% CI 1.042–1.311) | RDW levels at admission independently predict non-cardiac mortality in acute HFpEF. |
| [50] | 402 | Prospective | All-cause mortality at 1 year | All-cause mortality of all patients increased with quartiles of rising RDW (χ2 18; p < 0.001). | High RDW predicts mortality in acute HF. |
| [47] | 128 | Prospective (Follow up 3 months) | Cardiac death and/or readmission for HF | Adj. HR 4.610, (95% CI 1.935–10.981) | RDW and NT-proBNP are independent predictors of 90-day cardiovascular events in patients hospitalized with HF. RDW adds prognostic value to NT-proBNP. |
| [46] | 521 | Prospective (Median follow up 24 months) | In-hospital mortality, All-cause mortality and HF readmission (long term) | In-hospital mortality (for log RDW): coef. 5.21, p = 0.044, All-cause mortality and HF re-admission (long term): RDW (per SD increase, HR 2.19; 95% CI 1.92–2.50; p < 0.0001) | Higher RDW values in acute HF at admission are associated with worse short- and long-term outcomes and RDW values are more prognostically relevant than hemoglobin levels. |
| [39] | 100 | Retrospective | Slow diuretic response | Adj. OR 1.47, (95 % CI 1.07–2.02) | High RDW at admission is a predictor of slower diuretic response. |
| [41] | 907 | Retrospective | All-cause mortality at 30 days | Adj.HR 1.23, (95% CI 1.11–1.36) | RDW measured at ED is an independent predictor of early mortality. |
| [45] | 789 | Retrospective (Median follow up 573 days) | All-cause mortality | Adj. HR 3.21, (95% CI 1.77–5.83) | Discharge RDW is an independent predictor of all-cause mortality in predominantly African American patients. |
| [48] | 205 | Retrospective (Follow-up 1 year) | All-cause mortality | Adj. HR = 1.03 per 1% increase in RDW, (95% CI 1.02–1.07, p = 0.04) | RDW independently predicted 1-year mortality in acute HF. |
| [43] | 628 | Prospective (Median follow up 38.1 months) | All-cause mortality | Adj. HR 1.072, (95% CI 1.023–1.124) | Higher RDW levels at discharge are associated with a worse long-term outcome, irrespective of hemoglobin levels. |
| [44] | 707 | Prospective (Median follow up 421 days) | All-cause mortality | Adj. HR 1.06, (95% CI 1.01–1.11) | RDW provides incremental prognostic value to BNP in acute heart failure. The prognostic ability of RDW is independent of hemoglobin concentration. |
| [40] | 100 | Prospective | Relation between RDW and echocardiographic parameters | RDW was independently correlated with E/E (β-coefficient 0.431, p = 0.001) | RDW may be associated with elevated LV filling pressures in patients with acute HF. |
| (d) Advanced Heart Failure | |||||
| [59] | 409 patients with cf-LVADs | Retrospective | All-cause mortality at 90 days | Adj. OR 1.16 for 1% increase, (95% CI: 1.04–1.31) | RDW is an independent predictor of 90-days mortality in cf-LVAD patients. |
| [53] | 367 | Retrospective (Mean follow up 4.4 years) | All-cause mortality | Adj. HR 1.0492 (95 % CI 1.0247–1.0743) | RDW is an independent predictor of all-cause mortality in advanced HF patients with concomitant diabetes mellitus. |
| [62] | 173 | Retrospective (Mean follow up 45.5 months) | All-cause mortality | Adj. HR 1.381 (95% CI 1.251–1.467) | RDW immediately before OHT is an independent predictor of all-cause mortality in heart transplant recipients. |
| [54] | 432 patients with ICDs | Retrospective (Follow up ≤ 5 years) | First appropriate ICD therapy and death | Adj. HR 2.045 for RDW > 15.2 (95% CI 1.145–3.65) | RDW may be useful in risk stratification of patients selected for ICD implantation. |
| [58] | 188 cf-LVADs | Retrospective (Follow-up ≥ 1 year) | All-cause mortality | Adj. HR (for RDW > 18.1% vs. RDW < 15.7%) 4.61 (95% CI 1.74–12.21) | Preimplant RDW is independently associated with postimplant mortality. |
| [61] | 44 | Prospective | Parameters associated with bone marrow dysfunction in patients with advanced chronic non-ischemic HF | Adj. HR 8.64 (95% CI 1.242–60.021) | RDW is an independent predictor of poor mobilization of CD34+ cells. |
| [60] | 37 patients with cf-LVADs | Prospective (Median follow-up 136 days) | Changes in laboratory parameters/biomarkers in patients who underwent LVAD implantation | median RDW (pre-implant) 16.7% vs. 16.5% (post-implant), p = 0.98 | RDW is elevated but does not change (pre- vs. post-LVAD implant). |
| [57] | 156 patients with CRTs | Retrospective (Median follow up 61 months) | All-cause mortality | Adj. HR (baseline RDW) 1.33, (95%CI 1.16–1.53) HR (RDW 6 months after CRT implantation) 1.22, (95%CI 1.08–1.38) -HR (RDW 12 months after CRT implantation) 1.15, (95%CI 1.01–1.32) | Baseline RDW levels, as well as RDW after CRT implantation, are independently associated with mortality in patients who undergo CRT implantation. |
| [55] | 233 patients with CRTs | Retrospective (Mean follow up 11.5 months) | CRT response | Adj. OR 0.83, (95% CI 0.69–0.99) | Elevated RDW is associated with impaired reverse remodeling. |
| [56] | 66 patients with CRTs | Prospective (Follow up 6 months) | CRT response | Adj. OR 1.435, (95 % CI, 1.059–1.945) | Elevated RDW is associated with poor CRT response. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulos, A.; Giamouzis, G.; Dimos, A.; Skoularigki, E.; Starling, R.C.; Skoularigis, J.; Triposkiadis, F. Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. J. Clin. Med. 2022, 11, 1951. https://doi.org/10.3390/jcm11071951
Xanthopoulos A, Giamouzis G, Dimos A, Skoularigki E, Starling RC, Skoularigis J, Triposkiadis F. Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. Journal of Clinical Medicine. 2022; 11(7):1951. https://doi.org/10.3390/jcm11071951
Chicago/Turabian StyleXanthopoulos, Andrew, Grigorios Giamouzis, Apostolos Dimos, Evangelia Skoularigki, Randall C. Starling, John Skoularigis, and Filippos Triposkiadis. 2022. "Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas" Journal of Clinical Medicine 11, no. 7: 1951. https://doi.org/10.3390/jcm11071951





