Artificial Intelligence for COVID-19 Detection in Medical Imaging—Diagnostic Measures and Wasting—A Systematic Umbrella Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Definitions
2.4. Data Extraction and Quality Assessment
- Major flaws when the final score was ,
- Minor flaws when the final score was and ,
- Minimal flaws when the final score was .
2.5. Data Synthesis and Analysis
3. Results
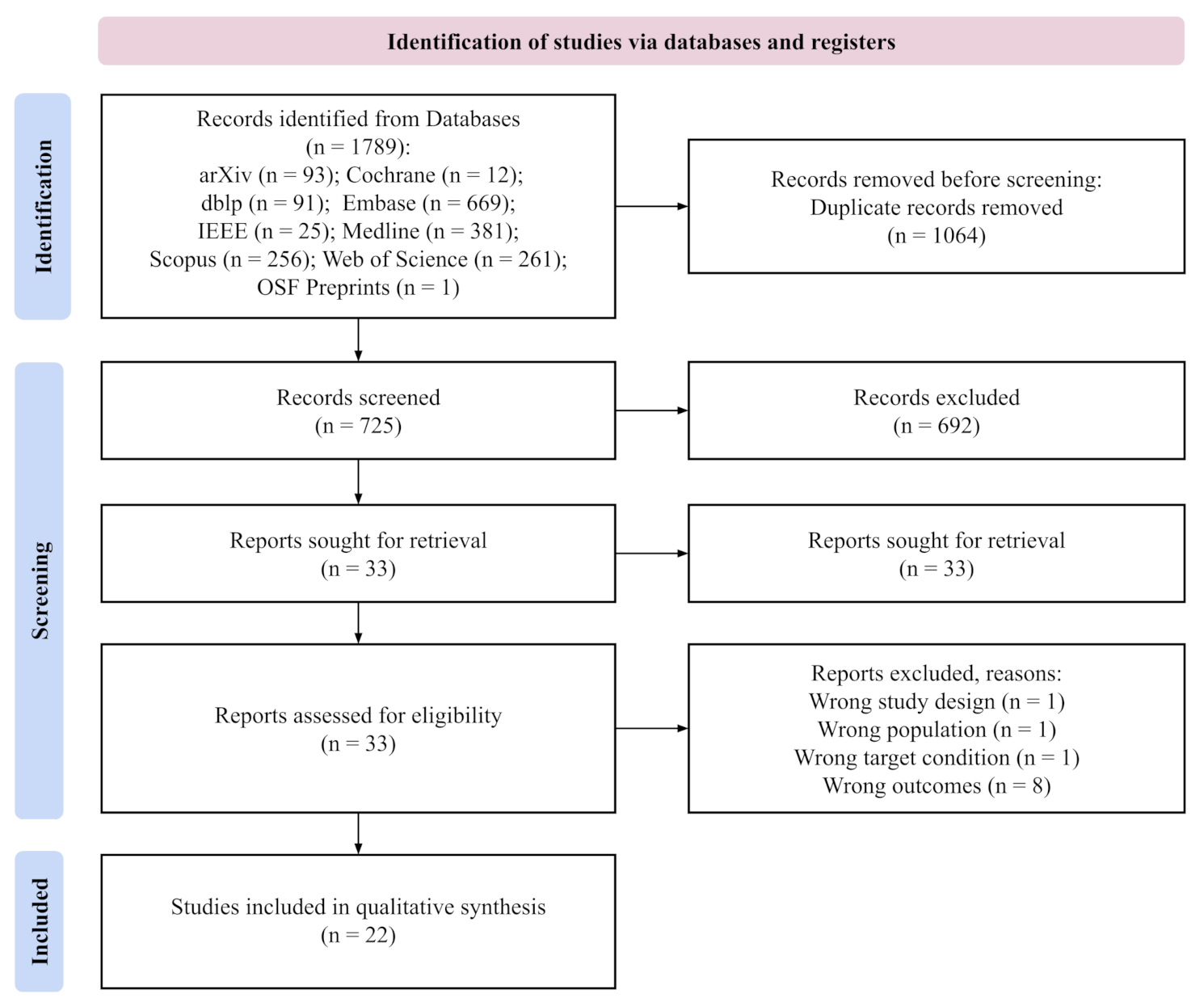
3.1. Included Studies
3.2. Quality of Included Studies
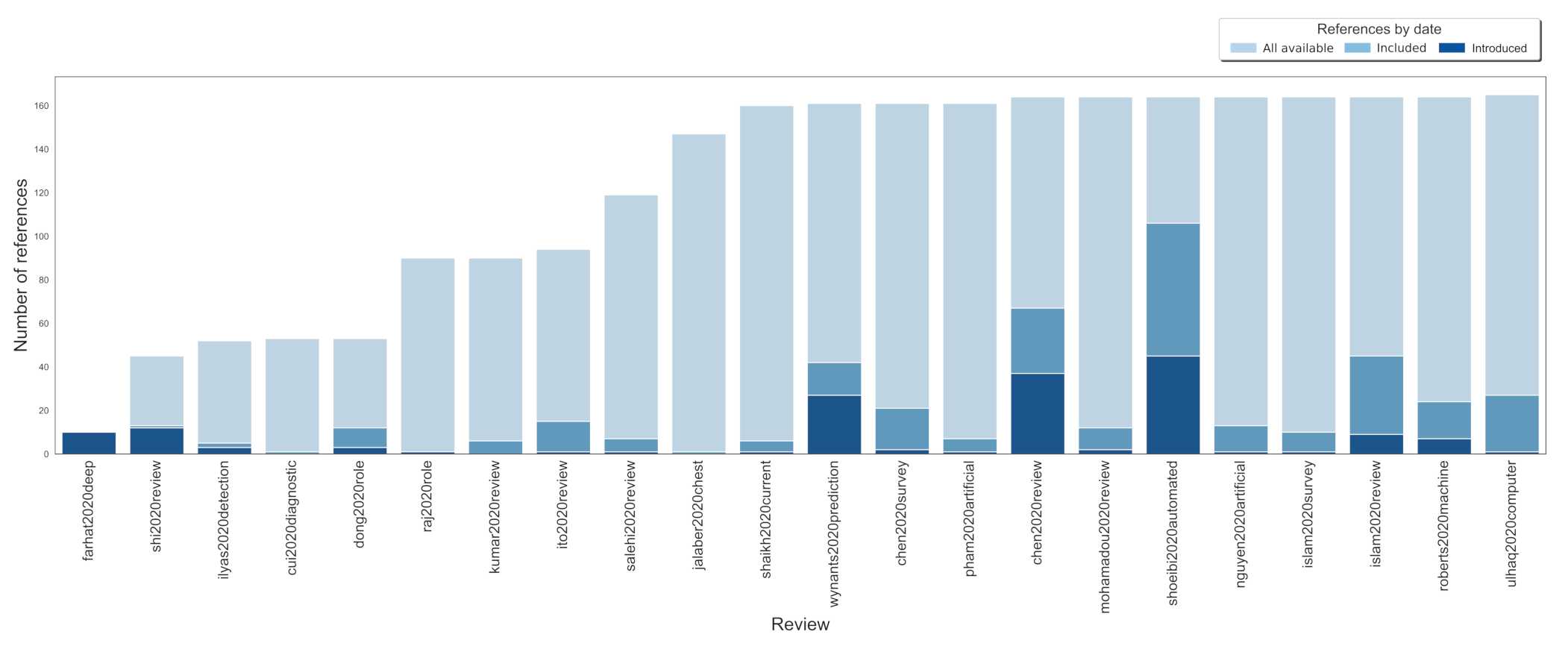
3.3. Resources and Time Wasting Analyses
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| BAL | bronchoalveolar lavage |
| COVID-19 | Coronavirus disease 2019 |
| CT | computed tomography |
| DL | deep learning |
| NAATs | Nucleic Acid Amplification Tests |
| PHEIC | public health emergency of international concern |
| X-ray | radiography |
| RT-PCR | reverse transcription-polymerase chain reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| WHO | World Health Organisation |
References
- Kahn, N. New virus Discovered by Chinese Scientists Investigating Pneumonia Outbreak 2020. Available online: https://www.wsj.com/articles/new-virus-discovered-by-chinese-scientists-investigating-pneumonia-outbreak-11578485668 (accessed on 23 February 2022).
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020. Available online: https://reliefweb.int/report/china/report-who-china-joint-mission-coronavirus-disease-2019-covid-19?gclid=EAIaIQobChMI2vX_nJro9gIVVj5gCh2LDQKuEAAYASAAEgLn9PD_BwE (accessed on 23 February 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 23 February 2022).
