Fungal Infections in Critically Ill COVID-19 Patients: Inevitabile Malum
Abstract
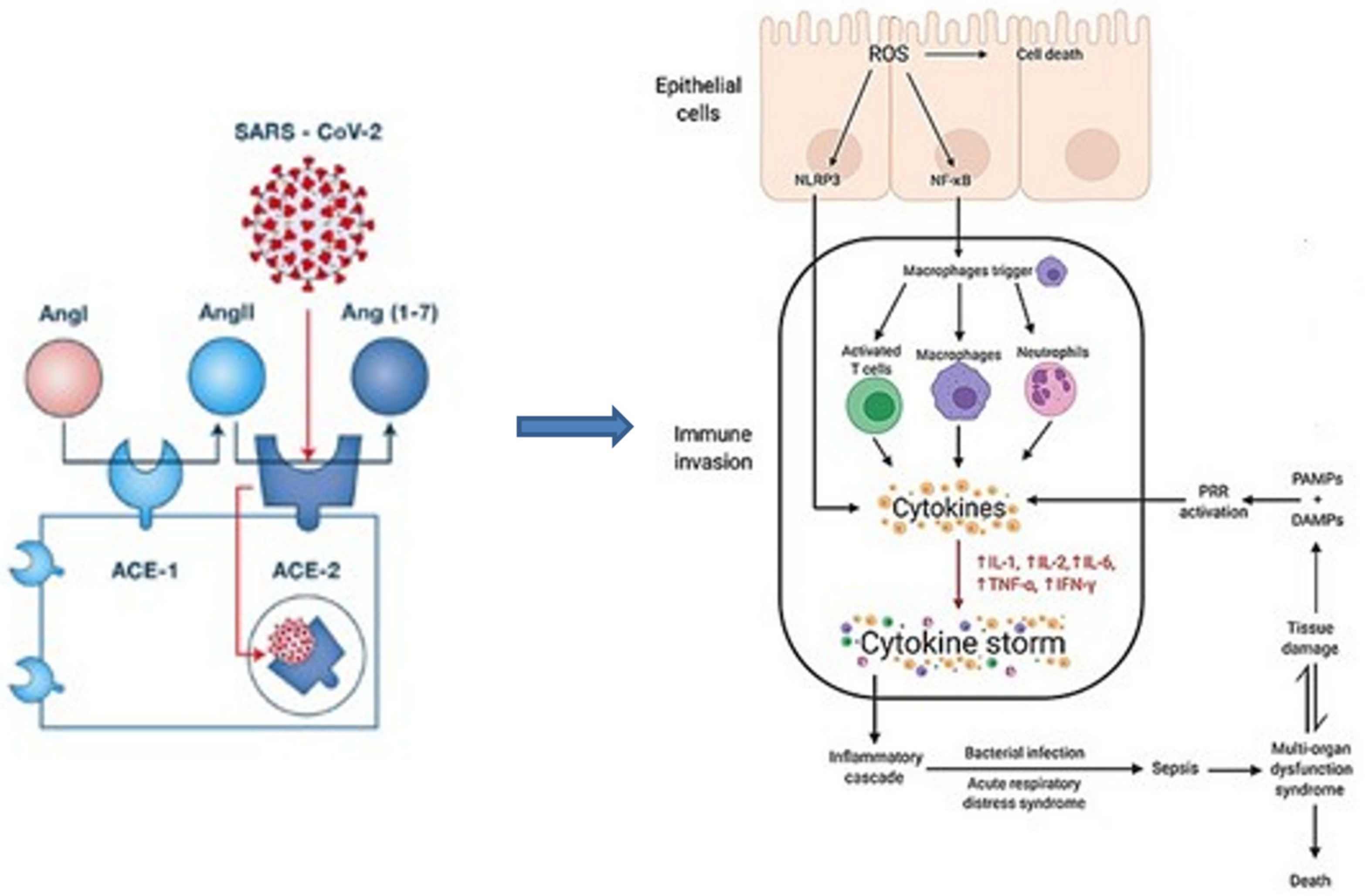
:1. Introduction
2. Pathophysiology and Risk Factors
2.1. Host Factors
2.2. Healthcare Associated Factors
3. Prevalence of Invasive Fungal Infections in Critically Ill COVID-19 Patients
3.1. Candidiasis
3.2. Aspergillosis
3.3. Mucormycosis
3.4. Pneumocystis
| Literature | Trial Design/Population | Type of IFI | Incidence |
|---|---|---|---|
| Fekkar A. et al. [24] | R, SC, n = 145 COVID-19 ICU MV pts screened for fungal superinfection; 54% on ECMO | prob/putat IFI (1 Fusarium case) | 4.8% |
| Chong W.H. et al. [25] | Literature review; 28 O studies, 21 cr/s | Secondary FI | 6.4% |
| Chen N. et al. [26] | R, SC, 99 hospital pts | Secondary FI | 5% |
| Yang X. et al. [27] | R, SC, O, 52 ICU pts | 5.8% | |
| Musuuza J.S. et al. [28] | MA of 118 studies | Fungal co- and superinfections | 4% and 8%, respect |
| Bardi T. et al. [29] | R, SC, 140 ICU pts | FI | 15% |
| White et al. [30] | MC, P, 137 ICU pts screened for IFI | IFI | 26.7% |
| Peng J. et al. [31] | SRMA of 9 studies | IFI | 0.12 (opp) |
| Bishburg E. et al. [34] | SC, R, 89 COVID-19 ICU pts | CAC | 8.9% |
| Nucci M. et al. [35] | SC | CAC | ×5 comp to prepandemic |
| Seagle E.E. et al. [36] | Surveillance data | candidemia | Among 251 candidemia pts, 25.5% were SARS-CoV-2 |
| Gouzien L. et al. [42] | R, O, COVID-19 ICU pts | CAPA | 1.5% |
| Hoenigl M. et al. [43] | Review of 80 CAM cases | CAM | 0.3–0.8% prevalence in COVID-19 ICU pts |
| Meawed T.E. et al. [46] | Cross-sectional study of 197 critically-ill MV COVID-19 pts | Fungal VAP | 16.4% Aspergillus 8.2% mucor |
| Selarka L. et al. [47] | P, O, MC | CAM | 1.8% |
| Alanio et al. [50] | O, 108 critically-ill COVID-19 pts | PJP | 9.3% |
| Blaize et al. [51] | PCR assays on severe COVID-19 pts | PJP | 1.4% |
4. Immunosuppressive Therapy as Risk Factor for Fungal Infections in Critically Ill COVID-19 Patients
4.1. Glucocorticosteroids
4.2. Tocilizumab
5. Mortality and Fungal Infections in Critically Ill COVID-19 Patients
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Live Updates COVID-19—World Health Organization—who.int. Available online: https://www.worldometers.info/coronavirus/ (accessed on 6 November 2021).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 6 November 2021).
