Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Study Population
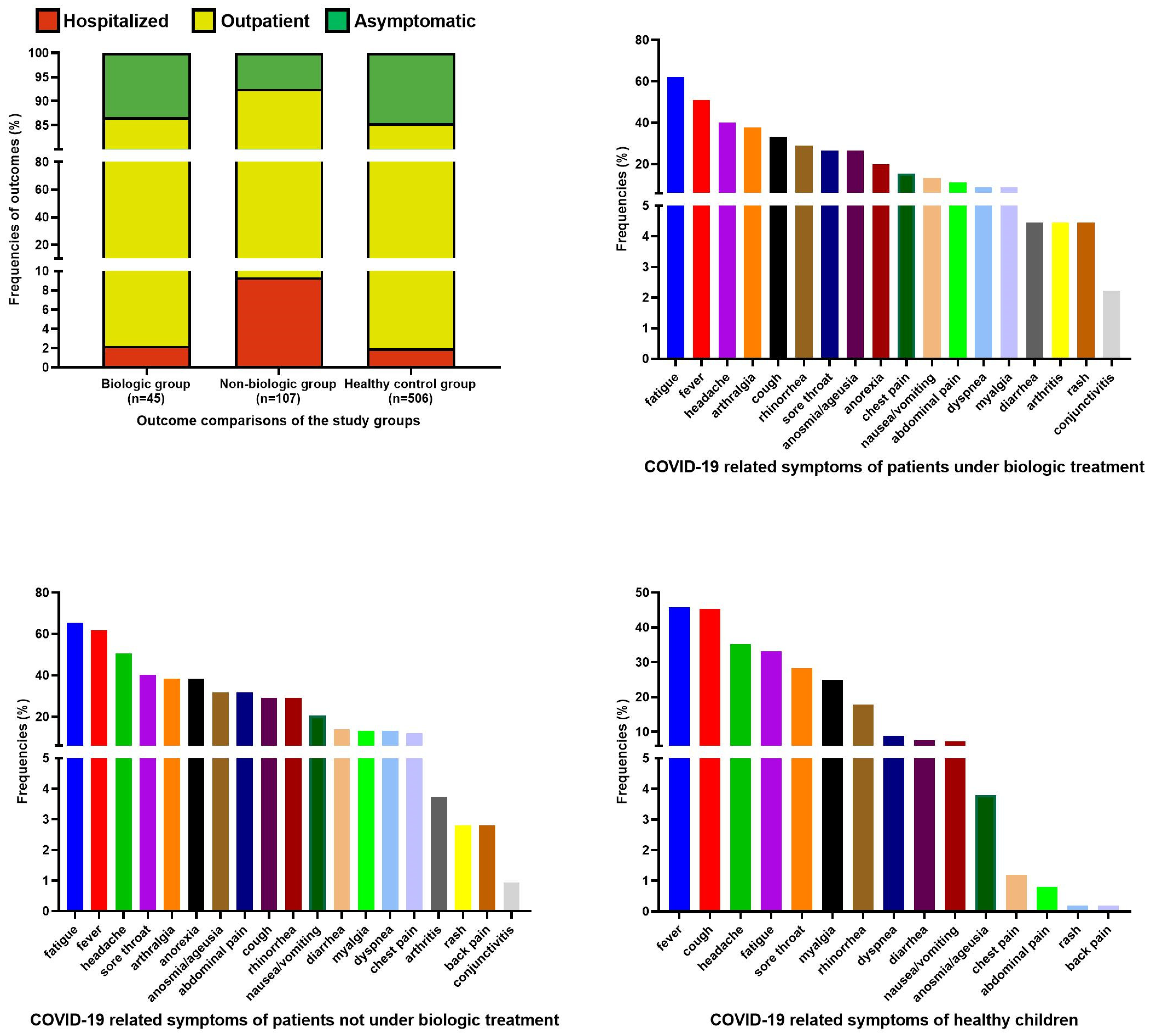
3.2. Comparison of Symptomatic and Asymptomatic Subjects
3.3. Comparison of Hospitalized and Non-Hospitalized Subjects
3.4. Risk Factor Assessment for Symptomatic Infection and Hospitalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Dergaa, I.; Abubaker, M.; Souissi, A.; Mohammed, A.R.; Varma, A.; Musa, S.; Al Naama, A.; Mkaouer, B.; Ben Saad, H. Age and clinical signs as predictors of COVID-19 symptoms and cycle threshold value. Libyan J. Med. 2022, 17, 2010337. [Google Scholar] [CrossRef] [PubMed]
- Teich, V.D.; Klajner, S.; Almeida, F.A.S.D.; Dantas, A.C.B.; Laselva, C.R.; Torritesi, M.G.; Canero, T.R.; Berwanger, O.; Rizzo, L.V.; Reis, E.P.; et al. Epidemiologic and clinical features of patients with COVID-19 in Brazil. Einstein 2020, 18, eAO6022. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Sankar, J.; Dhochak, N.; Kabra, S.K.; Lodha, R. COVID-19 in Children: Clinical Approach and Management. Indian J Pediatr. 2020, 87, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.B.; Gilani, Z.; Godfred-Cato, S.; Belay, E.D.; Feldstein, L.R.; Patel, M.M.; Randolph, A.G.; Newhams, M.; Thomas, D.; Magleby, R.; et al. Incidence of Multisystem Inflammatory Syndrome in Children Among US Persons Infected With SARS-CoV-2. JAMA Netw. Open 2021, 4, e2116420. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Haşlak, F.; Yıldız, M.; Adrovic, A.; Barut, K.; Kasapçopur, Ö. Childhood Rheumatic Diseases and COVID-19 Pandemic: An Intriguing Linkage and a New Horizon. Balkan Med. J. 2020, 37, 184–188. [Google Scholar] [CrossRef]
- Grainger, R.; Machado, P.M.; Robinson, P.C. Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: Epidemiology and outcomes. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101657. [Google Scholar] [CrossRef] [PubMed]
- Cordtz, R.; Lindhardsen, J.; Soussi, B.G.; Vela, J.; Uhrenholt, L.; Westermann, R.; Kristensen, S.; Nielsen, H.; Torp-Pedersen, C.; Dreyer, L. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: A nationwide cohort study from Denmark. Rheumatology 2021, 60, Si59–Si67. [Google Scholar] [CrossRef] [PubMed]
- Wahezi, D.M.; Lo, M.S.; Rubinstein, T.B.; Ringold, S.; Ardoin, S.P.; Downes, K.J.; Jones, K.B.; Laxer, R.M.; Pellet Madan, R.; Mudano, A.S.; et al. American College of Rheumatology Guidance for the Management of Pediatric Rheumatic Disease During the COVID-19 Pandemic: Version 2. Arthritis Rheumatol. 2021, 73, e46–e59. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xu, Y.; Xu, P.; Cao, X.; Wu, C.; Gu, C.; He, X.; Wang, X.; Huang, S.; Yuan, Q.; et al. Structures of the Omicron Spike trimer with ACE2 and an anti-Omicron antibody. Science 2022, 375, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Modes, M.E.; Directo, M.P.; Melgar, M.; Johnson, L.R.; Yang, H.; Chaudhary, P.; Bartolini, S.; Kho, N.; Noble, P.W.; Isonaka, S.; et al. Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance-One Hospital, California, July 15–September 23, 2021, and December 21, 2021–January 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 217–223. [Google Scholar] [PubMed]