- Simon, S.; Frank, B.J.; Aichmair, A.; Manolopoulos, P.P.; Dominkus, M.; Schernhammer, E.S.; Hofstaetter, J.G. Impact of the 1st and 2nd Wave of the COVID-19 Pandemic on Primary or Revision Total Hip and Knee Arthroplasty—A Cross-Sectional Single Center Study. J. Clin. Med. 2021, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, N.; Salehi, M.; Duarte, J.D.; Mollalo, A.; Michailidis, G. County-level longitudinal clustering of COVID-19 mortality to incidence ratio in the United States. Sci. Rep. 2021, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Asai, Y.; Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Tsuzuki, S.; Ohmagari, N. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics: Comparison of the two COVID-19 waves in Japan. J. Infect. Dis. 2020, 82, 84–123. [Google Scholar]
- Coccia, M. The Effects of the First and Second Wave of COVID-19 Pandemic on Public Health. 2020. Available online: https://www.researchsquare.com/article/rs-110013/latest.pdf (accessed on 23 February 2022).
- World Health Organization. World Health Organization coronavirus disease 2019 (COVID-19) Situation Report. 2020. Available online: https://apps.who.int/iris/handle/10665/331686 (accessed on 23 February 2022).
- Kimball, A.; Hatfield, K.M.; Arons, M.; James, A.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; Tanwar, S.; Chisty, Z.; et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR 2020, 69, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.j.; Ni, Z.y.; Hu, Y.; Liang, W.h.; Ou, C.q.; He, J.x.; Liu, L.; Shan, H.; Lei, C.l.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Gutiérrez-Sacristán, A.; Son, J.; Hong, C.; South, A.M.; Beaulieu-Jones, B.K.; Loh, N.H.W.; Luo, Y.; Morris, M.; Ngiam, K.Y.; et al. Multinational Prevalence of Neurological Phenotypes in Patients Hospitalized with COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: Asystematic review and meta-analysis. SSRN 2021, 11, 3769978. [Google Scholar]
- Greenhalgh, T.; Knight, M.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, H.; Huang, M.; Yang, Y. Lower mortality of COVID-19 by early recognition and intervention: Experience from Jiangsu Province. Ann. Intensive Care 2020, 10, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, N.; Salameh, J.P.; Leeflang, M.M.; Hooft, L.; McGrath, T.A.; Pol, C.B.; Frank, R.A.; Kazi, S.; Prager, R.; Hare, S.S.; et al. Thoracic Imaging Tests for the Diagnosis of COVID-19. Cochrane Database Syst. Rev. 2021, CD013639. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing. 2020. Available online: https:/cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html/ (accessed on 23 February 2022).
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.L.; Grenier, J.P.; Batte, C.; Spieler, B. A Characteristic Chest Radiographic Pattern in the Setting of COVID-19 Pandemic. J. Thorac Imaging 2020, 2, e200280. [Google Scholar] [CrossRef] [PubMed]
- Cleverley, J.; Piper, J.; Jones, M.M. The role of chest radiography in confirming covid-19 pneumonia. BMJ 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Palásti, P.; Veréb, D.; Bozsik, B.; Palkó, A.; Kincses, Z.T. The sensitivity and specificity of chest CT in the diagnosis of COVID-19. Eur. Radiol. 2020, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Freer, R.; Stevens, R.; Neil, S.; Jones, N. The Accuracy of Chest CT in the Diagnosis of COVID-19: An Umbrella Review. 2021. Available online: https://www.cebm.net/covid-19/the-accuracy-of-chest-ct-in-the-diagnosis-of-covid-19-an-umbrella-review/ (accessed on 23 February 2022).
- Chua, F.; Armstrong-James, D.; Desai, S.R.; Barnett, J.; Kouranos, V.; Kon, O.M.; José, R.; Vancheeswaran, R.; Loebinger, M.R.; Wong, J.; et al. The role of CT in case ascertainment and management of COVID-19 pneumonia in the UK: Insights from high-incidence regions. Lancet Respir Med. 2020, 8, 438–440. [Google Scholar] [CrossRef] [Green Version]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, L.R.; Sehgal, C.M. A review of early experience in lung ultrasound in the diagnosis and management of COVID-19. Ultrasound Med. Biol. 2020, 46, 2530–2545. [Google Scholar] [CrossRef]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.M.; Zietz, M.; Hoffman, M.M.; et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 2018, 15, 20170387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- McBee, M.P.; Awan, O.A.; Colucci, A.T.; Ghobadi, C.W.; Kadom, N.; Kansagra, A.P.; Tridandapani, S.; Auffermann, W.F. Deep Learning in Radiology. Acad. Radiol. 2018, 25, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Berzin, T.M.; Brown, J.R.G.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: A prospective randomised controlled study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit Health 2019, 1, e271–e297. [Google Scholar] [CrossRef]
- Loo, J.; Clemons, T.E.; Chew, E.Y.; Friedlander, M.; Jaffe, G.J.; Farsiu, S. Beyond performance metrics: Automatic deep learning retinal OCT analysis reproduces clinical trial outcome. Ophthalmology 2020, 127, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Luccioni, A.; Pham, K.H.; Lam, C.S.N.; Luengo-Oroz, M. Mapping the landscape of artificial intelligence applications against COVID-19. J. Artif. Intell. Res. 2020, 69, 807–845. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Zhang, Z.; Hou, L.; Qin, Y.; Hui, X.; Li, J.; Zhao, H.; Cui, G.; Cui, X.; et al. Reporting and methodological quality of COVID-19 systematic reviews needs to be improved: An evidence mapping. J. Clin. Epidemiol. 2021, 135, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Allot, A.; Lu, Z. Keep up with the latest coronavirus research. Nature 2020, 579, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. COVID-19 Portfolio. 2020. Available online: https://icite.od.nih.gov/covid19/search/ (accessed on 23 February 2022).