- Michelen, M.; Jones, N.; Stavropoulou, C. In Patients of COVID-19, What Are the Symptoms and Clinical Features of Mild and Moderate Cases? Available online: https://www.cebm.net/covid-19/in-patients-of-covid-19-what-are-the-symptoms-and-clinical-features-of-mild-and-moderate-case/CEBM (accessed on 20 November 2021).
- Atzrodt, C.L.; Maknojia, I.; McCarthy, R.D.; Oldfield, T.M.; Po, J.; Ta, K.T.; Stepp, H.E.; Clements, T.P. A Guide to COVID-19: A global pandemic caused by the novel coronavirus SARS-CoV-2. FEBS J. 2020, 287, 3633–3650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coron- avirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 6, 568–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Karaba, S.M.; Jones, G.; Helsel, T.; Smith, L.L.; Avery, R.; Dzintars, K.; Salinas, A.B.; Keller, S.C.; Townsend, J.L.; Klein, E.; et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients. A Multicenter Study. Open Forum Infect. Dis. 2020, 8, ofaa578. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Demoule, A.; Pham, T.; Combes, A.; Dres, M.; Lebbah, S.; Kimmoun, A.; Mercat, A.; Beduneau, G.; et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Cataldo, M.A.; Tetaj, N.; Selleri, M.; Marchioni, L.; Capone, A.; Caraffa, E.; Di Caro, A.; Petrosillo, N. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming “collateral effect”. J. Glob. Antimicrob. Resist. 2020, 23, 290–291. [Google Scholar] [CrossRef]
- Chiurlo, M.; Mastrangelo, A.; Ripa, M.; Scarpellini, P. Invasive fungal infections in patients with COVID-19: A review on pathogenesis, epidemiology, clinical features, treatment, and outcomes. New Microbiol. 2021, 44, 71–83. [Google Scholar]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Van De Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; Perlin, D.S.; Lass-Flörl, C.; Hoenigl, M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)—From Immunology to Treatment. J. Fungi 2020, 6, 91. [Google Scholar] [CrossRef]
- Ezeokoli, O.; Gcilitshana, O.; Pohl, C. Risk Factors for Fungal Co-Infections in Critically Ill COVID-19 Patients, with a Focus on Immunosuppressants. J. Fungi 2021, 7, 545, PMID:34356924. PMCID:PMC8304654. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Sufaru, I.G.; Jelihovschi, I.; Luchian, I.; Hurjui, L.; Martu, I.; Pasarin, L. COVID-19 and periodontal disease: Etiopathogenic and clinical immplications. Rom. J. Oral Rehabil. 2020, 12, 116–124. [Google Scholar]
- Cunha, C.; Carvalho, A.; Esposito, A.; Esposito, F.; Bistoni, F.; Romani, L. DAMP signaling in fungal infections and diseases. Front. Immunol. 2012, 3, 286. [Google Scholar] [CrossRef] [Green Version]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis Complicating Severe Coronavirus Disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef]
- Verweij, P.E.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef]
- Montrucchio, G.; Lupia, T.; Lombardo, D.; Stroffolini, G.; Corcione, S.; De Rosa, F.G.; Brazzi, L. Risk factors for invasive aspergillosis in ICU patients with COVID-19: Current insights and new key elements. Ann. Intensive Care 2021, 11, 136. [Google Scholar] [CrossRef]
- Szabo, B.G.; Lakatos, B.; Bobek, I.; Szabo, E.; Szlavik, J.; Vályi-Nagy, I. Invasive fungal infections among critically ill adult COVID-19 patients: First experiences from the national centre in Hungary. J. Med. Mycol. 2021, 31, 101198. [Google Scholar] [CrossRef]
- Fekkar, A.; Lampros, A.; Mayaux, J.; Poignon, C.; Demeret, S.; Constantin, J.-M.; Marcelin, A.-G.; Monsel, A.; Luyt, C.-E.; Blaize, M. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021, 203, 307–317. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481, Erratum in Lancet Respir. Med. 2020, 8, e26. [Google Scholar] [CrossRef] [Green Version]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019–Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2020, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Mei, H.; Zheng, H.; Liang, G.; She, X.; Liu, W. Fungal co-infection in COVID-19 patients: Evidence from a systematic review and meta-analysis. Aging 2021, 13, 7745–7757. [Google Scholar] [CrossRef]
- White, P.L. Diagnosis of invasive fungal disease in coronavirus disease 2019: Approaches and pitfalls. Curr. Opin. Infect. Dis. 2021, 34, 573–580. [Google Scholar] [CrossRef]
- Calandra, T.; Roberts, J.A.; Antonelli, M.; Bassetti, M.; Vincent, J.-L. Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit. Care 2016, 20, 125. [Google Scholar] [CrossRef] [Green Version]