- Cheng, W.A.; Turner, L.; Marentes Ruiz, C.J.; Tanaka, M.L.; Congrave-Wilson, Z.; Lee, Y.; Jumarang, J.; Perez, S.; Peralta, A.; Pannaraj, P.S. Clinical manifestations of COVID-19 differ by age and obesity status. Influenza Other Respir. Viruses 2022, 16, 255–264. [Google Scholar] [CrossRef]
- Dai, X.J.; Shao, Y.; Ren, L.; Tao, W.; Wang, Y. Risk factors of COVID-19 in subjects with and without mental disorders. J. Affect. Disord. 2022, 297, 102–111. [Google Scholar] [CrossRef]
- Salzberger, B.; Buder, F.; Lampl, B.; Ehrenstein, B.; Hitzenbichler, F.; Holzmann, T.; Schmidt, B.; Hanses, F. Epidemiology of SARS-CoV-2. Infection 2021, 49, 233–239. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr. Infect Dis. J. 2020, 39, 355–368. [Google Scholar] [CrossRef]
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.D.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [Green Version]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Carducci, F.I.C.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef]
- Monti, S.; Balduzzi, S.; Delvino, P.; Bellis, E.; Quadrelli, V.S.; Montecucco, C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann. Rheum. Dis. 2020, 79, 667–668. [Google Scholar] [CrossRef] [Green Version]
- Fredi, M.; Cavazzana, I.; Moschetti, L.; Andreoli, L.; Franceschini, F. COVID-19 in patients with rheumatic diseases in northern Italy: A single-centre observational and case-control study. Lancet Rheumatol. 2020, 2, e549–e556. [Google Scholar] [CrossRef]
- Haslak, F.; Yildiz, M.; Adrovic, A.; Sahin, S.; Koker, O.; Aliyeva, A.; Barut, K.; Kasapcopur, O. Management of childhood-onset autoinflammatory diseases during the COVID-19 pandemic. Rheumatol. Int. 2020, 40, 1423–1431. [Google Scholar] [CrossRef]
- Yildiz, M.; Haslak, F.; Adrovic, A.; Sahin, S.; Barut, K.; Kasapcopur, O. The frequency and clinical course of COVID-19 infection in children with juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 2020, 38, 1271–1272. [Google Scholar]
- Welzel, T.; Samba, S.D.; Klein, R.; van den Anker, J.N.; Kuemmerle-Deschner, J.B. COVID-19 in Autoinflammatory Diseases with Immunosuppressive Treatment. J. Clin. Med. 2021, 10, 605. [Google Scholar] [CrossRef]
- Maritsi, D.N.; Krepis, P.; Vartzelis, G.; Syggelou, A.; Tsolia, M. The impact of SARS-CoV-2 infection in children with rheumatic/autoinflammatory diseases on immunosuppressive treatment: A single centre experience. Clin. Exp. Rheumatol. 2022. [Google Scholar] [CrossRef]
