Extracorporeal Shockwave Therapy in the Treatment of Nonunion in Long Bones: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Selection of the Studies
2.5. Data Extraction
2.6. Assessment of Quality
2.7. Statistical Analysis
3. Results
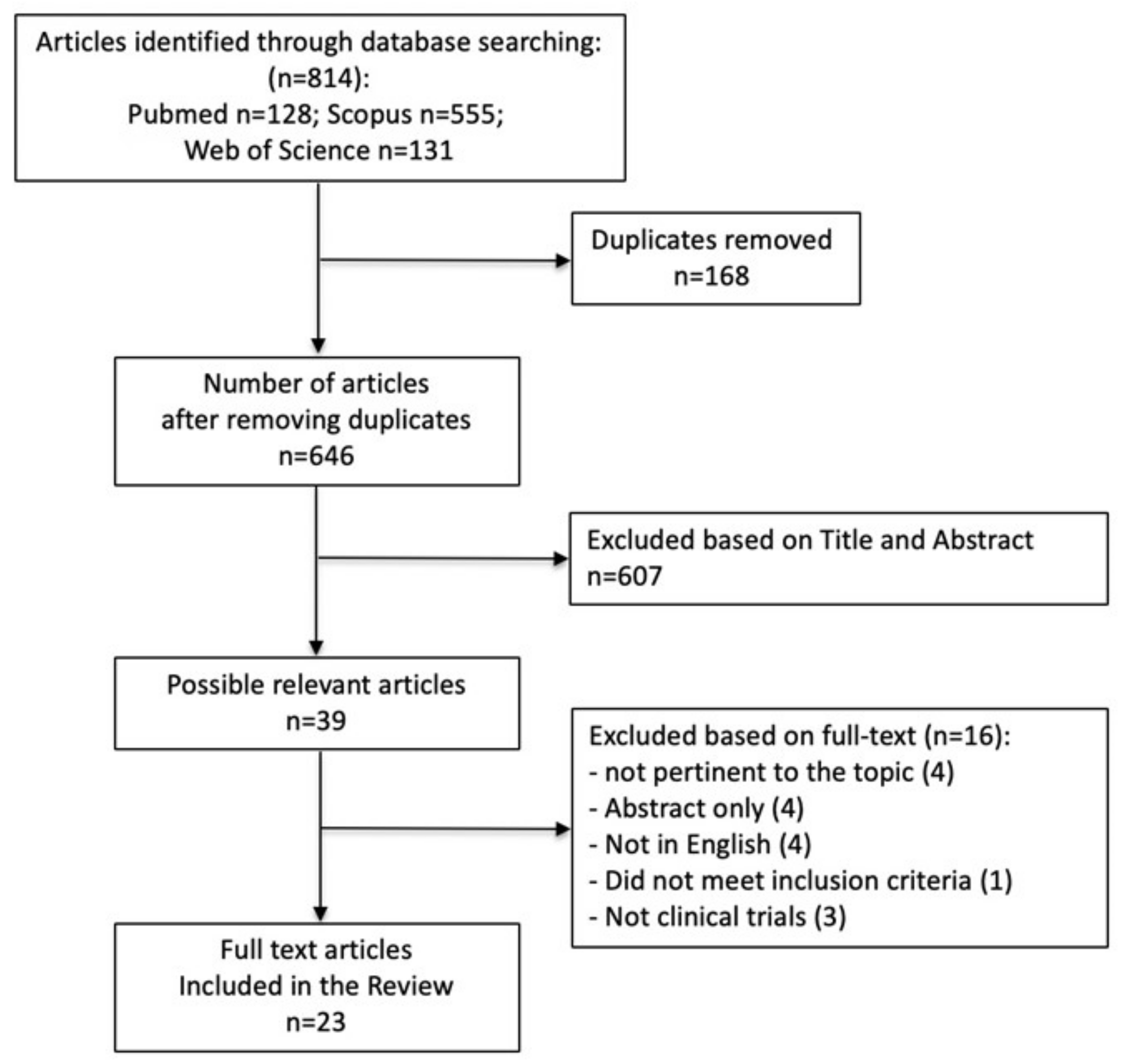
3.1. Study Selection Process
3.2. Study Characteristics
3.3. Overall Healing Rates for ESWT
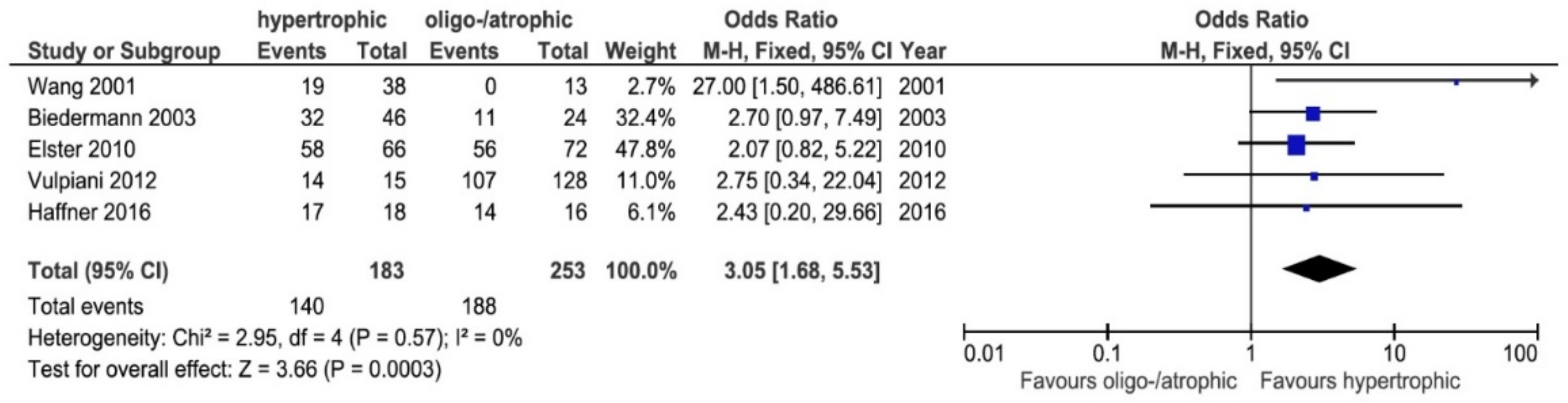
3.4. Healing Rates: Atrophic/Oligotrophic vs. Hypertrophic
3.5. Healing Rates by Anatomical Site
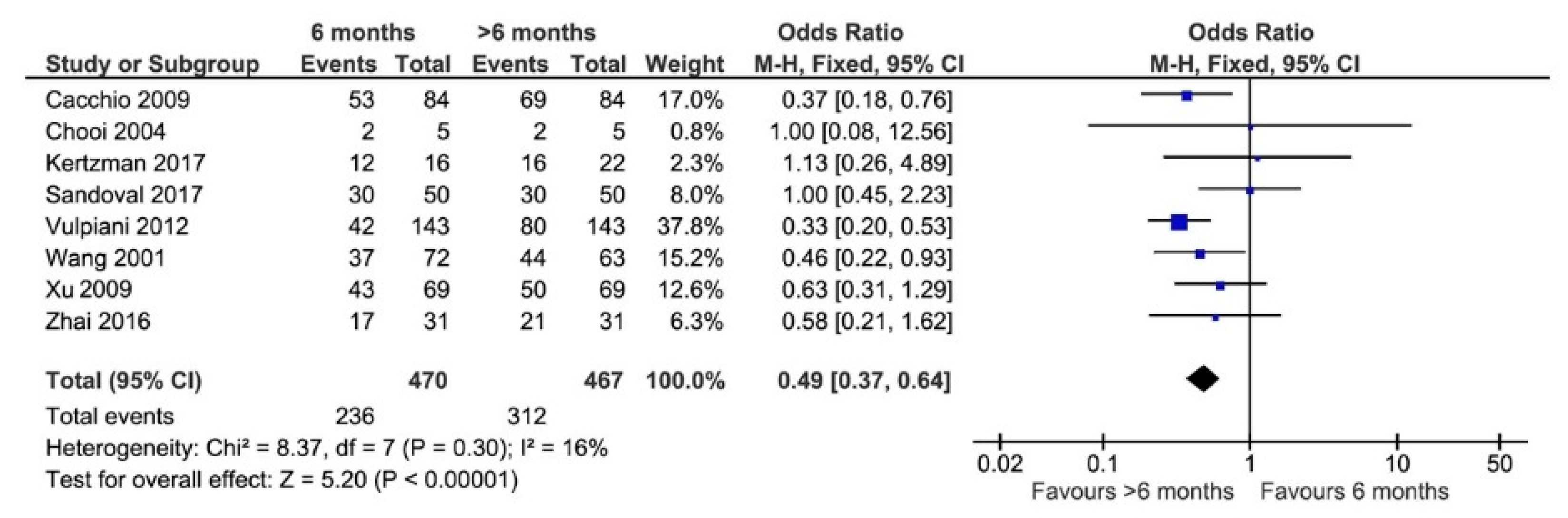
3.6. Elapsed Time between Injury and Treatment
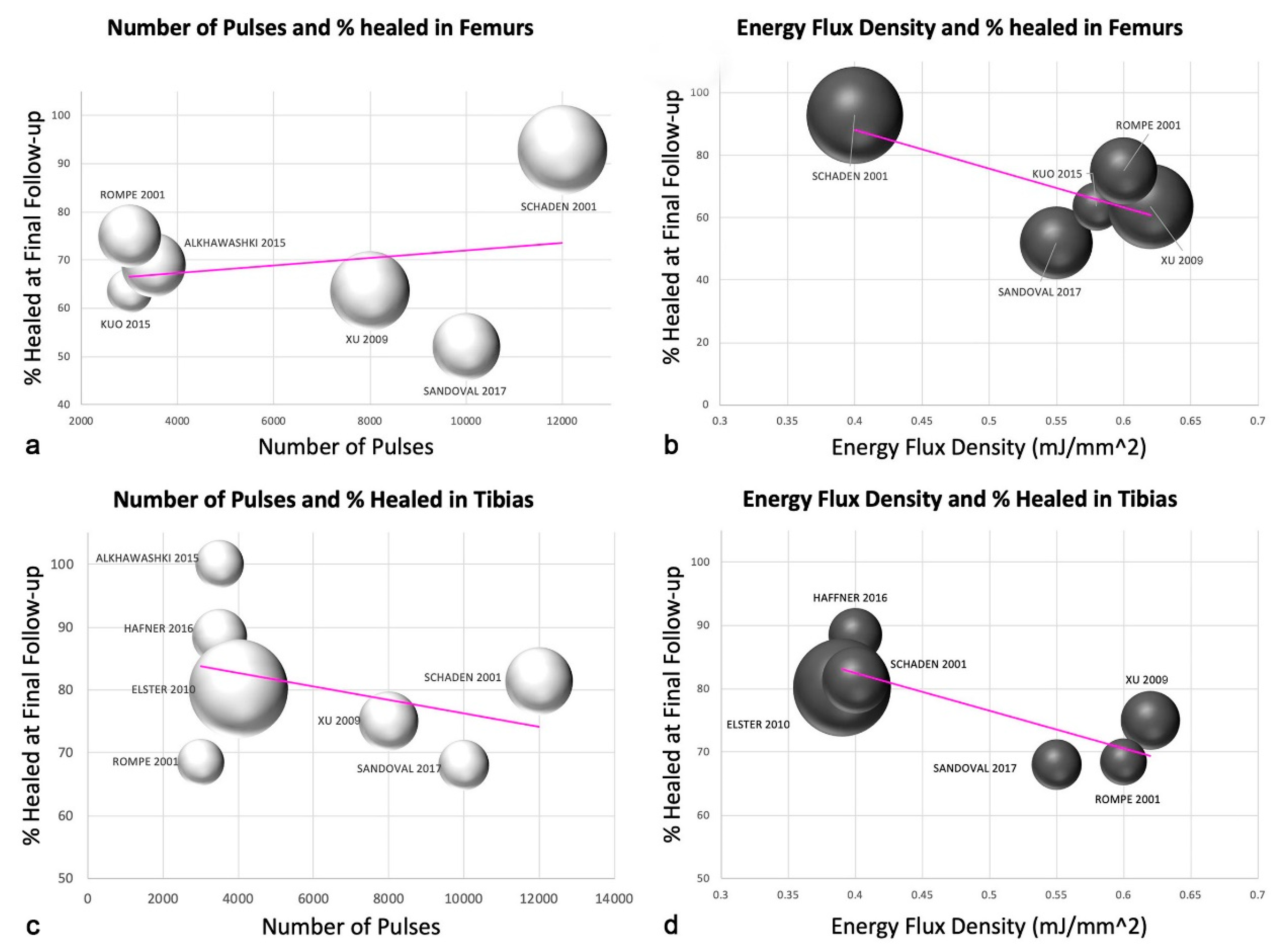
3.7. Number of Pulses and Energy Flux Density for Femurs and Tibias
3.8. Number of ESWT Sessions
3.9. Time of Last Follow-Up
3.10. Assessment of Quality
4. Discussion
4.1. Healing Rates: Atrophic/Oligotrophic vs. Hypertrophic
4.2. Energy Flux Density
4.3. Healing Rates by Anatomical Site
4.4. Elapsed Time between Injury and Treatment
4.5. Risk of Bias
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Search Strategies for Literature Searches
References
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38 (Suppl. 2), S3–S9. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Trauma: Non-Union: New Trends. In European Instructional Lectures, 10th ed.; Bentley, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 10, pp. 15–19. [Google Scholar]
- Pfeifer, R.; Tarkin, I.S.; Rocos, B.; Pape, H.C. Patterns of mortality and causes of death in polytrauma patients—Has anything changed? Injury 2009, 40, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.H.; de Steiger, R.; Richardson, M.; Gruen, R.; Balogh, Z.J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 2014, 45, 1653–1658. [Google Scholar] [CrossRef]
- Kertzman, P.; Császár, N.B.M.; Furia, J.P.; Schmitz, C. Radial extracorporeal shock wave therapy is efficient and safe in the treatment of fracture nonunions of superficial bones: A retrospective case series. J. Orthop. Surg. Res. 2017, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; Forriol, F. Nonunion: General principles and experimental data. Clin. Orthop. Relat. Res. 2004, 419, 4–12. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. 2), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Morshed, S.; Corrales, L.; Genant, H.; Miclau, T., III. Outcome assessment in clinical trials of fracture-healing. J. Bone Jt. Surg. 2008, 90, 62–67. [Google Scholar] [CrossRef]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions: Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Haupt, G. Use of extracorporeal shock waves in the treatment of pseudarthrosis, tendinopathy and other orthopedic diseases. J. Urol. 1997, 158, 4–11. [Google Scholar] [CrossRef]
- Wang, C.J.; Chen, H.S.; Chen, C.E.; Yang, K.D. Treatment of non-unions of long bone fractures with shock waves. Clin. Orthop. Relat. Res. 2001, 387, 95–101. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wurtz, T.; Wang, C.J.; Kuo, Y.R.; Yang, K.D.; Huang, H.C.; Wang, F.S. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J. Orthop. Res. 2004, 22, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Wang, C.J.; Huang, H.C.; Chung, H.; Chen, R.F.; Yang, K.D. Physical shock wave mediates membrane hyperpolarization and Ras activation for osteogenesis in human bone marrow stromal cells. Biochem. Biophy. Res. Commun. 2001, 287, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Yang, K.D.; Chen, R.F.; Wang, C.J.; Sheen-Chen, S.M. Extracorporeal shock wave promotes growth and differentiation of bone-marrow stromal cells towards osteoprogenitors associated with induction of TGF-beta1. J. Bone Jt. Surg. Br. 2002, 84, 457–461. [Google Scholar] [CrossRef]
- Sun, D.; Junger, W.G.; Yuan, C.; Zhang, W.; Bao, Y.; Qin, D.; Wang, C.; Tan, L.; Qi, B.; Zhu, D.; et al. Shockwaves Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells Through ATP Release and Activation of P2X7 Receptors. Stem Cells 2013, 31, 1170–1180. [Google Scholar] [CrossRef]
- Aicher, A.; Heeschen, C.; Sasaki, K.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: A new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006, 114, 2823–2830. [Google Scholar] [CrossRef]
