Acid-Base Disorders in COVID-19 Patients with Acute Respiratory Distress Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol and Data Collection
- -
- A pH of less than 7.38 was categorized as acidemia; a pH of more than 7.42 was categorized as alkalemia; a pH between 7.38 and 7.42, with PaCO2 between 38 and 42 mmHg and [HCO3-] between 22 and 26 mMol/L was categorized as no disorder;
- -
- Respiratory acidosis pH < 7.38 and PaCO2 > 42 mmHg; respiratory acidosis with the secondary acute metabolic response if [HCO3−] is increased by 1 mMol/liter for each PaCO2 increase of 10 mmHg above 40 mm Hg; respiratory acidosis with the secondary chronic metabolic response if [HCO3−] is increased by 4–5 mMol/liter for each PaCO2 increase of 10 mmHg above 40 mmHg; superimposed metabolic alkalosis or acidosis may be diagnosed if the calculated [HCO3−] is greater or less than predicted;
- -
- Metabolic acidosis pH < 7.38 and bicarbonate [HCO3−] < 22 mMol/L; metabolic acidosis with secondary respiratory response if PaCO2 = 1.5 × [HCO3−] + 8 ± 2 mmHg; superimposed respiratory acidosis or alkalosis may be diagnosed if the calculated PaCO2 is greater or less than predicted;
- -
- Respiratory alkalosis pH > 7.42 and PaCO2 < 38 mmHg; respiratory alkalosis with the secondary acute metabolic response if is decreased by 2 mMol/L for each PaCO2 decrease of 10 mmHg below 40 mmHg; respiratory alkalosis with the secondary chronic metabolic response if [HCO3−] is decreased by 4–5 mMol/L for each PaCO2 decrease of 10 mmHg below 40 mmHg; superimposed metabolic alkalosis or acidosis may be diagnosed if the calculated [HCO3−] is greater or less than predicted;
- -
- Metabolic alkalosis pH > 7.42 and [HCO3−] > 26 mMol/L; metabolic alkalosis with secondary respiratory response if PaCO2 = 0.7 × ([HCO3−] − 24) + 40 ± 2 mmHg; superimposed respiratory acidosis or alkalosis may be diagnosed if the calculated PaCO2 is greater or less than predicted.
- -
- A pH of less than 7.38 was categorized as acidemia; a pH of more than 7.42 was categorized as alkalemia; a pH between 7.38 and 7.42, with PaCO2 between 38 and 42 mmHg and [aSID] between 38 and 42 mEq/L was categorized as no disorder;
- -
- Respiratory acidosis: pH < 7.38, PaCO2 > 42 mm Hg and [aSID] between 38 and 42 mEq/L;
- -
- Metabolic acidosis secondary to aSID: pH < 7.38, PaCO2 between 38 and 42 and [aSID] < 38 mEq/L;
- -
- Other metabolic acidosis: pH < 7.38, PaCO2 between 38 and 42 and [aSID] between 38 and 42 mEq/L;
- -
- Respiratory alkalosis: pH > 7.42, PaCO2 < 38 mmHg and [aSID] 38–42 mEq/L;
- -
- Metabolic alkalosis secondary to aSID: pH > 7.42, PaCO2 between 38 and 42 mmHg and [aSID] > 42 mEq/L;
- -
- Other metabolic alkalosis: pH > 7.42, PaCO2 between 38 and 42 mmHg and [aSID] between 38 and 42 mEq/L;
- -
- Mixed disorder pH 7.38–7.42 with PaCO2 > 42 and [aSID] > 42 mEq/L or PaCO2 < 38 and [aSID] < 38 mEq/L.
