Cardiac Tomography and Cardiac Magnetic Resonance to Predict the Absence of Intracardiac Thrombus in Anticoagulated Patients Undergoing Atrial Fibrillation Ablation
Abstract
:1. Introduction
2. Methods
2.1. Population
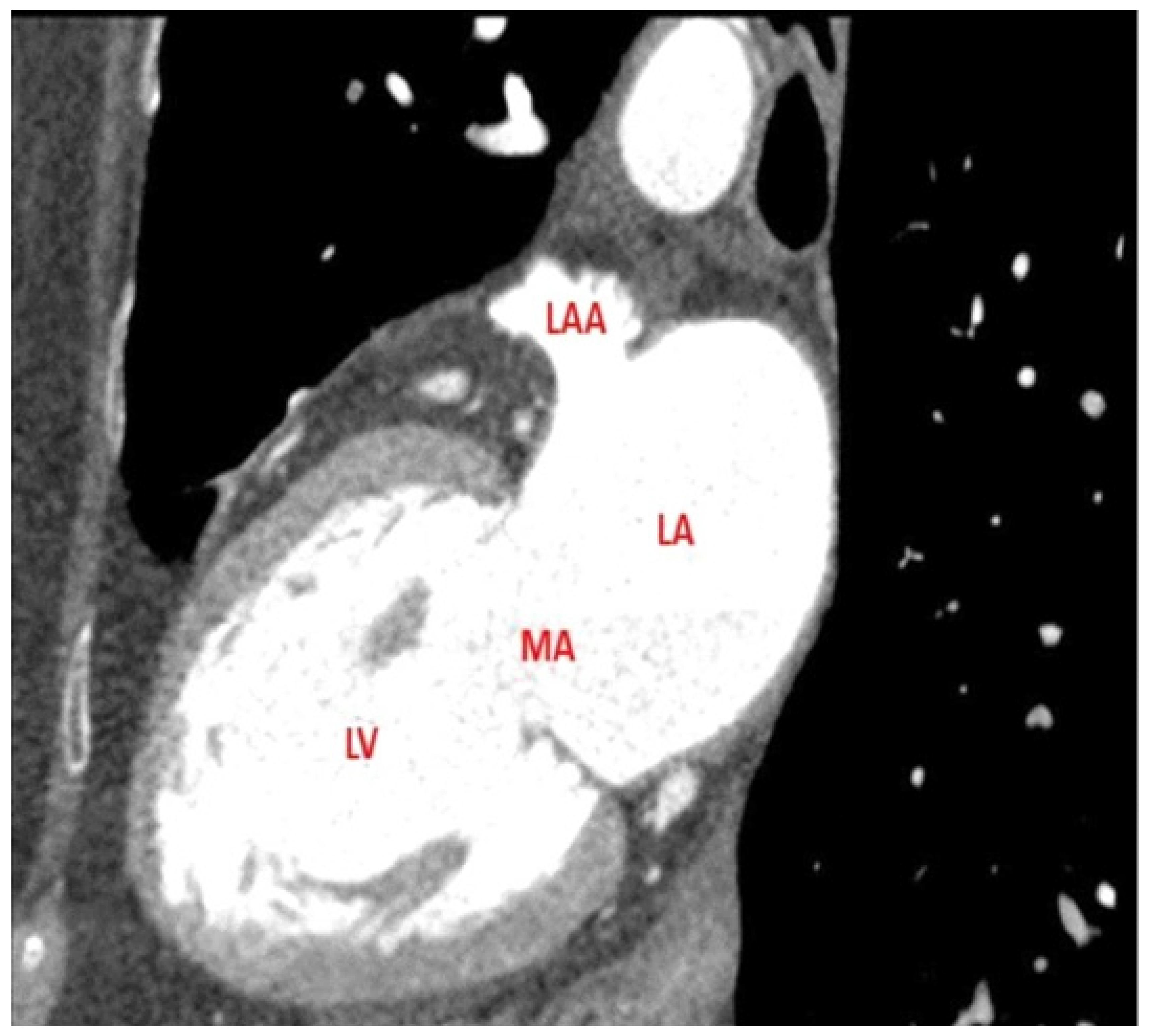
2.2. Imaging Protocols
2.3. Procedural Characteristics
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Anticoagulation Therapy
3.3. Thrombus Detection
3.4. Cost Analyses
3.5. Follow-Up
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Manning, W.J.; Weintraub, R.M.; Waksmonski, C.A.; Haering, J.M.; Rooney, P.S.; Maslow, A.D.; Johnson, R.G.; Douglas, P.S. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 1995, 123, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef] [PubMed]
- Hilberath, J.N.; Oakes, D.A.; Shernan, S.K.; Bulwer, B.E.; D’Ambra, M.N.; Eltzschig, H.K. Safety of transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Sriram, C.S.; Banchs, J.E.; Moukabary, T.; Moradkhan, R.; Gonzalez, M.D. Detection of left atrial thrombus by intracardiac echocardiography in patients undergoing ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2015, 43, 227–236. [Google Scholar] [CrossRef]
- Choi, Y.R.; Kim, H.L.; Kwon, H.M.; Chun, E.J.; Ko, S.M.; Yoo, S.M.; Choi, S.-I.; Jin, K.N. Cardiac CT and MRI for assessment of cardioembolic stroke. Cardiovasc. Imaging Asia 2017, 1, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Romero, J.; Husain, S.A.; Kelesidis, I.; Sanz, J.; Medina, H.M.; Garcia, M.J. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: A meta-analysis. Circ. Cardiovasc. Imaging 2013, 6, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018, 20, e1–e160. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Obeng-Gyimah, E.; Nazarian, S. Advancements in Imaging for Atrial Fibrillation Ablation: Is There a Potential to Improve Procedural Outcomes? J. Innov. Card. Rhythm. Manag. 2020, 11, 4172–4178. [Google Scholar] [CrossRef]
- Rathi, V.K.; Reddy, S.T.; Anreddy, S.; Belden, W.; Yamrozik, J.A.; Williams, R.B.; Doyle, M.; Thompson, D.V.; Biederman, R.W.W. Contrast-enhanced CMR is equally effective as TEE in the evaluation of left atrial appendage thrombus in patients with atrial fibrillation undergoing pulmonary vein isolation procedure. Heart Rhythm 2013, 10, 1021–1027. [Google Scholar] [CrossRef]
- Tops, L.F.; Bax, J.J.; Zeppenfeld, K.; Jongbloed, M.R.; Lamb, H.J.; van der Wall, E.E.; Schalij, M.J. Fusion of multislice computed tomography imaging with three-dimensional electroanatomic mapping to guide radiofrequency catheter ablation procedures. Heart Rhythm 2005, 2, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Kistler, P.M.; Rajappan, K.; Jahngir, M.; Earley, M.J.; Harris, S.; Abrams, D.; Gupta, D.; Liew, R.; Ellis, S.; Sporton, S.C.; et al. The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006, 17, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Charalampos, K.; Roettgen, R.; Wellnhofer, E.; Gebker, R.; Paetsch, I.; Jahnke, C.; Schnackenburg, B.; Tang, M.; Gerds-Li, H.; et al. Magnetic resonance imaging versus computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am. J. Cardiol. 