- Tepeköylü, C.; Wang, F.S.; Kozaryn, R.; Albrecht-Schgoer, K.; Theurl, M.; Schaden, W.; Ke, H.J.; Yang, Y.; Kirchmair, R.; Grimm, M.; et al. Shock wave treatment induces angiogenesis and mobilizes endogenous CD31/CD34-positive endothelial cells in a hindlimb ischemia model: Implications for angiogenesis and vasculogenesis. J. Thorac. Cardiovasc. Surg. 2013, 146, 971–978. [Google Scholar] [CrossRef]
- Lei, P.P.; Tao, S.M.; Shuai, Q.; Bao, Y.X.; Wang, S.W.; Qu, Y.Q.; Wang, D.H. Extracorporeal cardiac shock wave therapy ameliorates myocardial fibrosis by decreasing the amount of fibrocytes after acute myocardial infarction in pigs. Coron. Artery Dis. 2013, 24, 509–515. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ha, K.Y.; Kim, J.W.; Seo, J.Y.; Kim, Y.H. Does extracorporeal shock wave introduce alteration of microenvironment in cell therapy for chronic spinal cord injury? Spine 2014, 39, E1553–E1559. [Google Scholar] [CrossRef]
- Caron, J.; Michel, P.; Dussaule, J.; Chatziantoniou, C.; Ronco, P.; Boffa, J. Extracorporeal shock wave therapy does not improve hypertensive nephropathy. Physiol. Rep. 2016, 4, e12699. [Google Scholar] [CrossRef]
- Moll, N.M.; Ransohoff, R.M. CXCL12 and CXCR4 in bone marrow physiology. Expert Rev. Hematol. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Van der Jagt, O.P.; Piscaer, T.M.; Schaden, W.; Li, J.; Kops, N.; Jahr, H.; van der Linden, J.C.; Waarsing, J.H.; Verhaar, J.A.N.; de Jong, M.; et al. Unfocused extracorporeal shock waves induce anabolic effects in rat bone. J. Bone Jt. Surg. 2011, 93, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.F.; Cheung, W.H.; Lee, K.M.; Qin, L.; Leung, K.S. Shockwave exerts osteogenic effect on osteoporotic bone in an ovariectomized goat model. Ultrasound Med. Biol. 2009, 35, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Schaden, W.; Fischer, A.; Sailler, A. Extracorporeal shock wave therapy of nonunion or delayed osseous union. Clin. Orthop. Relat. Res. 2001, 387, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Elster, E.A.; Stojadinovic, A.; Forsberg, J.; Shawen, S.M.; Andersen, R.C.; Schaden, W. Extracorporeal shock wave therapy for nonunion of the tibia. J. Orthop. Trauma 2010, 24, 133–141. [Google Scholar] [CrossRef]