2.3. Statistical Analysis
3. Results
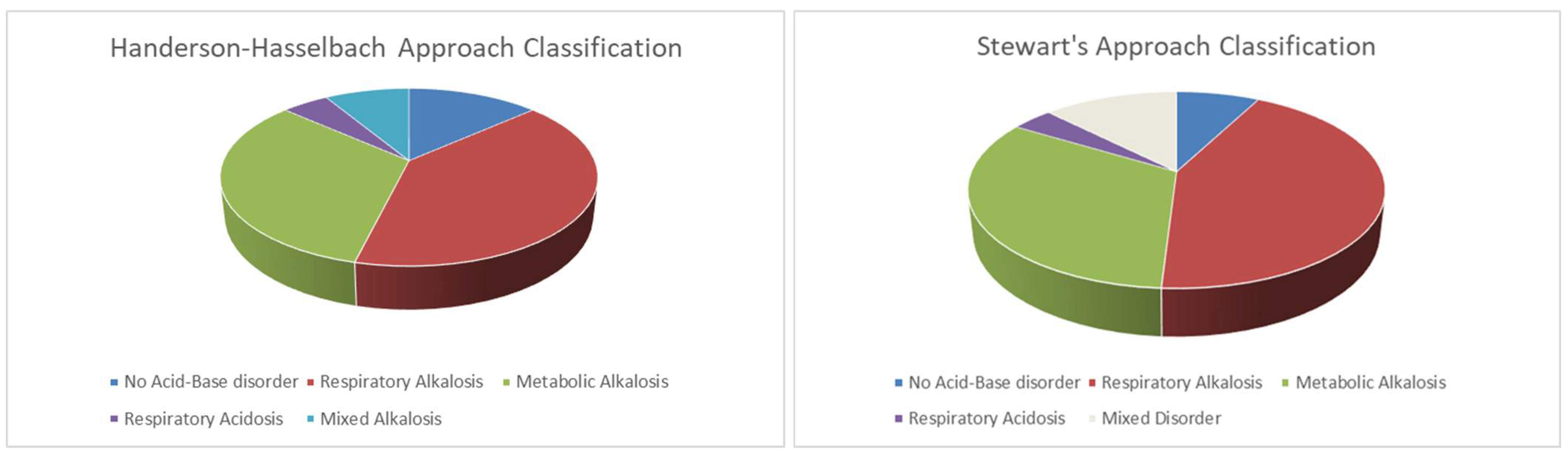
3.1. Acid-Base Disturbance According to Henderson–Hasselbalch Approach
3.2. Acid-Base Disturbance According to Stewart’s Method
3.3. Hypoxemia and Acid-Base Disturbance
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State. JAMA—J. Am. Med. Assoc. 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Xu, D.; Fu, S.; Zhang, J.; Yang, X.; Xu, L.; Xu, J.; Wu, Y.; Huang, C.; Ouyang, Y.; et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care 2020, 24, 219. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T.; Husain-syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T.; et al. Clinical Course and outcomes of critically ill patients with COVID19 in Wuhan China. Lancet Respir Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Zanella, A.; Florio, G.; Antonelli, M.; Bellani, G.; Berselli, A.; Bove, T.; Cabrini, L.; Carlesso, E.; Castelli, G.P.; Cecconi, M.; et al. Time course of risk factors associated with mortality of 1260 critically ill patients with COVID-19 admitted to 24 Italian intensive care units. Intensive Care Med. 2021, 47, 995–1008. [Google Scholar] [CrossRef]
- Jung, B.; Rimmele, T.; Le Goff, C.; Chanques, G.; Corne, P.; Jonquet, O.; Muller, L.; Lefrant, J.Y.; Guervilly, C.; Papazian, L.; et al. Severe metabolic or mixed acidemia on intensive care unit admission: Incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit. Care 2011, 15, R238. [Google Scholar] [CrossRef] [Green Version]
- Alfano, G.; Fontana, F.; Mori, G.; Giaroni, F.; Ferrari, A.; Giovanella, S.; Ligabue, G.; Ascione, E.; Cazzato, S.; Ballestri, M.; et al. Acid base disorders in patients with COVID-19. Int. Urol. Nephrol. 2021, 54, 405–410. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Berend, K.; de Vries, A.P.J.; Gans, R.O.B. Physiological Approach to Assessment of Acid–Base Disturbances. N. Engl. J. Med. 2014, 371, 1434–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastegar, A. Clinical utility of Stewart’s method in diagnosis and management of acid-base disorders. Clin. J. Am. Soc. Nephrol. 2009, 4, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.A. Modern quantitative acid-base chemistry. Can. J. Physiol. Pharmacol. 1983, 61, 1444–1461. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A. Independent and dependent variables of acid-base control. Respir. Physiol. 1978, 33, 9–26. [Google Scholar] [CrossRef]
- Boniatti, M.M.; Cardoso, P.R.C.; Castilho, R.K.; Vieira, S.R.R. Acid-base disorders evaluation in critically ill patients: We can improve our diagnostic ability. Intensive Care Med. 2009, 35, 1377–1382. [Google Scholar] [CrossRef]