- Bishburg, E.; Okoh, A.; Nagarakanti, S.R.; Lindner, M.; Migliore, C.; Patel, P. Fungemia in COVID-19 ICU patients, a single medical center experience. J. Med. Virol. 2021, 93, 2810–2814. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2020, 64, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The Landscape of Candidemia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2021, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van De Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Yang, S.; Hua, M.; Liu, X.; Du, C.; Pu, L.; Xiang, P.; Wang, L.; Liu, J. Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. 2021, 23, 104806. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Brown, L.-A.K.; Ellis, J.; Gorton, R.; De, S.; Stone, N. Surveillance for COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e152. [Google Scholar] [CrossRef]
- Gouzien, L.; Cocherie, T.; Eloy, O.; Legriel, S.; Bedos, J.-P.; Simon, C.; Marque-Juillet, S.; Ferré, A.; Bruneel, F. Invasive Aspergillosis associated with Covid-19: A word of caution. Infect. Dis. Now 2021, 51, 383–386. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19- & associated mucormycosis: Analysis of cases from 18 countries. Lancet Microbe 2022. [CrossRef]
- Khan, N.; Gutierrez, C.G.; Martinez, D.V.; Proud, K.C. A case report of COVID-19 associated pulmonary mucormycosis. Arch. Clin. Cases 2020, 07, 46–51. [Google Scholar] [CrossRef]
- Pasero, D.; Sanna, S.; Liperi, C.; Piredda, D.; Branca, G.P.; Casadio, L.; Simeo, R.; Buselli, A.; Rizzo, D.; Bussu, F.; et al. A challenging complication following SARS-CoV-2 infection: A case of pulmonary mucormycosis. Infection 2020, 49, 1055–1060. [Google Scholar] [CrossRef]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef]
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260. [Google Scholar] [CrossRef]
- Rubiano, C.; Tompkins, K.; Sellers, S.A.; Bramson, B.; Eron, J.; Parr, J.B.; Schranz, A.J. Pneumocystis and Severe Acute Respiratory Syndrome Coronavirus 2 Coinfection: A Case Report and Review of an Emerging Diagnostic Dilemma. Open Forum Infect. Dis. 2020, 8, ofaa633. [Google Scholar] [CrossRef]
- Choy, C.Y.; Wong, C.S. It’s not all about COVID-19: Pneumocystis pneumonia in the era of a respiratory outbreak. J. Int. AIDS Soc. 2020, 23, e25533. [Google Scholar] [CrossRef]
- Alanio, A.; Dellière, S.; Voicu, S.; Bretagne, S.; Mégarbane, B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J. Infect. 2020, 82, 84–123. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Luyt, C.-E.; Lampros, A.; Fekkar, A. COVID-19–related Respiratory Failure and Lymphopenia Do Not Seem Associated with Pneumocystosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1734–1736. [Google Scholar] [CrossRef]
- Jeican, I.I.; Inișca, P.; Gheban, D.; Tăbăran, F.; Aluaș, M.; Trombitas, V.; Cristea, V.; Crivii, C.; Junie, L.M.; Albu, S. COVID-19 and Pneumocystis jirovecii Pulmonary Coinfection-The First Case Confirmed through Autopsy. Medicina 2021, 57, 302. [Google Scholar] [CrossRef]
- Poignon, C.; Blaize, M.; Vezinet, C.; Lampros, A.; Monsel, A.; Fekkar, A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin. Microbiol. Infect. 2020, 26, 1582–1584. [Google Scholar] [CrossRef]
- Nobrega de Almeida, J., Jr.; Moreno, L.; Francisco, E.C.; Noronha Marques, G.; Mendes, A.V.; Barberino, M.G.; Colombo, A.L. Trichosporon asahii superinfections in critically ill COVID-19 patients overexposed to antimicro- bials and corticosteroids. Mycoses 2021, 64, 817–822. [Google Scholar] [CrossRef]
- Khatib, M.Y.; Ahmed, A.A.; Shaat, S.B.; Mohamed, A.S.; Nashwan, A.J. Cryptococcemia in a patient with COVID-19: A case report. Clin. Case Rep. 2020, 9, 853–855. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Land, W.G. Role of DAMPs in respiratory virus-induced acute respiratory distress syndrome—With a preliminary reference to SARS-CoV-2 pneumonia. Genes Immun. 2021, 22, 141–160. [Google Scholar] [CrossRef]
- Galani, I.-E.; Rovina, N.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2020, 22, 32–40. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [Green Version]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- World Health Organization Clinical Management of COVID-19. 2020. Available online: https://apps.who.int/iris/rest/bitstreams/1278777/retrieve (accessed on 1 April 2021).
- IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management (accessed on 15 December 2021).
- COVID-19 Rapid Guideline: Managing COVID-19. Available online: https://www.guidelines.co.uk/infection/nice-covid-19-management (accessed on 15 December 2021).