- Glasziou, P.P.; Sanders, S.; Hoffmann, T. Waste in COVID-19 Research. BMJ 2020, 369, m1847. [Google Scholar]
- London, A.J.; Kimmelman, J. Against pandemic research exceptionalism. Science 2020, 368, 476–477. [Google Scholar] [CrossRef] [Green Version]
- Quinn, T.J.; Burton, J.K.; Carter, B.; Cooper, N.; Dwan, K.; Field, R.; Freeman, S.C.; Geue, C.; Hsieh, P.H.; McGill, K.; et al. Following the science? Comparison of methodological and reporting quality of covid-19 and other research from the first wave of the pandemic. BMC Med. 2021, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: 146 researchers raise concerns over chloroquine study that halted WHO trial. BMJ 2020, 369, 2197. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Coronavirus Disease 2019: The Harms of Exaggerated Information and Non-Evidence-Based Measures. Eur. J. Clin. Invest. 2020, 50, e13222. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T. Preparing for the Next Pandemic. N. Engl. J. Med. 2005, 352, 1839–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemioło, P.; Storman, D.; Moore, J.H.; Orzechowski, P. Diagnosing COVID-19 from Medical Images with Artificial Intelligence—An Umbrella Survey. 2020. Available online: https://osf.io/kxrmh/ (accessed on 23 February 2022).
- Jemioło, P.; Storman, D.; Moore, J.H.; Orzechowski, P. Diagnosing COVID-19 from Medical Images with Artificial Intelligence—An Umbrella Survey (Registration). 2020. Available online: https://osf.io/hkwfq/ (accessed on 23 February 2022).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, B. Artificial Intelligence: Definition, Examples, and Applications. 2020. Available online: https://www.britannica.com/technology/artificial-intelligence (accessed on 23 February 2022).
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef] [PubMed]
- Leondes, C.T. Medical Imaging Systems Techniques and Applications: Computational Techniques; CRC Press: Boca Raton, FL, USA, 1998; Volume 6. [Google Scholar]
- Santosh, K.; Antani, S.; Guru, D.S.; Dey, N. Medical Imaging: Artificial Intelligence, Image Recognition, and Machine Learning Techniques; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Cambridge Dictionary English Dictionary, Translations & Thesaurus. 2021. Available online: https://dictionary.cambridge.org/ (accessed on 23 February 2022).
- Botchkarev, A. Performance Metrics (Error Measures) in Machine Learning Regression, Forecasting and Prognostics: Properties and Typology. arXiv 2018, arXiv:1809.03006. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemioło, P.; Storman, D. Quality Assessment of Systematic Reviews (QASR). 2020. Available online: https://osf.io/dhtw3/ (accessed on 23 February 2022).