- Fencl, V.; Jabor, A.; Kazda, A.; Figge, J. Diagnosis of metabolic acid-base disturbances in critically III patients. Am. J. Respir. Crit. Care Med. 2000, 162, 2246–2251. [Google Scholar] [CrossRef]
- Dubin, A.; Menises, M.M.; Masevicius, F.D.; Moseinco, M.C.; Kutscherauer, D.O.; Ventrice, E.; Laffaire, E.; Estenssoro, E. Comparison of three different methods of evaluation of metabolic acid-base disorders. Crit. Care Med. 2007, 35, 1264–1270. [Google Scholar] [CrossRef]
- Apigo, M.; Schechtman, J.; Dhliwayo, N.; Al Tameemi, M.; Gazmuri, R.J. Development of a work of breathing scale and monitoring need of intubation in COVID-19 pneumonia. Crit. Care. 2020, 24, 4–6. [Google Scholar] [CrossRef]
- Raphael, K.L.; Zhang, Y.; Wei, G.; Greene, T.; Cheung, A.K.; Beddhu, S. Serum bicarbonate and mortality in adults in NHANES III. Nephrol. Dial. Transplant. 2013, 28, 1207–1213. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Wildenthal, K.; Johnson, R.L. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972, 1, 375–389. [Google Scholar] [CrossRef] [Green Version]
- Berend, K. Diagnostic use of base excess in acid-base disorders. N. Engl. J. Med. 2018, 378, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA-J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, G.; Zhang, Q.; Yu, B.; Lv, J.; Zhang, S.; Wu, G.; Wu, S.; Zhong, Y. Association Between Respiratory Alkalosis and the Prognosis of COVID-19 Patients. Front. Med. 2021, 8, 564635. [Google Scholar] [CrossRef]
- Laffey, J.G. Hypocapnia. N. Engl. J. Med. 2002, 347, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef]
- Bulfamante, G.; Chiumello, D.; Canevini, M.P.; Priori, A.; Mazzanti, M.; Centanni, S.; Felisati, G. First ultrastructural autoptic findings of SARS-CoV-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020, 86, 678–679. [Google Scholar] [CrossRef]
- Alfano, G.; Guaraldi, G.; Fontana, F.; Ferrari, A.; Magistroni, R.; Mussini, C.; Cappelli, G. The Role of the Renin-Angiotensin System in Severe Acute Respiratory Syndrome-CoV-2 Infection. Blood Purif. 2021, 50, 263–267. [Google Scholar] [CrossRef]
- Coppola, S.; Santus, P.; Sotgiu, G.; Mondoni, M.; Gandola, A.; Saad, M.; Sferrazza Papa, G.F.; Centanni, S.; Saderi, L.; Chiumello, D.A.; et al. Feasibility and clinical outcomes of a step up noninvasive respiratory support strategy in patients with severe COVID-19 pneumonia. J. Clin. Med. 2021, 10, 5444. [Google Scholar] [CrossRef] [PubMed]