- Olnes, M.J.; Kotliarov, Y.; Biancotto, A.; Cheung, F.; Chen, J.; Shi, R.; Zhou, H.; Wang, E.; Tsang, J.S.; Nussenblatt, R. Effects of Systemically Administered Hydrocortisone on the Human Immunome. Sci. Rep. 2016, 6, 23002. [Google Scholar] [CrossRef] [Green Version]
- Romanou, V.; Koukaki, E.; Chantziara, V.; Stamou, P.; Kote, A.; Vasileiadis, I.; Koutsoukou, A.; Rovina, N. Dexamethasone in the treatment of COVID-19: Primus inter pares? J. Pers. Med. 2021, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Chen, C.; Bhagat, S.S.; Parker, R.A.; Östör, A.J.K. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatology 2010, 50, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, H.R.; Gaffen, S.L. IL-17–Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffner, A.; Douglas, H.; Braude, A. Selective protection against conidia by mononuclear and against mycelia by polymorpho-nuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 1982, 69, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Kirkpatrick, W.R.; White, M.; Hiemenz, J.W.; Wingard, J.R.; Dupont, B.; Rinaldi, M.G.; Stevens, D.A.; Graybill, J.R. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine 2000, 79, 250–260. [Google Scholar] [CrossRef]
- Luvanda, M.; Posch, W.; Vosper, J.; Zaderer, V.; Noureen, A.; Lass-Flörl, C.; Wilflingseder, D. Dexamethasone Promotes Aspergillus fumigatus Growth in Macrophages by Triggering M2 Repolarization via Targeting PKM2. J. Fungi 2021, 7, 70. [Google Scholar] [CrossRef]
- Clemons, K.V.; Calich, V.L.G.; Burger, E.; Filler, S.G.; Graziutti, M.; Murphy, J.; Roilides, E.; Campa, A.; Dias, M.R.; Edwards, J.E.; et al. Pathogenesis I: Interactions of host cells and fungi. Med. Mycol. 2000, 38 (Suppl. 1), 99–111. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.T.C.; Robson, G.D.; Denning, D.W. Hydrocortisone-enhanced growth of Aspergillus spp.: Implications for pathogenesis. Microbiology 1994, 140, 2475–2479. [Google Scholar] [CrossRef] [Green Version]
- Schaffner, A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J. Clin. Investig. 1985, 76, 1755–1764. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Netea, M.G. Candida and Host Determinants of Susceptibility to Invasive Candidiasis. PLOS Pathog. 2013, 9, e1003079. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 2000, 30, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Spellber, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S16–S22. [Google Scholar] [CrossRef]
- Slivka, A.; Wen, P.Y.; Shea, W.; Loeffler, J.S. Pneumocystis carinii pneumonia during steroid taper in patients with primary brain tumors. Am. J. Med. 1993, 94, 216–219. [Google Scholar] [CrossRef]
- Youssef, J.; Novosad, S.A.; Winthrop, K.L. Infection Risk and Safety of Corticosteroid Use. Rheum. Dis. Clin. N. Am. 2016, 42, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Baddley, J.W.; Stephens, J.M.; Ji, X.; Gao, X.; Schlamm, H.T.; Tarallo, M. Aspergillosis in Intensive Care Unit (ICU) patients: Epidemiology and economic outcomes. BMC Infect. Dis. 2013, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Garnacho-Montero, J.; Amaya-Villar, R.; Ortiz-Leyba, C.; León, C.; Alvarez-Lerma, F.; Nolla-Salas, J.; Iruretagoyena, J.R.; Barcenilla, F. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: Risk factors, clinical presentation and outcome. Crit. Care 2005, 9, R191–R199. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [Green Version]
- Poissy, J.; Funginos, T.; Damonti, L.; Bignon, A.; Khanna, N.; Von Kietzell, M.; Boggian, K.; Neofytos, D.; Vuotto, F.; Coiteux, V.; et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Swamydas, M.; Fischer, B.G.; Plantinga, T.S.; Johnson, M.D.; Jaeger, M.; Green, N.M.; Masedunskas, A.; Weigert, R.; Mikelis, C.; et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J. Clin. Investig. 2013, 123, 5035–5051. [Google Scholar] [CrossRef] [Green Version]
- Alanio, A.; Dellière, S.; Fodil, S.; Bretagne, S.; Mégarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, e48–e49. [Google Scholar] [CrossRef]
- Van Arkel, A.L.E.; Rijpstra, T.A.; Belderbos, H.N.A.; Van Wijngaarden, P.; Verweij, P.E.; Bentvelsen, R.G. COVID-19-associated Pulmonary Aspergillosis. Am. J. Respir. Crit. Care Med. 2020, 202, 132–135. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: A prospective study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef]
