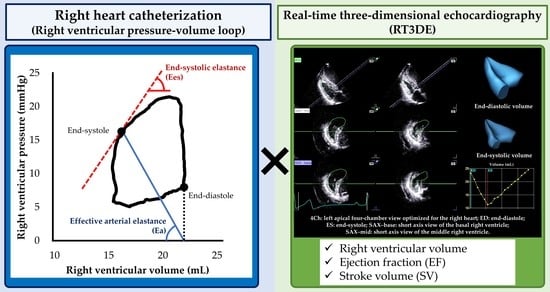
Utility of Real-Time Three-Dimensional Echocardiography for the Assessment of Right Ventricular Morphology and Function in Large Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Protocol
2.3. Hemodynamic Measurement
2.4. Two-Dimensional and Doppler Echocardiography
2.5. RT3DE
2.6. Intra- and Interobserver Measurement Variability
2.7. Statistical Analysis
3. Results
3.1. Dobutamine Infusion
3.2. Changes in Volume Loading Condition
3.3. Single and Multiple Regression Analysis
3.4. Agreements between Right Heart Catheterization and RT3DE
3.5. Intra- and Interobserver Measurement Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Douglas, I.S.; Archer, S.L.; Bogaard, H.J.; Chesler, N.C.; Haddad, F.; Hemnes, A.R.; Kawut, S.M.; Kline, J.A.; Kolb, T.M.; et al. Assessment of right ventricular function in the research setting: Knowledge gaps and pathways forward an official American thoracic society research statement. Am. J. Respir. Crit. Care Med. 2018, 198, e15–e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemann, P.S.; Pinho, L.; Balbach, T.; Galuschky, C.; Blankenhagen, M.; Silberbach, M.; Broberg, C.; Jerosch-Herold, M.; Sahn, D.J. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-Tesla magnetic resonance imaging. J. Am. Coll. Cardiol. 2007, 50, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Li, H.; Wan, X.; Zhong, Y.; Zhang, Y.; Liu, J.; Gao, Y.; Qian, M.; Lin, Y.; Yi, L.; et al. Feasibility and Accuracy of a Fully Automated Right Ventricular Quantification Software With Three-Dimensional Echocardiography: Comparison With Cardiac Magnetic Resonance. Front. Cardiovasc. Med. 2021, 8, 732893. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Wu, V.C.C.; Kado, Y.; Otani, K.; Lin, F.C.; Otsuji, Y.; Negishi, K.; Takeuchi, M. Prognostic Value of Right Ventricular Ejection Fraction Assessed by Transthoracic 3D Echocardiography. Circ. Cardiovasc. Imaging 2017, 10, e005384. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Golfín, C.; Zamorano, J.L. Three-Dimensional Echocardiography and Right Ventricular Function. Circ. Cardiovasc. Imaging 2017, 10, e006099. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, C.; Chan, J.; Bricknell, K.; Strudwick, M.; Marwick, T.H. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: Comparison with cardiac MRI. Chest 2007, 131, 1844–1851. [Google Scholar] [CrossRef]
- Brimioulle, S.; Wauthy, P.; Ewalenko, P.; Rondelet, B.; Vermeulen, F.; Kerbaul, F.; Naeije, R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am. J. Physiol. Circ. Physiol. 2003, 284, H1625–H1630. [Google Scholar] [CrossRef]
- Yuchi, Y.; Suzuki, R.; Teshima, T.; Matsumoto, H.; Koyama, H. Investigation of the influence of manual ventilation-controlled respiration on right ventricular pressure-volume loops and echocardiographic variables in healthy anesthetized dogs. Am. J. Vet. Res. 2021, 82, 865–871. [Google Scholar] [CrossRef]