- Lorenz, R.C.; Matthias, K.; Pieper, D.; Wegewitz, U.; Morche, J.; Nocon, M.; Rissling, O.; Schirm, J.; Jacobs, A. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J. Clin. Epidemiol. 2019, 114, 133–140. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.l.; Ge, L.; Ma, J.c.; Zeng, Q.l.; Yao, L.; An, N.; Ding, J.x.; Gan, Y.h.; Tian, J.h. Quality of reporting of systematic reviews published in “evidence-based” Chinese journals. Syst. Rev. 2014, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, J.; Shi, J.; Wu, Z.; Wang, Q.; Tang, Z.; He, K.; Shi, Y.; Shen, D. Review of artificial intelligence techniques in imaging data acquisition, segmentation and diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2020, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilyas, M.; Rehman, H.; Naït-Ali, A. Detection of covid-19 from chest X-ray images using artificial intelligence: An early review. arXiv 2020, arXiv:2004.05436. [Google Scholar]
- Dong, D.; Tang, Z.; Wang, S.; Hui, H.; Gong, L.; Lu, Y.; Xue, Z.; Liao, H.; Chen, F.; Yang, F.; et al. The role of imaging in the detection and management of COVID-19: A review. IEEE Rev. Biomed. Eng. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Iwano, S.; Naganawa, S. A review on the use of artificial intelligence for medical imaging of the lungs of patients with coronavirus disease 2019. Diagn. Interv. Radiol. 2020, 26, 443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, P.K.; Srivastava, A. A review of modern technologies for tackling COVID-19 pandemic. Diabetes Metab. Syndr. 2020, 14, 569–573. [Google Scholar] [CrossRef]
- Raj, V. Role of Chest Radiograph (CXR) in COVID-19 Diagnosis and Management. J. Indian Med. Assoc. 2020, 118, 14–19. [Google Scholar]
- Cui, F.; Zhou, H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef]
- Jalaber, C.; Lapotre, T.; Morcet-Delattre, T.; Ribet, F.; Jouneau, S.; Lederlin, M. Chest CT in COVID-19 pneumonia: A review of current knowledge. Diagn. Interv. Imaging 2020, 101, 431–437. [Google Scholar] [CrossRef]
- Salehi, A.W.; Baglat, P.; Gupta, G. Review on machine and deep learning models for the detection and prediction of Coronavirus. Mater. Today Proc. 2020, 33, 3896–3901. [Google Scholar] [CrossRef]
- Farhat, H.; Sakr, G.E.; Kilany, R. Deep learning applications in pulmonary medical imaging: Recent updates and insights on COVID-19. Mach. Vis. Appl. 2020, 31, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.; Anderson, M.; Sohail, M.R.; Mulero, F.; Awan, O.; Dupont-Roettger, D.; Kubassova, O.; Dehmsehki, J.; Bisdas, S. Current landscape of imaging and the potential role for artificial intelligence in the management of COVID-19. Curr. Probl. Diagn. Radiol. 2020, 50. [Google Scholar] [CrossRef] [PubMed]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.; Dahly, D.L.; Damen, J.A.; Debray, T.P.; et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 2020, 369, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, K.; Zhang, Z.; Li, K.; Yu, P.S. A Survey on Applications of Artificial Intelligence in Fighting against COVID-19. arXiv 2020, arXiv:2007.02202. [Google Scholar] [CrossRef]
- Pham, Q.V.; Nguyen, D.C.; Huynh-The, T.; Hwang, W.J.; Pathirana, P.N. Artificial Intelligence (AI) and Big Data for Coronavirus (COVID-19) Pandemic: A Survey on the State-of-the-Arts. IEEE Access 2020, 8, 130820–130839. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ji, S.; Liu, F.; Li, Z.; Zhou, X. A review of Automated Diagnosis of COVID-19 Based on Scanning Images. arXiv 2020, arXiv:2006.05245. [Google Scholar]
- Mohamadou, Y.; Halidou, A.; Kapen, P.T. A review of mathematical modeling, artificial intelligence and datasets used in the study, prediction and management of COVID-19. Appl. Intell. 2020, 50, 3913–3925. [Google Scholar] [CrossRef]
- Shoeibi, A.; Khodatars, M.; Alizadehsani, R.; Ghassemi, N.; Jafari, M.; Moridian, P.; Khadem, A.; Sadeghi, D.; Hussain, S.; Zare, A.; et al. Automated Detection and Forecasting of COVID-19 Using Deep Learning Techniques: A Review. arXiv 2020, arXiv:2007.10785. [Google Scholar]
- Nguyen, T.T. Artificial Intelligence in the Battle Against Coronavirus (COVID-19): A Survey and Future Research Directions. arXiv 2020, arXiv:2008.07343. [Google Scholar]
- Islam, M.N.; Inan, T.T.; Rafi, S.; Akter, S.S.; Sarker, I.H.; Islam, A. A Survey on the Use of AI and ML for Fighting the COVID-19 Pandemic. arXiv 2020, arXiv:2008.07449. [Google Scholar]
- Islam, M.M.; Karray, F.; Alhajj, R.; Zeng, J. A Review on Deep Learning Techniques for the Diagnosis of Novel Coronavirus (COVID-19). IEEE Access 2021, 9, 30551–30572. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Driggs, D.; Thorpe, M.; Gilbey, J.; Yeung, M.; Ursprung, S.; Aviles-Rivero, A.I.; Etmann, C.; McCague, C.; Beer, L.; et al. Machine Learning for COVID-19 Detection and Prognostication Using Chest Radiographs and CT Scans: A Systematic Methodological Review. 2020. Available online: https://www.researchgate.net/publication/343689629_Machine_learning_for_COVID-19_detection_and_prognostication_using_chest_radiographs_and_CT_scans_a_systematic_methodological_review (accessed on 23 February 2022).