- Benedetti, M.F.; Alava, K.H.; Sagardia, J.; Cadena, R.C.; Laplume, D.; Capece, P.; Posse, G.; Nusblat, A.D.; Cuestas, M.L. COVID-19 associated pulmonary aspergillosis in ICU patients: Report of five cases from Argentina. Med. Mycol. Case Rep. 2020, 31, 24–28. [Google Scholar] [CrossRef]
- Dupont, D.; Menotti, J.; Turc, J.; Miossec, C.; Wallet, F.; Richard, J.C.; Argaud, L.; Paulus, S.; Wallon, M.; Ader, F.; et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID-19). Med. Mycol. 2021, 59, 110–114. [Google Scholar] [CrossRef]
- Dellière, S.; Dudoignon, E.; Fodil, S.; Voicu, S.; Collet, M.; Oillic, P.-A.; Salmona, M.; Dépret, F.; Ghelfenstein-Ferreira, T.; Plaud, B.; et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: A French multicentric retrospective cohort. Clin. Microbiol. Infect. 2020, 27, 790.e1–790.e5. [Google Scholar] [CrossRef]
- Meijer, E.F.J.; Dofferhoff, A.S.M.; Hoiting, O.; Meis, J.F. COVID-19-associated pulmonary aspergillosis: A prospective single-center dual case series. Mycoses 2021, 64, 457–464. [Google Scholar] [CrossRef]
- Fortarezza, F.; Boscolo, A.; Pezzuto, F.; Lunardi, F.; Acosta, M.J.; Giraudo, C.; Del Vecchio, C.; Sella, N.; Tiberio, I.; Godi, I.; et al. Proven COVID-19–associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses 2021, 64, 1223–1229. [Google Scholar] [CrossRef]
- Obata, R.; Maeda, T.; Rizk, D.; Kuno, T. Increased Secondary Infection in COVID-19 Patients Treated with Steroids in New York City. Jpn. J. Infect. Dis. 2021, 74, 307–315. [Google Scholar] [CrossRef]
- Riche, C.V.W.; Cassol, R.; Pasqualotto, A.C. Is the Frequency of Candidemia Increasing in COVID-19 Patients Receiving Corticosteroids? J. Fungi 2020, 6, 286. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.S.; Narasimhan, B.; Difabrizio, L.; Rogers, L.; Bose, S.; Li, L.; Chen, R.; Sheehan, J.; El-Halabi, M.A.; Sarosky, K.; et al. Impact of corticosteroids in hospitalised COVID-19 patients. BMJ Open Respir. Res. 2021, 8, e000766. [Google Scholar] [CrossRef] [PubMed]
- Rutsaert, L.; Steinfort, N.; Van Hunsel, T.; Bomans, P.; Naesens, R.; Mertes, H.; Dits, H.; Van Regenmortel, N. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensive Care 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, S.; Kwa, D.; Bosman, R.J.; Juffermans, N.P. Detection of Invasive Pulmonary Aspergillosis in COVID-19 with Non-directed Bronchoalveolar Lavage. Am. J. Respir. Crit. Care Med. 2020, 202, 1171–1173. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Zhang, P.; Sheng, J.; Zhou, J.; Qu, T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: A retrospective case series. Crit. Care 2020, 24, 299. [Google Scholar] [CrossRef]
- Janssen, N.A.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.B.; van Dijk, K.; Altenburg, J.; Bouman, C.S.; van der Spoel, H.I.; et al. Multinational Observational Cohort Study of COVID-19–Associated Pulmonary Aspergillosis1. Emerg. Infect. Dis. 2021, 27, 2892–2898. [Google Scholar] [CrossRef]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef]
- Mishra, N.; Mutya, V.S.S.; Thomas, A.; Rai, G.; Reddy, B.; Mohanan, A.A.; Ray, S.; Thiruvengadem, A.V.; Siddini, V.; Hegde, R. A case series of invasive mucormycosis in patients with COVID-19 infection. Int. J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 867–870. [Google Scholar] [CrossRef]
- Meher, R.; Wadhwa, V.; Kumar, V.; Phanbuh, D.S.; Sharma, R.; Singh, I.; Rathore, P.K.; Goel, R.; Arora, R.; Garg, S.; et al. COVID associated mucormycosis: A preliminary study from a dedicated COVID Hospital in Delhi. Am. J. Otolaryngol. 2021, 43, 103220. [Google Scholar] [CrossRef]
- Moorthy, A.; Gaikwad, R.; Krishna, S.; Hegde, R.; Tripathi, K.K.; Kale, P.G.; Rao, P.S.; Haldipur, D.; Bonanthaya, K. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids-An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J. Maxillofac. Oral Surg. 2021, 20, 418–425. [Google Scholar] [CrossRef]
- Sen, M.; Honavar, S.G.; Bansal, R.; Sengupta, S.; Rao, R.; Kim, U.; Sharma, M.; Sachdev, M.; Grover, A.K.; Surve, A.; et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021, 69, 1670. [Google Scholar]
- Chong, W.H.; Saha, B.K.; Chopra, A. Narrative review of the relationship between COVID-19 and PJP: Does it represent coinfection or colonization? Infection 2021, 49, 1079–1090. [Google Scholar] [CrossRef]