- Nechipurenko, Y.D.; Semyonov, D.A.; Lavrinenko, I.A.; Lagutkin, D.A.; Generalov, E.A.; Zaitceva, A.Y.; Matveeva, O.V.; Yegorov, Y.E. The role of acidosis in the pathogenesis of severe forms of COVID-19. Biology 2021, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Baldwin, I. Introduction to an alternate view of acid/base balance: The strong ion difference or Stewart approach. Aust. Crit. Care 2002, 15, 14–20. [Google Scholar] [CrossRef]
| Number = 104 | |
|---|---|
| Age, years | 58 (52–64) |
| Male gender, % (n) | 73 (77) |
| Weight, kg | 83 (72–97) |
| BMI, kg/m2 | 28 (25–33) |
| Time from symptoms onset to hospital admission, days | 6 (4–8) |
| Time from hospital admission to respiratory support start, days | 1 (0–3) |
| Arterial pH | 7.45 (7.43–7.48) |
| PaCO2, mmHg | 31.8 (28.4–34.2) |
| PaO2, mmHg | 63.0 (55.7–74.0) |
| PaO2/FiO2 | 264 (204–301) |
| White blood cell count, cells/μL | 6600 (5200–8780) |
| Haemoglobin, g/dL | 14.4 (13.1–15.4) |
| Platelets, cells/μL | 198 (152–241) |
| INR | 1.14 (1.08–1.22) |
| GOT, U/L | 55 (41–76) |
| GPT, U/L | 46 (31–71) |
| Total bilirubin, mg/dL | 0.6 (0.4–1.0) |
| Creatinine, mg/dL | 0.8 (0.7–1.0) |
| LDH, mg/dL | 382 (292–472) |
| D-dimer, ng/mL | 300 (226–394) |
| SOFA score | 2 (2–3) |
| Study Population | No Acid-Base Disorder | Respiratory Alkalosis | Metabolic Alkalosis | Respiratory Acidosis | Mixed Alkalosis | p | |
|---|---|---|---|---|---|---|---|
| Number (%) | 104 (100) | 14 (13) | 42 (40) | 34 (32) | 5 (5) | 9 (10) | - |
| Age, years | 60 (53–69) | 59 (52–71) | 61 (55–68) | 59 (52–66) | 58 (57–69) | 63 (59–70) | 0.824 |
| Female gender, n (%) | 28 (27) | 21 (3) | 17 (7) | 41 (14) | 100 (5) | 13 (1) | 0.845 |
| BMI, kg/m2 | 28 (25–33) | 25 (25–28) | 28 (25–31) | 28 (26–34) | 30 (30–30) | 30 (26–36) | 0.804 |
| Time from symptoms to ED, days | 6 (4–8) | 6 (4–7) | 5 (4–8) | 6 (3–8) | 7 (5–7) | 6 (5–8) | 0.989 |
| Respiratory rate, bpm | 19 (17–24) | 20 (17–24) | 20 (17–23) | 18 (16–22) | 18 (16–20) | 20 (18–24) | 0.791 |
| FiO2 | 70 (60–70) | 70 (60–80) | 70 (60–80) | 65 (60–70) | 70 (60–75) | 60 (60–70) | 0.286 |
| PEEP, cmH2O | 8 (7.5–10) | 10 (8–10) | 7.5 (7.5–10) | 8 (7.5–10) | 7.5 (7.5–10) | 7.5 (7.5–8) | 0.220 |
| Borg Score | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.203 |
| WOB Score | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–4) | 2 (1–3) | 0.684 |
| Arterial pH | 7.44 (7.43–7.46) | 7.40 (7.39–7.42) | 7.44 (7.44–7.46) | 7.45 (7.44–7.46) | 7.36 (7.36–7.36) | 7.48 (7.48–7.49) | <0.001 |
| PaCO2, mmHg | 38 (35–41) | 40 (39–42) | 35 (33–36) | 41 (40–44) | 48 (44–51) | 36 (35–37) | <0.001 |
| PaO2, mmHg | 123 (92–155) | 139 (101–180) | 125 (94–177) | 108 (92–150) | 98 (74–149) | 130 (108–146) | 0.762 |