- Ulhaq, A.; Born, J.; Khan, A.; Gomes, D.P.S.; Chakraborty, S.; Paul, M. Covid-19 control by computer vision approaches: A survey. IEEE Access 2020, 8, 179437–179456. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.G.; Di Santo, P.; Clifford, C.; Prosperi-Porta, G.; Skanes, S.; Hung, A.; Parlow, S.; Visintini, S.; Ramirez, F.D.; Simard, T.; et al. Methodological quality of COVID-19 clinical research. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shi, Q.; Zheng, P.; Gao, L.; Li, H.; Tao, P.; Gu, B.; Wang, D.; Chen, H. Assessment of the quality of systematic reviews on COVID-19: A comparative study of previous coronavirus outbreaks. J. Med. Virol. 2020, 92, 883–890. [Google Scholar] [CrossRef]
- Al-Ryalat, N.; Al-Rashdan, O.; Alaaraj, B.; Toubasi, A.A.; Alsghaireen, H.; Yaseen, A.; Mesmar, A.; AlRyalat, S.A. Assessment of COVID-19-Related Meta-Analysis Reporting Quality. Ir. J. Med. Sci. 2021, 1–5. [Google Scholar] [CrossRef]
- Nagendran, M.; Chen, Y.; Lovejoy, C.A.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; Ioannidis, J.P.; Collins, G.S.; Maruthappu, M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ 2020, 368, m689. [Google Scholar] [CrossRef] [Green Version]
- Storman, M.; Storman, D.; Jasinska, K.W.; Swierz, M.J.; Bala, M.M. The quality of systematic reviews/meta-analyses published in the field of bariatrics: A cross-sectional systematic survey using AMSTAR 2 and ROBIS. Obes. Rev. 2020, 21, e12994. [Google Scholar] [CrossRef]
- Leclercq, V.; Beaudart, C.; Tirelli, E.; Bruyère, O. Psychometric measurements of AMSTAR 2 in a sample of meta-analyses indexed in PsycINFO. J. Clin. Epidemiol. 2020, 119, 144–145. [Google Scholar] [CrossRef] [Green Version]
- Pieper, D.; Lorenz, R.C.; Rombey, T.; Jacobs, A.; Rissling, O.; Freitag, S.; Matthias, K. Authors should clearly report how they derived the overall rating when applying AMSTAR 2—A cross-sectional study. J. Clin. Epidemiol. 2021, 129, 97–103. [Google Scholar] [CrossRef]
- Adadi, A.; Lahmer, M.; Nasiri, S. Artificial Intelligence and COVID-19: A Systematic Umbrella Review and Roads Ahead. J. King Saud Univ. Comput. Inf. Sci. 2021. [Google Scholar] [CrossRef]
- ESHRE Capri Workshop Group. Protect us from poor-quality medical research. Hum. Reprod. 2018, 33, 770–776. [Google Scholar] [CrossRef] [PubMed]
- International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals: Writing and editing for biomedical publication. Indian J. Pharmacol. 2006, 38, 149. [Google Scholar]
- Johnson, C. Repetitive, duplicate, and redundant publications: A review for authors and readers. J. Manipulative Physiol. Ther. 2006, 29, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Yank, V.; Barnes, D. Consensus and contention regarding redundant publications in clinical research: Cross-sectional survey of editors and authors. J. Med. Ethics 2003, 29, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huth, E.J. Repetitive and divided publication. In Ethical Issues in Biomedical Publication; JHU Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Naudé, W. Artificial intelligence vs COVID-19: Limitations, constraints and pitfalls. AI Soc. 2020, 35, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Corrado, E.M. The Importance of Open Access, Open Source, and Open Standards for Libraries. 2005. Available online: https://library.educause.edu/resources/2005/1/the-importance-of-open-access-open-source-and-open-standards-for-libraries (accessed on 23 February 2022).