2009, 104, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, J.; Martinho, S.; Frankel, D.; Slim, A.M.; Eckart, R.E. Exclusion of Left Atrial Appendage Thrombus Using Single Phase Coronary Computed Tomography as Compared to Transesophageal Echocardiography in Patients Undergoing Pulmonary Vein Isolation. Int. Sch. Res. Not. 2014, 4, 838727. [Google Scholar] [CrossRef]
- Hur, J.; Pak, H.-N.; Kim, Y.J.; Lee, H.-J.; Chang, H.-J.; Hong, Y.J.; Choi, B.W. Dual-enhancement cardiac computed tomography for assessing left atrial thrombus and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. Am. J. Cardiol. 2013, 112, 238–244. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Tong, J.; Liu, Z. Multidetector computed tomography for detecting left atrial/left atrial appendage thrombus: A meta-analysis. Intern. Med. J. 2015, 45, 1044–1053. [Google Scholar] [CrossRef]
- Caponi, D.; Corleto, A.; Scaglione, M.; Blandino, A.; Biasco, L.; Cristoforetti, Y.; Cerrato, N.; Toso, E.; Morello, M.; Gaita, F. Ablation of atrial fibrillation: Does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome? A randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace 2010, 12, 1098–1104. [Google Scholar] [CrossRef]
- Lundqvist, C.B.; Auricchio, A.; Brugada, J.; Boriani, G.; Bremerich, J.; Cabrera, J.A.; Frank, H.; Gutberlet, M.; Heidbuchel, H.; Kuck, K.-H.; et al. The use of imaging for electrophysiological and devices procedures: A report from the first European Heart Rhythm Association Policy Conference, jointly organized with the European Association of Cardiovascular Imaging (EACVI), the Council of Cardiovascular Imaging and the European Society of Cardiac Radiology. Europace 2013, 15, 927–936. [Google Scholar] [CrossRef]
- Stocker, T.J.; Deseive, S.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Heckner, M.; Bax, J.J.; Fang, X.-M.; Grove, E.L.; et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: Results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur. Heart J. 2018, 39, 3715–3723. [Google Scholar] [CrossRef] [Green Version]
- Baran, J.; Stec, S.; Pilichowska-Paszkiet, E.; Zaborska, B.; Sikora-Frąc, M.; Kryński, T.; Michałowska, I.; Łopatka, R.; Kułakowski, P. Intracardiac echocardiography for detection of thrombus in the left atrial appendage: Comparison with transesophageal echocardiography in patients undergoing ablation for atrial fibrillation: The Action-Ice I Study. Circ. Arrhythmia Electrophysiol. 2013, 6, 1074–1081. [Google Scholar] [CrossRef] [Green Version]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology Working With the Anatomy Rather Than Fighting It. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef] [PubMed]