- Lamoth, F.; Glampedakis, E.; Boillat-Blanco, N.; Oddo, M.; Pagani, J.-L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1706–1708. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Wu, D.; Gold, M.; Pettit, N.N.; Pitrak, D.; Mueller, J.; Husain, A.N.; Mutlu, E.A.; Mutlu, G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated with Increased Secondary Infections. Front. Med. 2020, 7, 583897. [Google Scholar] [CrossRef]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Salmanton-García, J.; Maertens, J.; Bourgeois, M.; Reynders, M.; Rutsaert, L.; Van Regenmortel, N.; Lormans, P.; et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—A multinational observational study by the European Confederation of Medical Mycology. Clin. Microbiol. Infect. 2021, 28, 580–587. [Google Scholar] [CrossRef]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef]
- Guaraldi, G.; Meschiari, M.; Cozzi-Lepri, A.; Milic, J.; Tonelli, R.; Menozzi, M.; Franceschini, E.; Cuomo, G.; Orlando, G.; Borghi, V.; et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e474–e484. [Google Scholar] [CrossRef]
- Segrelles-Calvo, G.; de SAraújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.R.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida spp. co-infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir. Med. 2021, 188, 106619. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Hermine, O.; Mariette, X.; Tharaux, P.L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Bureau, S.; Dougados, M.; Tibi, A.; Azoulay, E.; et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 32–40. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Almyroudi, M.-P.; Myrianthefs, P.; Rello, J. COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Intensiv. Med. 2021, 1, 71–80. [Google Scholar] [CrossRef]
- Permpalung, N.; Chiang, T.P.; Massie, A.B.; Zhang, S.X.; Avery, R.K.; Nematollahi, S.; Ostrander, D.; Segev, D.L.; Marr, K.A. COVID-19 Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients. Clin. Infect. Dis. 2021, 74, 83–91. [Google Scholar] [CrossRef]
- Ergün, M.; Brüggemann, R.J.M.; Alanio, A.; Dellière, S.; van Arkel, A.; Bentvelsen, R.G.; Rijpstra, T.; Brugge, S.V.D.S.-V.D.; Lagrou, K.; Janssen, N.A.F.; et al. Aspergillus Test Profiles and Mortality in Critically Ill COVID-19 Patients. J. Clin. Microbiol. 2021, 59, e0122921. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Dimopoulos, G.; Koliakos, N.; Siopi, M.; Vourli, S.; Pournaras, S.; Meletiadis, J. Epidemiology and Incidence of COVID-19-Associated Pulmonary Aspergillosis (CAPA) in a Greek Tertiary Care Academic Reference Hospital. Infect. Dis. Ther. 2021, 10, 1779–1792. [Google Scholar] [CrossRef]
- Omrani, A.S.; Koleri, J.; Ben Abid, F.; Daghfal, J.; Odaippurath, T.; Peediyakkal, M.Z.; Baiou, A.; Sarsak, E.; Elayana, M.; Kaleeckal, A.; et al. Clinical characteristics and risk factors for COVID-19-associated Candidemia. Med. Mycol. 2021, 59, 1262–1266. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tezer Tekce, Y.; Erdem, D.; Turan, S.; et al. Characteristics of candidemia in COVID-19 patients; Increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021, 64, 1083–1091. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive Fungal Infections Complicating COVID-19: A Narrative Review. J. Fungi 2021, 7, 921. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.Á.; Pemán, J. What Do We Know about Candida auris? State of the Art, Knowledge Gaps, and Future Directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef] [PubMed]
- Macauley, P.; Epelbaum, O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021, 64, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, e0114621. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Forsberg, K.; Reuben, J.; Dang, T.; Free, R.; Seagle, E.E.; Sexton, D.J.; Soda, E.; Jones, H.; Hawkins, D.; et al. Notes from the Field: Transmission of Pan-Resistant and Echinocandin-Resistant Candida auris in Health Care Facilities—Texas and the District of Columbia, January–April 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1022–1023. [Google Scholar] [CrossRef]
- Allaw, F.; Zahreddine, N.K.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.; Dbaibo, G.; Kanj, S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef]
- Almeida, J.N.D.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Presta Dias, P.H.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; de Groot, T.; Colombo, A.L. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Hoenigl, M.; Meis, J.F.; Cornely, O.A.; Muthu, V.; Gangneux, J.P.; Perfect, J.; Chakrabarti, A.; Isham, E.A. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses 2021, 64, 1028–1037. [Google Scholar] [CrossRef]
| CAPA/Invasive Aspergillus Tracheobronchitis |
|---|