- Nicholson, J.A.; Makaram, N.; Simpson, A.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52 (Suppl. 2), S3–S11. [Google Scholar] [CrossRef]
- Cacchio, A.; Giordano, L.; Colafarina, O.; Rompe, J.D.; Tavernese, E.; Ioppolo, F.; Flamini, S.; Spacca, G.; Santilli, V. Extracorporeal shock-wave therapy compared with surgery for hypertrophic long-bone non-unions. J. Bone Jt. Surg. 2009, 91, 2589–2597. [Google Scholar] [CrossRef]
- Xu, Z.H.; Jiang, Q.; Chen, D.Y.; Xiong, J.; Shi, D.Q.; Yuan, T.; Zhu, X.L. Extracorporeal shock wave treatment in non-unions of long bone fractures. Int. Orthop. 2009, 33, 789–793. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 2535. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.; Deeks, J.; Higgins, J.; Wells, G. Including non-randomized studies. In Cochrane Handbook for Systematic Reviews of 69 Interventions; Version 5.1.0.; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Zhai, L.; Ma, X.L.; Jiang, C.; Zhang, B.; Liu, S.T.; Xing, G.Y. Human autologous mesenchymal stem cells with extracorporeal shock wave therapy for nonunion of long bones. Indian J. Orthop. 2016, 50, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Furia, J.P.; Juliano, P.J.; Wade, A.M.; Schaden, W.; Mittermayr, R. Shock wave therapy compared with intramedullary screw fixation for nonunion of proximal fifth metatarsal metaphyseal-diaphyseal fractures. J. Bone Jt. Surg. 2010, 92, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Czarnowska-Cubała, M.; Gwoździewicz, K.; Studniarek, M.; Lasek, J. Predictive role of scintigraphy (BS) in bone union induction using extracorporeal shock wave treatment (ESWT). J. Orthop. 2013, 10, 70–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.J.; Yang, K.D.; Ko, J.Y.; Huang, C.C.; Huang, H.Y.; Wang, F.S. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-beta1, VEGF and BMP-2 in long bone non-unions. Nitric Oxide 2009, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Haffner, N.; Antonic, V.; Smolen, D.; Slezak, P.; Schaden, W.; Mittermayr, R.; Stojadinovic, A. Extracorporeal shockwave therapy (ESWT) ameliorates healing of tibial fracture non-union unresponsive to conventional therapy. Injury 2016, 47, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.J.; Su, I.C.; Wang, C.J.; Ko, J.Y. Extracorporeal shockwave therapy (ESWT) in the treatment of atrophic non-unions of femoral shaft fractures. Int. J. Surg. 2015, 24 Pt B, 131–134. [Google Scholar] [CrossRef]
- Moretti, B.; Notarnicola, A.; Moretti, L.; Patella, S.; Tatò, I.; Patella, V. Bone healing induced by ESWT. Clin. Cases Miner. Bone Metab. 2009, 6, 155–158. [Google Scholar]
- Vogel, J.; Hopf, C.; Eysel, P.; Rompe, J.D. Application of extracorporeal shock-waves in the treatment of pseudarthrosis of the lower extremity. Preliminary results. Arch. Orthop. Trauma Surg. 1997, 116, 480–483. [Google Scholar] [CrossRef]
- Valchanou, V.D.; Michailov, P. High energy shock waves in the treatment of delayed and nonunion of fractures. Int. Orthop. 1991, 15, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.; Valenzuela, A.; Rojas, C.; Brañes, M.; Guiloff, L. Extracorporeal shockwave therapy for atrophic and oligotrophic nonunion of tibia and femur in high energy trauma patients. Case series. Int. J. Surg. Open 2017, 9, 36–40. [Google Scholar] [CrossRef]
- Alkhawashki, H.M. Shock wave therapy of fracture nonunion. Injury 2015, 46, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, R.; Martin, A.; Handle, G.; Auckenthaler, T.; Bach, C.; Krismer, M. Extracorporeal shock waves in the treatment of nonunions. J. Trauma Acute Care Surg. 2003, 54, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Vulpiani, M.C.; Vetrano, M.; Conforti, F.; Minutolo, L.; Trischitta, D.; Furia, J.P.; Ferretti, A. Effects of extracorporeal shock wave therapy on fracture nonunions. Am. J. Orthop. 2012, 41, E122–E127. [Google Scholar] [PubMed]
- Chooi, Y.S.; Penafort, R. Extra-corporeal shock-wave therapy in the treatment of non-unions. Med. J. Malays. 2004, 59, 674–677. [Google Scholar]