- Yuchi, Y.; Suzuki, R.; Teshima, T.; Matsumoto, H.; Koyama, H. Utility of tricuspid annular plane systolic excursion normalized by right ventricular size indices in dogs with postcapillary pulmonary hypertension. J. Vet. Intern. Med. 2021, 35, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Yuchi, Y.; Suzuki, R.; Teshima, T.; Matsumoto, H.; Koyama, H. Right ventricular systolic and diastolic function assessed by two-dimensional speckle tracking echocardiography in dogs with myxomatous mitral valve disease. J. Vet. Med. Sci. 2021, 83, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Gentile-Solomon, J.M.; Abbott, J.A. Conventional echocardiographic assessment of the canine right heart: Reference intervals and repeatability. J. Vet. Cardiol. 2016, 18, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Scansen, B.A.; Schober, K.E.; Bonagura, J.D. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: Repeatability and reference intervals. J. Vet. Cardiol. 2015, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Sintov, D.J.; Oldach, M.S. Evaluation of tricuspid annular plane systolic excursion measured by two-dimensional echocardiography in healthy dogs: Repeatability, reference intervals, and comparison with M-mode assessment. J. Vet. Cardiol. 2018, 20, 165–174. [Google Scholar] [CrossRef]
- Yuchi, Y.; Suzuki, R.; Kanno, H.; Teshima, T.; Matsumoto, H.; Koyama, H. Right Ventricular Myocardial Adaptation Assessed by Two-Dimensional Speckle Tracking Echocardiography in Canine Models of Chronic Pulmonary Hypertension. Front. Vet. Sci. 2021, 8, 727155. [Google Scholar] [CrossRef]
- Suzuki, R.; Yuchi, Y.; Kanno, H.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Pulmonary Vascular Resistance Estimated by Echocardiography in Dogs With Myxomatous Mitral Valve Disease and Pulmonary Hypertension Probability. Front. Vet. Sci. 2021, 8, 771726. [Google Scholar] [CrossRef]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Leibundgut, G.; Rohner, A.; Grize, L.; Bernheim, A.; Kessel-Schaefer, A.; Bremerich, J.; Zellweger, M.; Buser, P.; Handke, M. Dynamic Assessment of Right Ventricular Volumes and Function by Real-Time Three-Dimensional Echocardiography: A Comparison Study With Magnetic Resonance Imaging in 100 Adult Patients. J. Am. Soc. Echocardiogr. 2010, 23, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.S.; Young, A.; Occleshaw, C.; Cowan, B.; Zeng, I.S.L.; Gentles, T.L. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults with congenital heart disease: Comparison with cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2009, 22, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef]
- Bleeker, G.B.; Steendijk, P.; Holman, E.R.; Yu, C.M.; Breithardt, O.A.; Kaandorp, T.A.M.; Schalij, M.J.; Van Der Wall, E.E.; Nihoyannopoulos, P.; Bax, J.J. Assessing right ventricular function: The role of echocardiography and complementary technologies. Heart 2006, 92, i19. [Google Scholar] [CrossRef] [Green Version]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef] [Green Version]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Braunwald, E. Heart Disease: A Textbook of Cardiovascular Medicine, 3rd ed.; Braunwald, E., Ed.; Saunders: Philadelphia, PA, USA, 1988; ISBN 0721619541. [Google Scholar]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J. Am. Coll. Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef]