| 1. High/long dose of corticosteroids; |
| 2. Underlying structural lung disease; |
| 3. Host factors, such as neutropenia, allogeneic stem cell transplant, immunosuppression, inherited severe immunodeficiency; |
| 4. Intubation and mechanical ventilation; |
| 5. Cancer/chemotherapy; |
| 6. Azithromycin (PMID: 33316401)/broad spectrum antibiotics; |
| 7. Severe lung damage due to COVID-19. |
| CAC |
| 1. Prolonged hospital stay; |
| 2. Mechanical ventilation; |
| 3. Central venous catheters; |
| 4. Surgical procedures; |
| 5. Broad-spectrum antibiotics. |
| MAC |
| 1. Diabetes, diabetic ketoacidosis |
| Literature | Trial Design and Population | Diagnostic Criteria Used | CS Used | CS Length | Other IST | Comorbidities | IA Incidence | Time to IA Dx | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Alanio A. et al. [87] France | P, O, n = 27 ICU pts, 9/27 CAPA, med age 63 [IQR 56–71] | EORTC-MSGERC or IAPA + ser β-D-glucan and qPCR (serum or pulm specimens) | 6/9 pts: dexa IV 20 mg/d (D1–5) then 10 mg/d (D6–10). 2/9 pts on prev GCS | 10 ds | NM | HPN more frequent in IPA (7/9 vs. 6/18, p = 0·046) | Probable IPAs: (4%) putative IPAs: 30% | NM | 4/9 |
| van Arkel A.E. et al. [88] Netherlands | O, n = 31 ICU pts on MV | A. fumigatus 5/6, A. Ag GM (+) BAL fluid: 3/6 | 3/6 pts: CS before IPA Dx, dose < 0.3 mg/kg/d | <3 wks | No | 3/6 Pre-existing lung disease | 6/31 (19.35%) presumed IPA | Sx onset—IPA: med 11.5 ds (8–42). ICU admis–IPA: med 5 ds (3–28) | 66.7% died, med 12 ICU ds (11–20) |
| Bartoletti et al. [89] Italy | P, MC, n = 822 | CAPA | MP 1 mg/kg | 5–7 ds | TOCI | 27.7% | Intub-CAPA: med 4ds (2–8). sx onset-CAPA: med 14ds (11–22) | ↑↑ ICU mortality after adj for age, RRT, admis severity scores | |
| Benedetti et al. [90] Argentina | n = 5 ICU pts | IAPA or EORTC-MSGERC serum markers, or AspICU | 5/5 CS (<0.3 mg/kg) | <3wks | No | 1/5 hematologic malignancy 2/5 diabetes | Sx onset–CAPA: 22 ds (13–52). ICU admis-CAPA: med 12 ds | 1/5 died (rest still on MV) | |
| Delliere et al. [92] France | R, O, MC, n = 360 ICU pts; 108 pts sampled on deterioration. 1 SOT. 1 myeloma | EORTC/MSGERC CAPA | NM | NM | Sarilumab 1 pt, eculizumab 6 pts, toci 4 pts | Azithromycin (>3 ds) and prob IPA (OR 3.1, 95% CI, 1.1–8.5, p = 0.02). HD dexa and IPA: 11.5% vs. 28.6%, (p = 0.08), cumul dose ≥100 mg and IPA (OR 3.7, 95% CI 1.0–9.7). | 5.7% in ICU pts 8.5% in MV pts 19.4% in deteriorated pts | Sx onset- IPA: 16 ds (10–23) ICU admis—IPA: 6 ds (1–15) | IPA pts vs. non-IPA: 71.4% vs. 36.8%, p < 0.01). |
| Dupont et al. [91] France | R, 153 ICU pts screened for fungi; 106 PCR SARS-Co-V2 (+) | AspICU + serum/BAL GM | 37% CS | short time | NM | HTN 36.8%, DM 36.8%, TB/COPD/asthma 36.8% | 17.9% putative IPA | MV-CAPA: 6 ds | 42% |
| Fekkar A. et al. [24] France | R, SC, n = 145 COVID-19 ICU MV pts screened for fungal superinfection; 54% on ECMO | EORTC/MSGERC, Mycology lab (microscopy, cultures, PCR respir samples and serum for Aspergillus, PJP, mucorales, GMI, β-D-glucan | Long-term (>3 wks) CS before COVID-19 and IFI (OR, 8.55; IQR, 6.8–10.3; p = 0.01), CS for COVID-19 (dexa 20 mg/d × 10 ds) no IFI | 10 ds | 6 Toci 3 saril 1 anti-IL1 | 100% MV, 68% ↑BW, 57% HTN, 32% DM, 14% preexisting immunosuppression | 4.8% prob/ putat IFI (1 fusarium case), 17.2% colonization | ICU admis-IFI: med 7 ds (IQR, 2–56) | Survival 74.5% |
| Fortarezza et al. [94] Italy | n = 45 COVID-19 autopsies | Histology | CS: 88% of CAPA vs. 54% non-CAPA CS: 12/28 pts 1st wave vs. 16/17 pts 2nd wave | NM | No Toci No antiIL-1 | 7/9 ICU 7/9 HTN 3/9 COPD | 20% proven CAPA, 1st wave 2/28 vs. 2nd wave 7/17 | NM | NA |
| Janssen et al. [102] Belgium, Netherlands, France | O, MC, 2 ICU cohorts: N1 = 512 N2 = 304 | ECCM/ISHAM | CS use not more prevalent in CAPA groups vs. non-CAPA | NM | Other IST < 90 ds before ICU admis | CAPA vs. nonCAPA: COPD 19% vs. 8% (p = 0.042). HIV (AIDS) 7% vs. 0.4% (p = 0.011) | 10–15% | ICU admis to CAPA: 6 ds (IQR 3–9) | 43–52% |
| Lamoth et al. [109] Switzerland | n = 80 ICU MV pts | IAPA | NM | NA | Toci—IPA Dx: 4 ds | No pt had any predisposing factors acc to EORTC/MSG | 3.8% 1 probable 2 putative | COVID dx- IPA: med 9 ds, ICU admis-IPA: 6 ds, MV start-IPA: med 5 ds | 1/3 died |
| Marr et al. [20] Spain, USA | R, MC n = 20 CAPA | Aspergillus recovery in BAS, sputum, BAL or GMI ≥ 1, imaging | NM Systemic and inh CS most common IST associated with CAPA | NM | NM | Age HTN Pulm dis underlying immunosuppressive disease/drugs | NA | Sx onset-CAPA: med 11 ds, ICU admis-CAPA: 9 ds | NM |