- Beaulieu-Jones, B.K.; Greene, C.S. Reproducibility of computational workflows is automated using continuous analysis. Nat. Biotechnol. 2017, 35, 342–346. [Google Scholar] [CrossRef]
- Born, J.; Beymer, D.; Rajan, D.; Coy, A.; Mukherjee, V.V.; Manica, M.; Prasanna, P.; Ballah, D.; Guindy, M.; Shaham, D.; et al. On the role of artificial intelligence in medical imaging of covid-19. Patterns 2021, 2, 100269. [Google Scholar] [CrossRef]
- Chee, M.L.; Ong, M.E.H.; Siddiqui, F.J.; Zhang, Z.; Lim, S.L.; Ho, A.F.W.; Liu, N. Artificial Intelligence Applications for COVID-19 in Intensive Care and Emergency Settings: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4749. [Google Scholar] [CrossRef]
- Syeda, H.B.; Syed, M.; Sexton, K.W.; Syed, S.; Begum, S.; Syed, F.; Prior, F.; Yu, F., Jr. Role of machine learning techniques to tackle the COVID-19 crisis: Systematic review. JMIR Med. Inform 2021, 9, e23811. [Google Scholar] [CrossRef]
- Soltan, A.A.; Kouchaki, S.; Zhu, T.; Kiyasseh, D.; Taylor, T.; Hussain, Z.B.; Peto, T.; Brent, A.J.; Eyre, D.W.; Clifton, D.A. Rapid triage for COVID-19 using routine clinical data for patients attending hospital: Development and prospective validation of an artificial intelligence screening test. Lancet Digit Health 2021, 3, e78–e87. [Google Scholar] [CrossRef]
- The Lancet Digital Health. Artificial intelligence for COVID-19: Saviour or saboteur? Lancet Digit Health 2021, 3, e1. [Google Scholar] [CrossRef]
- Mongan, J.; Moy, L.; Kahn, C.E. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A Guide for Authors and Reviewers. Radiol. Artif. Intell. 2020, 2, e200029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsiger, F.; Jungo, A.; Chen, J.; Ezhov, I.; Liu, S.; Ma, J.; Paetzold, J.C.; Sekuboyina, A.; Shit, S.; Suter, Y.; et al. MICCAI Hackathon on Reproducibility, Diversity, and Selection of Papers. In Proceedings of the MICCAI Conference, Strasburg, France, 27 September–1 October 2021. [Google Scholar]
- Gil, D.; Díaz-Chito, K.; Sánchez, C.; Hernández-Sabaté, A. A Early screening of sars-cov-2 by intelligent analysis of X-ray images. arXiv 2020, arXiv:2005.13928. [Google Scholar]
- Albahri, A.S.; Hamid, R.A.; Alwan, J.K.; Al-Qays, Z.; Zaidan, A.A.; Zaidan, B.B.; Alamoodi, A.H.; Khlaf, J.M.; Almahdi, E.M.; Thabet, E.; et al. Role of biological Data Mining and Machine Learning Techniques in Detecting and Diagnosing the Novel Coronavirus (COVID-19): A Systematic Review. J. Med. Syst. 2020, 44, 122. [Google Scholar] [CrossRef]
- Bressem, K.K.; Adams, L.C.; Erxleben, C.; Hamm, B.; Niehues, S.M.; Vahldiek, J.L. Comparing different deep learning architectures for classification of chest radiographs. Sci. Rep. 2020, 10, 13590. [Google Scholar] [CrossRef]
- Chamola, V.; Hassija, V.; Gupta, V.; Guizani, M. A Comprehensive Review of the COVID-19 Pandemic and the Role of IoT, Drones, AI, Blockchain, and 5G in Managing its Impact. IEEE Access 2020, 8, 90225–90265. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Dai, H.; Damiani, G.; Behzadifar, M.; Martini, M.; Wu, J. How Big Data and Artificial Intelligence Can Help Better Manage the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 3176. [Google Scholar] [CrossRef]
- Nagpal, P.; Narayanasamy, S.; Garg, C.; Vidholia, A.; Guo, J.; Shin, K.M.; Lee, C.H.; Hoffman, E.A. Imaging of COVID-19 pneumonia: Patterns, pathogenesis, and advances. Br. J. Radiol. 2020, 93, 20200538. [Google Scholar] [CrossRef]
- Bansal, A.; Padappayil, R.P.; Garg, C.; Singal, A.; Gupta, M.; Klein, A. Utility of Artificial Intelligence Amidst the COVID 19 Pandemic: A Review. J. Med. Syst. 2020, 44, 156. [Google Scholar] [CrossRef]
- Rezaei, M.; Shahidi, M. Zero-shot learning and its applications from autonomous vehicles to COVID-19 diagnosis: A review. Intell. Med. 2020, 3, 100005. [Google Scholar] [CrossRef]