- Richter, M.J.; Peters, D.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Sommer, N.; Gall, H.; Grimminger, F.; Seeger, W.; Tello, K. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 116–119. [Google Scholar] [CrossRef]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic Importance of Right Ventricular-Vascular Uncoupling in Acute Decompensated Heart Failure With Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2020, 13, 11430. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, R.; Rischard, F.; Naeije, R.; Hunter, K.; Simon, M.A. Simple functional imaging of the right ventricle in pulmonary hypertension: Can right ventricular ejection fraction be improved? Int. J. Cardiol. 2016, 223, 93–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trip, P.; Kind, T.; Van De Veerdonk, M.C.; Marcus, J.T.; De Man, F.S.; Westerhof, N.; Vonk-Noordegraaf, A. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J. Hear. Lung Transplant. 2013, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Maughan, W.L.; Shoukas, A.A.; Sagawa, K.; Weisfeldt, M.L. Instantaneous pressure-volume relationship of the canine right ventricle. Circ. Res. 1979, 44, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.M.L.; Periasamy, M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J. Mol. Cell. Cardiol. 2007, 43, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Støylen, A.; Ihlen, H.; Lima, J.A.C.; Smiseth, O.A.; et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Boettler, P.; Hartmann, M.; Watzl, K.; Maroula, E.; Schulte-Moenting, J.; Knirsch, W.; Dittrich, S.; Kececioglu, D. Heart Rate Effects on Strain and Strain Rate in Healthy Children. J. Am. Soc. Echocardiogr. 2005, 18, 1121–1130. [Google Scholar] [CrossRef]
- Weidemann, F.; Jamal, F.; Sutherland, G.R.; Claus, P.; Kowalski, M.; Hatle, L.; De Scheerder, I.; Bijnens, B.; Rademakers, F.E. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am. J. Physiol. Circ. Physiol. 2002, 283, H792–H799. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Piras, P.; Evangelista, A.; Nuzzi, V.; Nardinocchi, P.; Pannarale, G.; Torromeo, C.; Puddu, P.E. Improving performance of 3D speckle tracking in arterial hypertension and paroxysmal atrial fibrillation by using novel strain parameters. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]




| Variables | Baseline | Dobutamine | |
|---|---|---|---|
| 5 µg/kg/min | 10 µg/kg/min | ||
| End-systolic RV pressure (mmHg) | 12.6 ± 4.2 | 19.7 ± 3.5 a | 23.9 ± 5.5 a,b |
| End-diastolic RV pressure (mmHg) | 3.7 ± 2.3 | 5.0 ± 1.9 | 4.1 ± 2.7 |
| RVEDVcath (mL) | 20.3 ± 2.3 | 17.9 ± 3.0 a | 16.0 ± 3.4 a,b |
| RVESVcath (mL) | 10.1 ± 3.1 | 6.9 ± 1.7 a | 6.8 ± 2.5 a |
| EFcath (%) | 41.4 ± 14.3 | 61.6 ± 13.9 a | 57.6 ± 20.4 a |
| SVcath (mL) | 8.5 ± 2.9 | 11.0 ± 3.4 a | 9.2 ± 3.6 |
| Ees (mmHg/mL) | 1.7 ± 0.2 | 2.6 ± 0.6 a | 4.1 ± 1.2 a,b |
| Ea (mmHg/mL) | 1.6 ± 0.4 | 2.0 ± 0.7 | 3.3 ± 1.9 a,b |
| Ees/Ea | 1.2 ± 0.2 | 1.4 ± 0.3 a | 1.4 ± 0.4 |