- Alvarez, R.G.; Cincere, B.; Channappa, C.; Langerman, R.; Schulte, R.; Jaakkola, J.; Melancon, K.; Shereff, M.; Cross, G.L., 3rd. Extracorporeal shock wave treatment of non- or delayed union of proximal metatarsal fractures. Foot Ankle Int. 2011, 32, 746–754. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Kyle Potter, B.; Eberhardt, J.; Shawen, S.B.; Andersen, R.C.; Forsberg, J.A.; Shwery, C.; Ester, E.A.; Schaden, W. Development of a prognostic naive bayesian classifier for successful treatment of nonunions. J. Bone Jt. Surg. 2011, 93, 187–194. [Google Scholar] [CrossRef]
- Rompe, J.D.; Rosendahl, T.; Schöllner, C.; Theis, C. High-energy extracorporeal shock wave treatment of nonunions. Clin. Orthop. Relat. Res. 2001, 387, 102–111. [Google Scholar] [CrossRef]
- Jenny, G.; Jenny, J.Y.; Mosser, J.J. Ilizarov’s method in infected tibial pseudo-arthrosis and for reconstruction of bone defects. Orthop. Traumatol. 1993, 3, 55–58. [Google Scholar] [CrossRef]
- Jupiter, J.B.; First, K.; Gallico, G.G., 3rd; May, J.W. The role of external fixation in the treatment of post-traumatic osteomyelitis. J. Orthop. Trauma 1988, 2, 79–93. [Google Scholar] [CrossRef]
- Reed, A.A.; Joyner, C.J.; Brownlow, H.C.; Simpson, A.H. Human atrophic fracture non-unions are not avascular. J. Orthop. Res. 2002, 20, 593–599. [Google Scholar] [CrossRef]
- Tawonsawatruk, T.; Kelly, M.; Simpson, H. Evaluation of native mesenchymal stem cells from bone marrow and local tissue in an atrophic nonunion model. Tissue Eng. Part C Methods 2014, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Zelle, B.A.; Gollwitzer, H.; Zlowodzki, M.; Bühren, V. Extracorporeal shock wave therapy: Current evidence. J. Orthop. Trauma 2010, 24, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Tischer, T.; Milz, S.; Weiler, C.; Pautke, C.; Hausdorf, J.; Schmitz, C.; Maier, M. Dose-dependent new bone formation by extracorporeal shock wave application on the intact femur of rabbits. Eur. Surg. Res. 2008, 41, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, H.; Gloeck, T.; Roessner, M.; Langer, R.; Horn, C.; Gerdesmeyer, L.; Diehl, P. Radial extracorporeal shock wave therapy (rESWT) induces new bone formation in vivo: Results of an animal study in rabbits. Ultrasound Med. Biol. 2013, 39, 126–133. [Google Scholar] [CrossRef]