| PaO2/FiO2 | 199 (139–246) | 212 (173–227) | 199 (139–261) | 167 (140–234) | 163 (124–212) | 221 (180–246) | 0.780 |
| HCO3−, mMol/L | 25.8 (24.1–27.4) | 25.2 (23.8–26.0) | 24.2 (22.9–25.0) | 28.0 (27.1–29.7) | 27 (24.5–28) | 27.0 (26.1–27.4) | <0.001 |
| BE, mMol/L | 1.6 (0.1–3.6) | 1.2 (−0.6–1.7) | 0.2 (−1.0–0.8) | 4.1 (2.9–6.1) | 1.3 (−0.8–3.6) | 3.8 (2.1–4.1) | <0.001 |
| Apparent SID, mEq/L | 36.6 (34.9–38.2) | 37.8 (36.8–38.8) | 35.8 (33.9–37.2) | 37.6 (36.6–38.9) | 35.6 (35.0–36.5) | 36.2 (34.9–37.4) | 0.004 |
| Sodium, mEq/L | 136 (134–138) | 138 (135–139) | 136 (134–139) | 136 (135–138) | 137 (133–137) | 135 (133–137) | 0.333 |
| Potassium, mEq/L | 4.1 (3.8–4.3) | 4.1 (3.8–4.4) | 4.0 (3.8–4.4) | 4.1 (3.8–4.3) | 4.1 (4.0–4.5) | 3.8 (3.6–4.0) | 0.270 |
| Cloride, mEq/L | 103 (100–105) | 102 (100–105) | 104 (101–105) | 102 (100–104) | 100 (99–102) | 102 (100–103) | 0.151 |
| Lactates, mMol/L | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) | 1.4 (1.2–1.7) | 1.2 (1.0–1.4) | 1.5 (1.4–2.5) | 1.3 (1.0–1.4) | 0.075 |
| Anion Gap, mEq/L | 8.1 (6.3–10.0) | 9.9 (7.9–10.8) | 9.0 (8.0–10.7) | 6.6 (5.5–7.9) | 7.7 (6.0–8.8) | 6.9 (5.9–8.0) | <0.001 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–0.95) | 0.9 (0.8–1.1) | 0.7 (0.6–0.9) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 0.007 |
| Ventilation type, n (%) | 0.290 | ||||||
| CPAP | 84 (87) | 100 (14) | 81 (34) | 85 (29) | 80 (4) | 67 (6) | |
| NIV | 16 (17) | 0 (0) | 19 (8) | 15 (5) | 20 (1) | 33 (3) | |
| Endotracheal Intubation, n (%) | 19 (20) | 26 (4) | 19 (8) | 9 (3) | 2 (40) | 33 (3) | 0.223 |
| Mortality, n (%) | 14 (13) | 2 (14) | 5 (2) | 15 (5) | 40 (2) | 33 (3) | 0.065 |
| Study Population | No Acid-Base Disorder | Respiratory Alkalosis | Metabolic Alkalosis due to aSID | Other Alkalosis | Respiratory Acidosis | Mixed Disorder | p | |
|---|---|---|---|---|---|---|---|---|
| Number (%) | 104 (100) | 8 (8) | 45 (43) | 20 (19) | 14 (14) | 4 (4) | 13 (13) | - |
| Age, years | 60 (53–69) | 58 (53–64) | 60 (53–68) | 61 (50–70) | 60 (54–68) | 64 (58–69) | 62 (58–65) | 0.943 |
| Female gender, n (%) | 28 (27) | 1 (12) | 8 (18) | 11 (52) | 6 (43) | 25 (1) | 8 (1) | 0.654 |
| BMI, kg/m2 | 28 (25–33) | 25 (25–28) | 28 (25–31) | 28 (26–34) | 28 (26–33) | 30 (26–36) | 30 (30–30) | 0.734 |
| Time from symptoms to ED, days | 6 (4–8) | 6 (4–7) | 5 (4–8) | 4 (4–6) | 6 (4–8) | 6 (5–8) | 7 (5–7) | 0.783 |
| Respiratory rate, bpm | 19 (17–24) | 19 (17–26) | 19 (18–22) | 18 (16–22) | 18 (16–22) | 19 (18–22) | 19 (16–24) | 0.668 |