| RVEDV3D (mL) | 19.7 ± 1.9 | 17.5 ± 2.5 a | 16.0 ± 2.6 a,b |
| RVESV3D (mL) | 10.4 ± 1.3 | 7.1 ± 1.5 a | 6.9 ± 1.6 a,b |
| EF3D (%) | 46.1 ± 7.0 | 58.5 ± 9.4 a | 57.7 ± 11.2 a |
| SV3D (mL) | 9.2 ± 1.5 | 10.3 ± 2.7 a | 9.2 ± 2.8 |
| SV3D/RVESV3D | 0.9 ± 0.2 | 1.5 ± 0.6 a | 1.5 ± 0.6 a |
| Heart rate (bpm) | 66.7 ± 11.5 | 72.3 ± 16.7 | 83.3 ± 13.6 b |
| RVEDA (cm2) | 4.6 ± 0.6 | 4.6 ± 0.6 | 4.7 ± 0.9 |
| RVESA (cm2) | 2.6 ± 0.5 | 2.0 ± 0.3 a | 1.9 ± 0.4 a |
| RVIDd (mm) | 15.2 ± 1.4 | 15.5 ± 1.4 | 15.4 ± 1.4 |
| TAPSE (mm) | 7.7 ± 1.4 | 11.6 ± 1.6 a | 12.9 ± 1.6 a,b |
| RV FAC (%) | 43.6 ± 3.7 | 55.7 ± 5.1 a | 60.1 ± 2.9 a,b |
| RV s’ (cm/s) | 7.3 ± 1.6 | 13.0 ± 2.3 a | 16.3 ± 3.2 a,b |
| RV-SL3seg (%) | 20.1 ± 1.7 | 27.9 ± 2.8 a | 32.9 ± 3.1 a,b |
| RV-SL6seg (%) | 17.2 ± 1.6 | 24.3 ± 2.0 a | 27.4 ± 2.2 a,b |
| RV-SrL3seg (%/s) | 1.6 ± 0.2 | 3.5 ± 0.6 a | 4.6 ± 1.0 a,b |
| RV-SrL6seg (%/s) | 1.4 ± 0.2 | 3.1 ± 0.6 a | 3.7 ± 0.3 a,b |
| Variables | Baseline | Acute Volume Overload | Furosemide | ||
|---|---|---|---|---|---|
| 15 min | 30 min | 15 min | 30 min | ||
| End-systolic RV pressure (mmHg) | 15.2 ± 2.1 | 18.6 ± 2.6 a | 19.8 ± 3.3 a | 15.6 ± 1.4 b,c | 13.9 ± 2.1 b,c |
| End-diastolic RV pressure (mmHg) | 3.7 ± 1.1 | 10.8 ± 1.9 a | 10.0 ± 3.6 a | 5.7 ± 1.6 a,b,c | 3.3 ± 2.0 b,c,d |
| RVEDVcath (mL) | 19.7 ± 2.5 | 25.4 ± 3.6 a | 27.0 ± 3.3 a | 23.4 ± 2.7 a,c | 20.4 ± 2.9 b,c,d |
| RVESVcath (mL) | 10.2 ± 2.6 | 13.2 ± 2.5 | 15.0 ± 3.0 | 12.8 ± 2.1 | 10.8 ± 2.8 |
| EFcath (%) | 45.4 ± 12.4 | 47.7 ± 7.8 | 44.6 ± 11.7 | 44.4 ± 8.4 | 44.6 ± 13.5 |
| SVcath (mL) | 9.6 ± 2.0 | 12.2 ± 2.9 | 12.0 ± 3.4 | 10.7 ± 1.7 | 9.7 ± 2.3 |
| Ees (mmHg/mL) | 1.6 ± 0.2 | 1.8 ± 0.3 | 1.9 ± 0.4 | 2.0 ± 0.5 a | 1.7 ± 0.4 |
| Ea (mmHg/mL) | 1.8 ± 0.5 | 1.5 ± 0.3 | 1.7 ± 0.7 | 1.6 ± 0.5 | 1.6 ± 0.6 |
| Ees/Ea | 1.0 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.2 a | 1.2 ± 0.5 |
| RVEDV3D (mL) | 18.9 ± 2.0 | 23.5 ± 3.3 a | 25.5 ± 2.7 a | 21.8 ± 2.6 a,c | 19.6 ± 2.7 b,c,d |
| RVESV3D (mL) | 10.0 ± 1.3 | 11.9 ± 2.8 | 13.1 ± 2.5 | 11.5 ± 2.1 | 10.2 ± 2.3 c |
| EF3D (%) | 46.3 ± 6.6 | 49.6 ± 8.5 | 48.2 ± 8.3 | 47.5 ± 5.0 | 46.8 ± 9.4 |
| SV3D (mL) | 8.9 ± 1.9 | 11.6 ± 2.2 | 12.5 ± 2.4 | 10.3 ± 1.2 | 9.4 ± 2.1 |
| SV3D/ RVESV3D | 0.8 ± 0.3 | 1.1 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.4 |
| Heart rate (bpm) | 79.3 ± 8.1 | 96.0 ± 15.7 | 125.9 ± 29.2 a | 110.5 ± 32.5 a | 93.7 ± 21.4 |
| RVEDA (cm2) | 4.7 ± 0.5 | 5.1 ± 0.5 | 5.1 ± 0.7 a | 5.0 ± 0.4 | 4.7 ± 0.5 |
| RVESA (cm2) | 2.7 ± 0.4 | 2.9 ± 0.5 | 2.9 ± 0.4 | 2.7 ± 0.2 | 2.8 ± 0.3 |
| RVIDd (mm) | 15.6 ± 1.1 | 16.2 ± 1.0 | 16.5 ± 1.3 | 16.2 ± 1.3 | 15.6 ± 0.9 |
| TAPSE (mm) | 8.3 ± 0.9 | 11.7 ± 1.4 a | 12.8 ± 1.4 a | 10.7 ± 1.1 a,c | 9.3 ± 1.6 b,c,d |
| RV FAC (%) | 43.3 ± 4.1 | 42.8 ± 5.7 | 43.6 ± 4.8 | 46.1 ± 3.5 | 41.2 ± 3.8 |
| RV s’ (cm/s) | 7.7 ± 1.4 | 9.7 ± 1.2 a | 10.5 ± 1.2 a | 10.0 ± 1.5 a | 9.0 ± 1.7 |
| RV-SL3seg (%) | 21.4 ± 2.5 | 24.6 ± 1.8 | 26.4 ± 2.7 a | 24.2 ± 3.2 | 22.2 ± 3.1 c |
| RV-SL6seg (%) | 17.3 ± 2.1 | 20.5 ± 1.4 a | 21.7 ± 1.7 a | 19.7 ± 2.3 a | 17.7 ± 2.4 b,c,d |