- Ihara, B.P.; Strabelli, C.A.; Simon, J.R.; Viana, V.S.; Sallum, A.M.; Kozu, K.T.; Aikawa, N.E.; Leal, G.N.; Pereira, M.F.; Marques, H.H.; et al. Laboratory-confirmed pediatric COVID-19 in patients with rheumatic diseases: A case series in a tertiary hospital. Lupus 2021, 30, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Shao, R.; Han, X.; Su, C.; Lu, W. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Z.A.; Alghamdi, K.A.; Almaqati, A.S. Clinical characteristics and outcome of COVID-19 in patients with rheumatic diseases. Rheumatol. Int. 2021, 41, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Cai, S.; Shen, G.; Guan, H.; Zhou, L.; Hu, Y.; Tu, W.; Chen, Y.; Yu, Y.; Wu, X.; et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann. Rheum. Dis. 2020, 79, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, K.M.; Serling-Boyd, N.; Wallwork, R.; Hsu, T.; Fu, X.; Gravallese, E.M.; Choi, H.K.; Sparks, J.A.; Wallace, Z.S. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: A comparative cohort study from a US ‘hot spot’. Ann. Rheum. Dis. 2020, 79, 1156–1162. [Google Scholar] [CrossRef]
- Villacis-Nunez, D.S.; Rostad, C.A.; Rouster-Stevens, K.; Khosroshahi, A.; Chandrakasan, S.; Prahalad, S. Outcomes of COVID-19 in a cohort of pediatric patients with rheumatic diseases. Pediatr. Rheumatol. Online J. 2021, 19, 94. [Google Scholar] [CrossRef]
- Clemente, D.; Udaondo, C.; de Inocencio, J.; Nieto, J.C.; Del Río, P.G.; Fernández, A.G.; Palomo, J.A.; Bachiller-Corral, J.; Lopez Robledillo, J.C.; Millán Longo, C.; et al. Clinical characteristics and COVID-19 outcomes in a regional cohort of pediatric patients with rheumatic diseases. Pediatr. Rheumatol. Online J. 2021, 19, 162. [Google Scholar] [CrossRef]
- Sengler, C.; Eulert, S.; Minden, K.; Niewerth, M.; Horneff, G.; Kuemmerle-Deschner, J.; Siemer, C.; Berendes, R.; Girschick, H.; Hühn, R.; et al. Clinical manifestations and outcome of SARS-CoV-2 infections in children and adolescents with rheumatic musculoskeletal diseases: Data from the National Paediatric Rheumatology Database in Germany. RMD Open 2021, 7, e001687. [Google Scholar] [CrossRef]
- Barut, K.; Adrovic, A.; Şahin, S.; Kasapçopur, Ö. Juvenile Idiopathic Arthritis. Balkan Med. J. 2017, 34, 90–101. [Google Scholar] [CrossRef]
- Yildiz, M.; Adrovic, A.; Tasdemir, E.; Baba-Zada, K.; Aydin, M.; Koker, O.; Sahin, S.; Barut, K.; Kasapcopur, O. Evaluation of co-existing diseases in children with familial Mediterranean fever. Rheumatol. Int. 2020, 40, 57–64. [Google Scholar] [CrossRef]
- Yalçınkaya, F.; Özen, S.; Özçakar, Z.B.; Aktay, N.; Çakar, N.; Düzova, A.; Kasapçopur, Ö.; Elhan, A.H.; Doğanay, B.; Ekim, M.; et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology 2009, 48, 395–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, L.; Amiri, M.; Pouy, S. Clinical Characteristics of COVID-19 Infection in Newborns and Pediatrics: A Systematic Review. Arch Acad. Emerg. Med. 2020, 8, e50. [Google Scholar] [PubMed]