| FiO2 | 70 (60–70) | 70 (60–70) | 70 (60–70) | 70 (60–70) | 60 (60–70) | 75 (70–75) | 60 (60–70) | 0.532 |
| PEEP, cmH2O | 8 (7.5–10) | 10 (7.5–10) | 7.5 (7.5–10) | 8 (7.5–10) | 8 (7.5–10) | 10 (9–10) | 10 (7.5–10) | 0.141 |
| Borg Score | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0.145 |
| WOB Score | 1 (1–2) | 1 (1–3) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.971 |
| Arterial pH | 7.44 (7.43–7.46) | 7.41 (7.40–7.42) | 7.45 (7.44–7.48) | 7.45 (7.44–7.46) | 7.44 (7.45–7.46) | 7.36 (7.36–7.36) | 7.41 (7.40–7.42) | <0.001 |
| PaCO2, mmHg | 38 (35–41) | 41 (40–42) | 35 (33–36) | 40 (39–42) | 39 (38–42) | 48 (45–54) | 37 (34–43) | <0.001 |
| PaO2, mmHg | 123 (92–155) | 96 (81–190) | 120 (93–165) | 122 (90–152) | 126 (97–145) | 124 (92–195) | 138 (88–145) | 0.983 |
| PaO2/FiO2 | 199 (139–246) | 221 (118–298) | 199 (138–276) | 173 (150–230) | 168 (146–240) | 188 (148–278) | 198 (143–234) | 0.923 |
| HCO3−, mMol/L | 25.8 (24.1–27.4) | 25.6 (24.2–25.9) | 24.7 (23.6–25.6) | 27.4 (26.4–29.6) | 28.0 (26.9–29.4) | 26.2 (24.4–28.5) | 23.4 (22.0–27.0) | 0.001 |
| BE, mMol/L | 1.6 (0.1–3.6) | 1.6 (0.9–1.9) | 0.5 (−0.6–1.4) | 3.4 (2.4–6.0) | 3.6 (2.5–6.1) | 0.25 (−1.7–2.8) | 1.4 (−2–3.6) | 0.001 |
| Apparent SID, mEq/L | 36.6 (34.9–38.2) | 38.6 (38.0–39.1) | 38.9 (38.4–39.4) | 42.2 (42.1–43.0) | 40.0 (38.6–40.5) | 38.8 (38.0–39.6) | 35.6 (34.4–37.4) | 0.034 |
| Sodium, mEq/L | 136 (134–138) | 139 (136–139) | 136 (133–139) | 137 (135–138) | 137 (135–138) | 137 (136–138) | 137 (134–139) | 0.437 |
| Potassium, mEq/L | 4.1 (3.8–4.3) | 4.2 (4.0–4.4) | 4.0 (3.8–4.3) | 4.0 (3.7–4.2) | 4.1 (3.7–4.3) | 4.3 (4.1–4.5) | 4.3 (4.0–4.4) | 0.198 |
| Cloride, mEq/L | 103 (100–105) | 102 (99–103) | 103 (101–105) | 101 (100–104) | 101 (100–104) | 102 (100–104) | 103 (102–106) | 0.209 |
| Lactates, mMol/L | 1.3 (1.0–1.7) | 1.1 (0.9–1.3) | 1.3 (1.1–1.6) | 1.1 (1.0–1.4) | 1.1 (1.1–1.4) | 2.0 (1.4–2.6) | 1.7 (1.2–1.9) | 0.087 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–0.95) | 0.9 (0.8–1.1) | 1.0 (1.0–1.2) | 1.1 (0.9–1.1) | 1.0 (0.9–1.2) | 0.7 (0.6–0.9) | 0.004 |
| Ventilation type, n (%) | 0.489 | |||||||
| CPAP | 84 (87) | 8 (100) | 35 (78) | 10 (50) | 10 (71) | 3 (75) | 12 (92) | |
| NIV | 16 (17) | 0 (0) | 10 (22) | 10 (50) | 4 (29) | 1 (25) | 1 (8) | |
| Endotracheal Intubation, n (%) | 19 (20) | 4 (50) | 9 (20) | 2 (10) | 2 (14) | 2 (50) | 2 (15) | 0.132 |
| Mortality, n (%) | 13 (14) | 1 (12) | 5 (11) | 4 (20) | 1 (7) | 2 (50) | 1 (8) | 0.136 |
| Severe Hypoxemia | Moderate Hypoxemia | p | |
|---|---|---|---|
| Number, (%) | 51 (51) | 53 (49) | - |
| Age, years | 62 (54–68) | 59 (53–70) | 0.941 |