| RV-SrL3seg (%/s) | 1.9 ± 0.4 | 1.9 ± 0.3 | 2.4 ± 0.5 b | 2.2 ± 0.7 | 1.8 ± 0.4 c |
| RV-SrL6seg (%/s) | 1.5 ± 0.3 | 1.6 ± 0.2 | 1.9 ± 0.4 a | 1.8 ± 0.6 | 1.5 ± 0.3 c |
| Variables | Ees | Ees/Ea | ||
|---|---|---|---|---|
| Regression Coefficient (95% CI) | p | Regression Coefficient (95% CI) | p | |
| RVEDV3D (mL) | −0.1 (−0.15–−0.06) | <0.001 | 0.02 (0.00–0.04) | 0.079 |
| RVESV3D (mL) | −0.11 (−0.17–−0.04) | 0.002 | −0.01 (−0.04–0.01) | 0.360 |
| EF3D (%) | 0.01 (−0.02–0.03) | 0.641 | 0.01 (0.01–0.02) | <0.001 |
| SV3D (mL) | −0.10 (−0.18–−0.02) | 0.011 | 0.06 (0.04–0.09) | <0.001 |
| SV3D/RVESV3D | 0.20 (−0.24–0.65) | 0.371 | 0.33 (0.18–0.48) | <0.001 |
| RVEDA (cm2) | −0.19 (−0.51–0.13) | 0.246 | 0.07 (−0.05–0.20) | 0.219 |
| RVESA (cm2) | −0.93 (−1.27–−0.59) | <0.001 | −0.09 (−0.24–0.06) | 0.239 |
| RVIDd (mm) | −0.17 (−0.32–−0.01) | 0.033 | 0.02 (−0.04–0.08) | 0.416 |
| TAPSE (mm) | 0.17 (0.09–0.25) | <0.001 | 0.03 (−0.01–0.06) | 0.111 |
| RV FAC (%) | 0.08 (0.06–0.10) | <0.001 | 0.01 (0.00–0.02) | 0.006 |
| RV s’ (cm/s) | 0.19 (0.15–0.24) | <0.001 | 0.04 (0.02–0.06) | 0.001 |
| RV-SL3seg (%) | 0.13 (0.09–0.16) | <0.001 | 0.02 (0.00–0.03) | 0.038 |
| RV-SL6seg (%) | 0.16 (0.12–0.20) | <0.001 | 0.02 (0.00–0.04) | 0.041 |
| RV-SrL3seg (%/s) | 0.61 (0.49–0.74) | <0.001 | 0.06 (−0.01–0.13) | 0.099 |
| RV-SrL6seg (%/s) | 0.81 (0.67–0.96) | <0.001 | 0.09 (0.01–0.18) | 0.031 |
| Variables | Intra-Observer | Inter-Observer | ||
|---|---|---|---|---|
| CV (%) | ICC | CV (%) | ICC | |
| RVEDV3D (mL) | 4.2 | 0.93 * | 8.1 | 0.89 * |
| RVESV3D (mL) | 3.9 | 0.96 * | 6.9 | 0.90 * |
| EF3D (%) | 5.2 | 0.89 * | 3.7 | 0.92 * |
| SV3D (mL) | 8.1 | 0.90 * | 9.9 | 0.81 * |
| SV3D/RVESV3D | 9.6 | 0.82 * | 6.6 | 0.90 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuchi, Y.; Suzuki, R.; Higuchi, R.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Utility of Real-Time Three-Dimensional Echocardiography for the Assessment of Right Ventricular Morphology and Function in Large Animal Models. J. Clin. Med. 2022, 11, 2001. https://doi.org/10.3390/jcm11072001
Yuchi Y, Suzuki R, Higuchi R, Saito T, Teshima T, Matsumoto H, Koyama H. Utility of Real-Time Three-Dimensional Echocardiography for the Assessment of Right Ventricular Morphology and Function in Large Animal Models. Journal of Clinical Medicine. 2022; 11(7):2001. https://doi.org/10.3390/jcm11072001
Chicago/Turabian StyleYuchi, Yunosuke, Ryohei Suzuki, Riho Higuchi, Takahiro Saito, Takahiro Teshima, Hirotaka Matsumoto, and Hidekazu Koyama. 2022. "Utility of Real-Time Three-Dimensional Echocardiography for the Assessment of Right Ventricular Morphology and Function in Large Animal Models" Journal of Clinical Medicine 11, no. 7: 2001. https://doi.org/10.3390/jcm11072001
APA StyleYuchi, Y., Suzuki, R., Higuchi, R., Saito, T., Teshima, T., Matsumoto, H., & Koyama, H. (2022). Utility of Real-Time Three-Dimensional Echocardiography for the Assessment of Right Ventricular Morphology and Function in Large Animal Models. Journal of Clinical Medicine, 11(7), 2001. https://doi.org/10.3390/jcm11072001