- Koolen, M.K.E.; Kruyt, M.C.; Öner, F.C.; Schaden, W.; Weinans, H.; van der Jagt, O.P. Effect of unfocused extracorporeal shockwave therapy on bone mineral content of twelve distal forearms of postmenopausal women: A clinical pilot study. Arch. Osteoporos. 2019, 14, 113. [Google Scholar] [CrossRef]
- Siebert, W.; Buch, M. Extracorporeal Shock Waves in Orthopaedics; Springer Verlag: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Hausdorf, J.; Lutz, A.; Mayer-Wagner, S.; Birkenmaier, C.; Jansson, V.; Maier, M. Shock wave therapy for femoral head necrosis-Pressure measurements inside the femoral head. J. Biomech. 2010, 43, 2065–2069. [Google Scholar] [CrossRef]
- Wang, C.J.; Liu, H.C.; Fu, T.H. The effects of extracorporeal shockwave on acute high-energy long bone fractures of the lower extremity. Arch. Orthop. Trauma Surg. 2007, 127, 137–142. [Google Scholar] [CrossRef]
| ESWT Protocol | Patient Characteristics | Risk of Bias | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Type of Shock Waves | EFD (mJ/mm2) | Number of Pulses | Average and (Min–Max) # of Treatments | Last Follow-Up Mean (Months) | n Cases Treated by ESWT | n Long Bones | Pathology | Downs–Black Criteria |
| Kertzman et al., 2017 | radial | 0.18 | 3000 | 3.6 (2–6) | N.R. | 22 | 20 | NU | 16 |
| Sandoval et al., 2017 | focused | 0.55 | 10,000 | 2.8 (2–3) | 12 | 50 | 50 | NU | 16 |
| Haffner et al., 2016 | NR | 0.4 | 3000–4000 | NR | 6 | 58 | 58 | NU | 16 |
| Zhai et al., 2016 | focused | 0.7 | 2900 | 4.8 (4–5) | 18 | 31 | 31 | NU | 22 |
| Alkhawashki et al., 2015 | focused | NR | 2000–4000 * | 1.3 (1–3) | 21 | 49 | 45 | NU | 12 |
| Kuo et al., 2015 | focused | 0.58 | 3000 | 1 (1) | 12 | 22 | 22 | NU | 16 |
| Czarnowska-cubala et al., 2013 | NR | NR | 3000 | NR | 6 | 31 | 31 | DU/NU | 16 |
| Vulpiani et al., 2012 | focused | 0.25–0.84 | 2500–3000 | NR | 12 | 143 | 126 | NU | 18 |
| Alvarez et al., 2011 | focused | 0.22–0.51 | 2000 | 1 (1) | 12 | 34 | 34 | NU | 18 |
| Stojadinovic et al., 2011 | NR | NR | NR | NR | 6 | 349 | 269 | DU/NU | 18 |
| Elster et al., 2010 | focused | 0.38–0.40 | 4000 | 1.3 (1–4) | 24.7 | 192 | 192 | NU | 16 |