| BMI, kg/m2 | 28 (26–33) | 28 (25–33) | 0.366 |
| Time from symptoms to ED, days | 6 (4–7) | 5 (4–9) | 0.692 |
| Respiratory rate, bpm | 19 (17–24) | 19 (16–22) | 0.520 |
| FiO2 | 60 (60–70) | 70 (60–70) | 0.028 |
| PEEP, cmH2O | 8 (7.5–10) | 7.5 (7.5–10) | 0.380 |
| Borg Score | 0 (0–0) | 0 (0–0) | 0.410 |
| WOB Score | 1 (1–2) | 1 (1–2) | 0.126 |
| Arterial pH | 7.44 (7.43–7.46) | 7.44 (7.42–7.46) | 0.829 |
| PaCO2, mmHg | 38 (35–42) | 38 (34–41) | 0.185 |
| PaO2, mmHg | 92 (78–99) | 155 (140–204) | <0.001 |
| PaO2/FiO2 | 139 (115–162) | 246 (221–398) | <0.001 |
| HCO3−, mMol/L | 25.8 (24.4–27.4) | 25.8 (23.6–27.4) | 0.320 |
| BE, mMol/L | 1.6 (0.4–3.6) | 1.4 (−0.68–3.8) | 0.528 |
| Apparent SID, mEq/L | 36 (35–38) | 37 (35–38) | 0.761 |
| Sodium, mEq/L | 136 (135–139) | 137 (133–138) | 0.189 |
| Potassium, mEq/L | 4.1 (3.8–4.4) | 4.1 (3.8–4.2) | 0.199 |
| Cloride, mEq/L | 103 (101–105) | 102 (100–104) | 0.062 |
| Lactates, mMol/L | 1.3 (1.1–1.6) | 1.3 (1.0–1.7) | 0.780 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 0.448 |
| Endotracheal intubation, n (%) | 13 (25) | 7 (13) | 0.271 |
| Acid-base disorders, n (%) | 0.491 | ||
| No Acid-base disorder | 5 (10) | 9 (18) | |
| Respiratory Acidosis | 3 (5) | 2 (3) | |
| Respiratory Alkalosis | 20 (40) | 22 (41) | |
| Metabolic Alkalosis due to aSID | 10 (20) | 4 (9) | |
| Other Metabolic Alkalosis | 10 (20) | 10 (18) | |
| Mixed Alkalosis | 3 (5) | 6 (11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiumello, D.; Pozzi, T.; Fratti, I.; Modafferi, L.; Montante, M.; Papa, G.F.S.; Coppola, S. Acid-Base Disorders in COVID-19 Patients with Acute Respiratory Distress Syndrome. J. Clin. Med. 2022, 11, 2093. https://doi.org/10.3390/jcm11082093
Chiumello D, Pozzi T, Fratti I, Modafferi L, Montante M, Papa GFS, Coppola S. Acid-Base Disorders in COVID-19 Patients with Acute Respiratory Distress Syndrome. Journal of Clinical Medicine. 2022; 11(8):2093. https://doi.org/10.3390/jcm11082093
Chicago/Turabian StyleChiumello, Davide, Tommaso Pozzi, Isabella Fratti, Leo Modafferi, Marialaura Montante, Giuseppe Francesco Sferrazza Papa, and Silvia Coppola. 2022. "Acid-Base Disorders in COVID-19 Patients with Acute Respiratory Distress Syndrome" Journal of Clinical Medicine 11, no. 8: 2093. https://doi.org/10.3390/jcm11082093
APA StyleChiumello, D., Pozzi, T., Fratti, I., Modafferi, L., Montante, M., Papa, G. F. S., & Coppola, S. (2022). Acid-Base Disorders in COVID-19 Patients with Acute Respiratory Distress Syndrome. Journal of Clinical Medicine, 11(8), 2093. https://doi.org/10.3390/jcm11082093