- Pokorney, S.D.; Hammill, B.G.; Qualls, L.G.; Steinberg, B.A.; Curtis, L.H.; Piccini, J.P. Cost analysis of periprocedural imaging in patients undergoing catheter ablation for atrial fibrillation. Am. J. Cardiol. 2014, 114, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitkungvan, D.; Nabi, F.; Ghosn, M.G.; Dave, A.S.; Quinones, M.; Zoghbi, W.A.; Valderrabano, M.; Shah, D.J. Detection of LA and LAA Thrombus by CMR in Patients Referred for Pulmonary Vein Isolation. JACC Cardiovasc. Imaging 2016, 9, 809–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patients = 272 | |
|---|---|
| Age: years (Ave ± st dev) | 54.5 ± 4.94 |
| Gender, male: n (%) | 194 (71) |
| Hypertension: n (%) | 109 (40) |
| CHA2DS2VASC score < 2 | 197 (72) |
| CHA2DS2VASC (Ave ± st dev) | 0.9 ± 0.83 |
| Type, persistent AF: n (%) | 38 (14) |
| AF evolution time: months (Ave ± st dev) | 40 ± 41.4 |
| LV ejection fraction: (Ave ± st dev) | 59 ± 9.3 |
| LA diameter: mm (Ave ± st dev) | 42 ± 5.7 |
| Image tecnique: n (%) CT scan | 147 (54) |
| CMR | 125 (46) |
| PV anatomic variation: n (%) | 55 (20) |
| Total Patients = 272 | |
|---|---|
| Time from AC to ablation: days (Ave ± st dev) | 227 ± 392 |
| Time from CT/CMR to ablation: days (Ave ± st dev) | 291 ± 416 |
| Type of ablation: n (%) RF ablation | 89 (33) |
| Cryoballoon ablation | 183 (67) |
| Anticoagulant drugs: n (%) | 161(59) |
| Dabigratan | 38 (14) |
| Rivaroxaban | 55 (20) |
| Apixaban | 63 (23) |
| Edoxaban | 5 (2) |
| Acenocumarol | 111 (41) |
| Basal ACT: seconds (Ave ± st dev) | 162.2 ± 32.6 |
| 30 min ACT: seconds (Ave ± st dev) | 310.4 ± 73.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaraket, F.; Bas, D.; Jimenez, J.; Casteigt, B.; Benito, B.; Martí-Almor, J.; Conejos, J.; Tizón-Marcos, H.; Mojón, D.; Vallès, E. Cardiac Tomography and Cardiac Magnetic Resonance to Predict the Absence of Intracardiac Thrombus in Anticoagulated Patients Undergoing Atrial Fibrillation Ablation. J. Clin. Med. 2022, 11, 2101. https://doi.org/10.3390/jcm11082101
Zaraket F, Bas D, Jimenez J, Casteigt B, Benito B, Martí-Almor J, Conejos J, Tizón-Marcos H, Mojón D, Vallès E. Cardiac Tomography and Cardiac Magnetic Resonance to Predict the Absence of Intracardiac Thrombus in Anticoagulated Patients Undergoing Atrial Fibrillation Ablation. Journal of Clinical Medicine. 2022; 11(8):2101. https://doi.org/10.3390/jcm11082101
Chicago/Turabian StyleZaraket, Fatima, Deva Bas, Jesus Jimenez, Benjamin Casteigt, Begoña Benito, Julio Martí-Almor, Javi Conejos, Helena Tizón-Marcos, Diana Mojón, and Ermengol Vallès. 2022. "Cardiac Tomography and Cardiac Magnetic Resonance to Predict the Absence of Intracardiac Thrombus in Anticoagulated Patients Undergoing Atrial Fibrillation Ablation" Journal of Clinical Medicine 11, no. 8: 2101. https://doi.org/10.3390/jcm11082101