| Furia et al., 2010 | focused | 0.35 | 2000–4000 | 1 | 64.7 | 23 | 23 | NU | 20 |
| Moretti et al., 2009 | NR | 0.22–1.10 | 4000 | NR | 2.25 | 204 | 204 | NU | 9 |
| .Cacchio et al., 2009 | NR | 0.4; 0.7 # | 4000 | 4 | 21.7 | 84 | 126 | NU | 26 |
| Wang et al., 2009 | focused | 0.62 | 6000 | 1 (1) | 15.24 | 42 | 42 | NU | 18 |
| Xu et al., 2009 | focused | 0.56; 0.62 ◊ | 3000–10,000 ** | 1–2 | N.R. | 69 | 69 | NU | 14 |
| Chooi et al., 2004 | focused | NR | 4000 | 1 (1) | 7.75 | 5 | 5 | NU | 14 |
| Bidermann et al., 2003 | NR | 0.7 | 2900 | 1.2 (1–2) | N.R. | 70 | 58 | DU/NU | 12 |
| Rompe et al., 2001 | NR | 0.6 | 3000 | NR | 9 | 43 | 43 | NU | 12 |
| Schaden et al., 2001 | NR | 0.4 | 12,000 | 1 (1) | 18 | 115 | 89 | DU/NU | 8 |
| Wang et al., 2001 | focused | 0.47, 0.56; 0.62 ⌘ | 1000–6000 | NR | 12 | 72 | 72 | NU | 12 |
| Vogel et al., 1997 | NR | 0.6 | 3000 | NR | N.R. | 48 | 48 | NU | 12 |
| Valchanou et al., 1991 | focused | NR | 1000–4000 | 1 (1) | N.R. | 82 | NR | NU | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansone, V.; Ravier, D.; Pascale, V.; Applefield, R.; Del Fabbro, M.; Martinelli, N. Extracorporeal Shockwave Therapy in the Treatment of Nonunion in Long Bones: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1977. https://doi.org/10.3390/jcm11071977
Sansone V, Ravier D, Pascale V, Applefield R, Del Fabbro M, Martinelli N. Extracorporeal Shockwave Therapy in the Treatment of Nonunion in Long Bones: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(7):1977. https://doi.org/10.3390/jcm11071977
Chicago/Turabian StyleSansone, Valerio, Domenico Ravier, Valerio Pascale, Rachel Applefield, Massimo Del Fabbro, and Nicolò Martinelli. 2022. "Extracorporeal Shockwave Therapy in the Treatment of Nonunion in Long Bones: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 7: 1977. https://doi.org/10.3390/jcm11071977
APA StyleSansone, V., Ravier, D., Pascale, V., Applefield, R., Del Fabbro, M., & Martinelli, N. (2022). Extracorporeal Shockwave Therapy in the Treatment of Nonunion in Long Bones: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(7), 1977. https://doi.org/10.3390/jcm11071977