- Kharat, A.; Duddalwar, V.; Saoji, K.; Gaikwad, A.; Kulkarni, V.; Naik, G.; Lokwani, R.; Kasliwal, S.; Kondal, S.; Gupte, T.; et al. Role of edge device and cloud machine learning in point-of-care solutions using imaging diagnostics for population screening. arXiv 2020, arXiv:2006.13808. [Google Scholar]
- Albahri, O.S.; Zaidan, A.A.; Albahri, A.S.; Zaidan, B.B.; Abdulkareem, K.H.; Al-Qaysi, Z.T.; Alamoodi, A.H.; Aleesa, A.M.; Chyad, M.A.; Alesa, R.M.; et al. Systematic review of artificial intelligence techniques in the detection and classification of COVID-19 medical images in terms of evaluation and benchmarking: Taxonomy analysis, challenges, future solutions and methodological aspects. J. Infect. Public Health 2020, 13, 1381–1396. [Google Scholar]
- Manigandan, S.; Wu, M.-T.; Ponnusamy, V.K.; Raghavendra, V.B.; Pugazhendhi, A.; Brindhadevi, K. A systematic review on recent trends in transmission, diagnosis, prevention and imaging features of COVID-19. Process Biochem. 2020, 98, 233–240. [Google Scholar] [CrossRef]


| Variable | Number (Percentage) | Mean (Range)2 |
|---|---|---|
| Number of reviews with the authors from a specific country | ||
| United States of America | 8 (18%) | NA |
| Australia | 4 (9%) | NA |
| China | 4 (9%) | NA |
| India | 4 (9%) | NA |
| United Kingdom | 3 (7%) | NA |
| Other | 22 (49%) | NA |
| Total number of authors of the reviews | 171 | 8 (1-43) |
| Type of publication | ||
| Journal article (mean IF1: 4.14; range: 0–30.31) | 13 (59%) | NA |
| IEEE Access | 2 (9%) | NA |
| IEEE Reviews in Biomedical Engineering | 2 (9%) | NA |
| Diagnostic and Interventional Imaging | 2 (9%) | NA |
| Diabetes & Metabolic Syndrome: Clinical Research & Reviews | 1 (5%) | NA |
| Applied Intelligence | 1 (5%) | NA |
| British Medical Journal | 1 (5%) | NA |
| Biosensors and Bioelectronics | 1 (5%) | NA |
| Machine Vision and Applications | 1 (5%) | NA |
| Current Problems in Diagnostic Radiology | 1 (5%) | NA |
| Journal of the Indian Medical Association | 1 (5%) | NA |
| Preprint article | 8 (36%) | NA |
| Conference article | 1 (5%) | NA |
| Was the review specified as systematic by the authors? | ||
| No | 20 (91%) | NA |
| Yes | 2 (9%) | NA |
| Number of reviews that searched a given data source | 50 | 5 (3-7) |
| arXiv | 8 (36%) | NA |
| medRxiv | 6 (27%) | NA |
| Pubmed/Medline | 6 (27%) | NA |
| Google Scholar | 6 (27%) | NA |
| bioRxiv | 5 (23%) | NA |
| IEEE Xplore | 3 (14%) | NA |
| Science Direct | 3 (14%) | NA |
| ACM digital library | 2 (9%) | NA |
| Springer | 2 (9%) | NA |
| MICCAI conference | 1 (5%) | NA |
| IPMI conference | 1 (5%) | NA |
| Embase | 1 (5%) | NA |
| Web of Science | 1 (5%) | NA |
| Elsevier | 1 (5%) | NA |
| Nature | 1 (5%) | NA |
| Number of studies | ||
| Reported by review authors as included | 358 | 51 (20–107) |
| Applicable for this review question (total) | 451 | 21 (1–106) |
| Applicable for this review question (unique only) | 165 | 7.5 (0–11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jemioło, P.; Storman, D.; Orzechowski, P. Artificial Intelligence for COVID-19 Detection in Medical Imaging—Diagnostic Measures and Wasting—A Systematic Umbrella Review. J. Clin. Med. 2022, 11, 2054. https://doi.org/10.3390/jcm11072054
Jemioło P, Storman D, Orzechowski P. Artificial Intelligence for COVID-19 Detection in Medical Imaging—Diagnostic Measures and Wasting—A Systematic Umbrella Review. Journal of Clinical Medicine. 2022; 11(7):2054. https://doi.org/10.3390/jcm11072054
Chicago/Turabian StyleJemioło, Paweł, Dawid Storman, and Patryk Orzechowski. 2022. "Artificial Intelligence for COVID-19 Detection in Medical Imaging—Diagnostic Measures and Wasting—A Systematic Umbrella Review" Journal of Clinical Medicine 11, no. 7: 2054. https://doi.org/10.3390/jcm11072054
APA StyleJemioło, P., Storman, D., & Orzechowski, P. (2022). Artificial Intelligence for COVID-19 Detection in Medical Imaging—Diagnostic Measures and Wasting—A Systematic Umbrella Review. Journal of Clinical Medicine, 11(7), 2054. https://doi.org/10.3390/jcm11072054