| Meijer et al. [93] Netherlands | SC, P, 1st wave: 33 MV ICU pts vs. 2nd wave: 33 MV ICU pts | 2020 ECMM/ISHAM | All CAPA pts in 2nd wave on CS: Dexa 6 mg | 10 ds | no | CVD 4/13 DM 3/13 HTN 2/13 COPD 1/13 ARF 1/13 | 1st vs. 2nd wave poss and prob CAPA: 15.2% vs. 25% (p = 0.36) In total: 19.7% | NM | 40–50% mortality in both groups |
| Obata R. et al. [95] USA | R, 226 COVID-19 hosp pts, 57 on CS vs. 169 no-CS | NM | Dexa (48/57), P (6/57), MP (1/57), MP + P 1/57, HC 1/57 | Max 10ds | 20/57 Toci | NM | CAPA in CS vs. no-CS: 5.3% vs. 0.6% CAPA in toci vs. no-toci: 5% vs. 5.4% | NM | NM |
| Prattes et al. [111] Europe, USA | MC, P, MN 592 COVID-19 ICU pts | 2020 ECMM/ISHAM | Majority on GCS | NM | Toci | Age MV Toci | Proven: 1.9%, Prob 13.5% poss: 3% No-CAPA: 81.6% | ICU admis-CAPA: 8 ds (0–31) | Survival in CAPA pts vs. non-CAPA: 29% vs. 57% |
| Rutsaert et al. [99] Belgium | n = 20 MV pts med 66 yo (56–77) | AspICU | 1/7 CS (pemphigus) | NM | NM | 4/7 DL 2/7 obesity 3/7 DM 3/7 HTN | 7/20 (35%) proven IPA | Sx onset—IPA: 11–23 ds | 4/7 died |
| Van Biesen et al. [100] Netherlands | 42 MV ICU pts (9 IPA vs. 33 non-IPA) | AspICU + GMI ≥ 1 | No CS | NA | NM | 1/9 SOT COPD and asthma more common in IPA group | 9/42 | NM | 22% IPA vs. 15.1% non-IPA (p = 0.6) |
| White et al. [30] UK | MC, P n = 137 ICU pts screened for IFI | AspICU, IAPA, CAPA | 12/25 different CS | N/M | no | 12/25 CRD 8/25 HTN 6/25 DM 6/25 obesity 5/25 CA | 14.1% CAPA | ICU admiss- (+) Aspergillus tests: 8 ds (0–35) | CAPA mortality 57.9% depending on appropriate Tx |
| Literature | Trial Design | CS Used | CS Length | Other IST | Comorbidities/ Risk Factors | Candida Infection Incidence | Time to CAC Dx | Mortality |
|---|---|---|---|---|---|---|---|---|
| Antinori et al. [112] Italy | n = 43 severe COVID-19 pts; 3/43 candidemia | NM | NM | Toci | TPN (3/3), antibiotics (2/3), toci (3/3) | 6.9% BSI | Toci last dose—CAC: med 13 ds | Still hospitalized on publication |
| Chowdhary et al. [97] India | n = 596 COVID-19 ICU pts, 420 MV, 15 Candida BSI | NM | NM | NM | ↑ ICU LOS, HTN, DM, CKD, CS (10/15) | 2.5% BSI | Admis-CAC: 10–12 ds | 53% (60% for C. auris) |
| Ho et al. [98] USA | R, O, n = 4313 hospitalised, 574 (13.3%) received CS | MP > P > dexa | 6.34–9.53 ds | Toci | HTN 35.4% DM23.4% CKD 13% | BSI | NM | |
| Obata et al. [95] USA | R, 226 COVID-19 hosp pts, 57 on CS vs. 169 no-CS | See Table 1 | Max 10ds | 20/57 Toci | NM | CAC in CS vs. no-CS: 7% vs. 0% CAC in toci vs. no-toci: 15% vs. 2.7% | NM | NM |
| Riche et al. [96] Brazil | R, candidemia incidence between COVID and non-COVID inpatients | MP > dexa > P | 2–13 ds | No | HD CS CVC 90.9% ICU pts (72.7%) | ×10 increase in candidemia | ICU admis-CAC: 0–22 ds | 72.7% following CS use |
| Seagle et al. [36] USA | Candidemia in COVID-19 and non-COVID-19 pts, surveillance data | NM | NM | Toci more likely among pts with COVID-19 compared to non-COVID-19 pts | Candidemia RF in non-COVID pts: LD, malignancy, prior surgeries CAC each >1.3 times more common: ICU, MV, CVC, CS, IST. Common RF in COVID-19 pts: DM, obesity. | CS within 30 ds of CAC: ×2 vs. non-COVID-19 pts | SARS-CoV-2 (+) test-Candida culture: med 15 ds ([IQR]: 8–21 days) | CAC: ×2 mortality (62.5%) vs. candidemia in non-COVID-19 pts (32.1%) |
| Segrelles-Calvo [114] et al. Spain | O, P, n = 218 ICU pts | MP | 1–10 ds | Toci-CAC: RR 1.378, p = 0.05. Toci + MP/dexa (p = 0.01) | Malignancies more common in COVID-19 with candida co-infection. ICU, TPN, CVC, ↑LO ICU stay | 14.4% (+) Candida tests |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovina, N.; Koukaki, E.; Romanou, V.; Ampelioti, S.; Loverdos, K.; Chantziara, V.; Koutsoukou, A.; Dimopoulos, G. Fungal Infections in Critically Ill COVID-19 Patients: Inevitabile Malum. J. Clin. Med. 2022, 11, 2017. https://doi.org/10.3390/jcm11072017
Rovina N, Koukaki E, Romanou V, Ampelioti S, Loverdos K, Chantziara V, Koutsoukou A, Dimopoulos G. Fungal Infections in Critically Ill COVID-19 Patients: Inevitabile Malum. Journal of Clinical Medicine. 2022; 11(7):2017. https://doi.org/10.3390/jcm11072017
Chicago/Turabian StyleRovina, Nikoletta, Evangelia Koukaki, Vasiliki Romanou, Sevasti Ampelioti, Konstantinos Loverdos, Vasiliki Chantziara, Antonia Koutsoukou, and George Dimopoulos. 2022. "Fungal Infections in Critically Ill COVID-19 Patients: Inevitabile Malum" Journal of Clinical Medicine 11, no. 7: 2017. https://doi.org/10.3390/jcm11072017