- Swart, J.; Giancane, G.; Horneff, G.; Magnusson, B.; Hofer, M.; Alexeeva, E.; Panaviene, V.; Bader-Meunier, B.; Anton, J.; Nielsen, S.; et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: Combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res. Ther. 2018, 20, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozeri, B.; Ulu, K.; Kaya-Akça, U.; Haslak, F.; Pac-Kisaarslan, A.; Otar-Yener, G.; Baba, O.; Altug-Gucenmez, O.; Sahin, N.; Bağlan, E.; et al. The clinical course of SARS-CoV-2 infection among children with rheumatic disease under biologic therapy: A retrospective and multicenter study. Rheumatol. Int. 2022, 42, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Nuño, L.; Navarro, M.N.; Bonilla, G.; Franco-Gómez, K.; Aguado, P.; Peiteado, D.; Monjo, I.; Tornero, C.; Villalba, A.; Miranda-Carus, M.E.; et al. Clinical course, severity and mortality in a cohort of patients with COVID-19 with rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 1659–1661. [Google Scholar] [CrossRef]
| Healthy Control (n = 506) | Patients with AIDs (n = 81) | Patients with JIA (n = 39) | Patients with CTD (n = 22) | Patients with Vasculitis (n = 10) | p | |
|---|---|---|---|---|---|---|
| Age (years) (median, min–max) | 13 (0.33–17.9) | 13.7 (2.94–20.86) | 14.05 (3.09–20.95) | 17.61 (6.8–20.59) | 15.22 (3.87–20.79) | <0.001 |
| Gender | 0.283 | |||||
| Female (n, %) | 252 (49.8%) | 42 (51.9%) | 24 (61.5%) | 15 (68.2%) | 6 (60%) | |
| Male (n, %) | 254 (50.2%) | 39 (48.1%) | 15 (38.5%) | 7 (31.8%) | 4 (40%) | |
| Rheumatic diagnoses (n) | - | FMF (71) CAPS (3) PFAPA (3) HIDS (2) CRMO (1) BS (1) | oJIA (17) ERA (9) pJIA (8) sJIA (5) | SLE (10) DM (6) SD (4) Sjögren (2) | KD (3) BD (2) GPA (2) DADA2 (1) TA (1) HSP (1) | |
| Follow-up duration * (months) (median, min–max) | - | 66 (6–182) | 54 (8–192) | 66 (12–170) | 46.5 (16–204) | 0.119 |
| Ongoing treatments | ||||||
| Colchicine (n, %) | - | 75 (92.6%) | - | - | 2 (10%) | |
| Steroid (n, %) | - | 1 (1.2%) | 7 (17.9%) | 10 (45.5%) | 3 (30%) | |
| bDMARDs | ||||||
| Anakinra (n, %) | - | 2 (2.5%) | - | - | - | |
| Canakinumab (n, %) | - | 11 (13.6%) | 3 (7.7%) | - | - | |
| Tocilizumab (n, %) | - | 1 (1.2%) | - | 1 (4.5%) | - | |
| Etanercept (n, %) | - | 1 (1.2%) | 6 (15.4%) | 3 (13.6%) | 1 (10%) | |
| Adalimumab (n, %) | - | - | 12 (30.8%) | - | 1 (10%) | |
| Infliximab (n, %) | - | - | 1 (2.6%) | - | - | |
| Rituximab (n, %) | - | - | - | 1 (4.5%) | - | |
| Baricitinib (n, %) | - | - | 1 (2.6%) | - | - | |
| cDMARDs | ||||||
| MTX (n, %) | - | - | 14 (35.9%) | 5 (22.7%) | - | |
| Leflunomide (n, %) | - | - | 1 (2.6%) | - | - | |
| AZT (n, %) | - | - | - | 1 (4.5%) | 2 (20%) | |
| Cyclosporine (n, %) | - | - | 1 (2.6%) | - | - | |
| Cyclophosphamide (n, %) | - | - | - | 1 (4.5%) | - | |
| HCQ (n, %) | - | - | - | 14 (63.6%) | - | |
| MMF (n, %) | - | - | 1 (2.6%) | 9 (40.9%) | 1 (10%) | |
| Additional non-rheumatic disease * (n, %) | - | 10 (12.3%) | 4 (10.3%) | 4 (18.2%) | 2 (20%) | 0.674 |
| Family contact history of COVID-19 (n, %) | 427 (84.4%) | 67 (82.7%) | 33 (84.6%) | 20 (90.9%) | 10 (100%) | 0.603 |
| Chest CT features of COVID-19 (n, %) | 2 (0.4%) | 10 (12.3%) | 1 (2.6%) | 4 (18.2%) | 1 (10%) | <0.001 |
| Outcome | ||||||
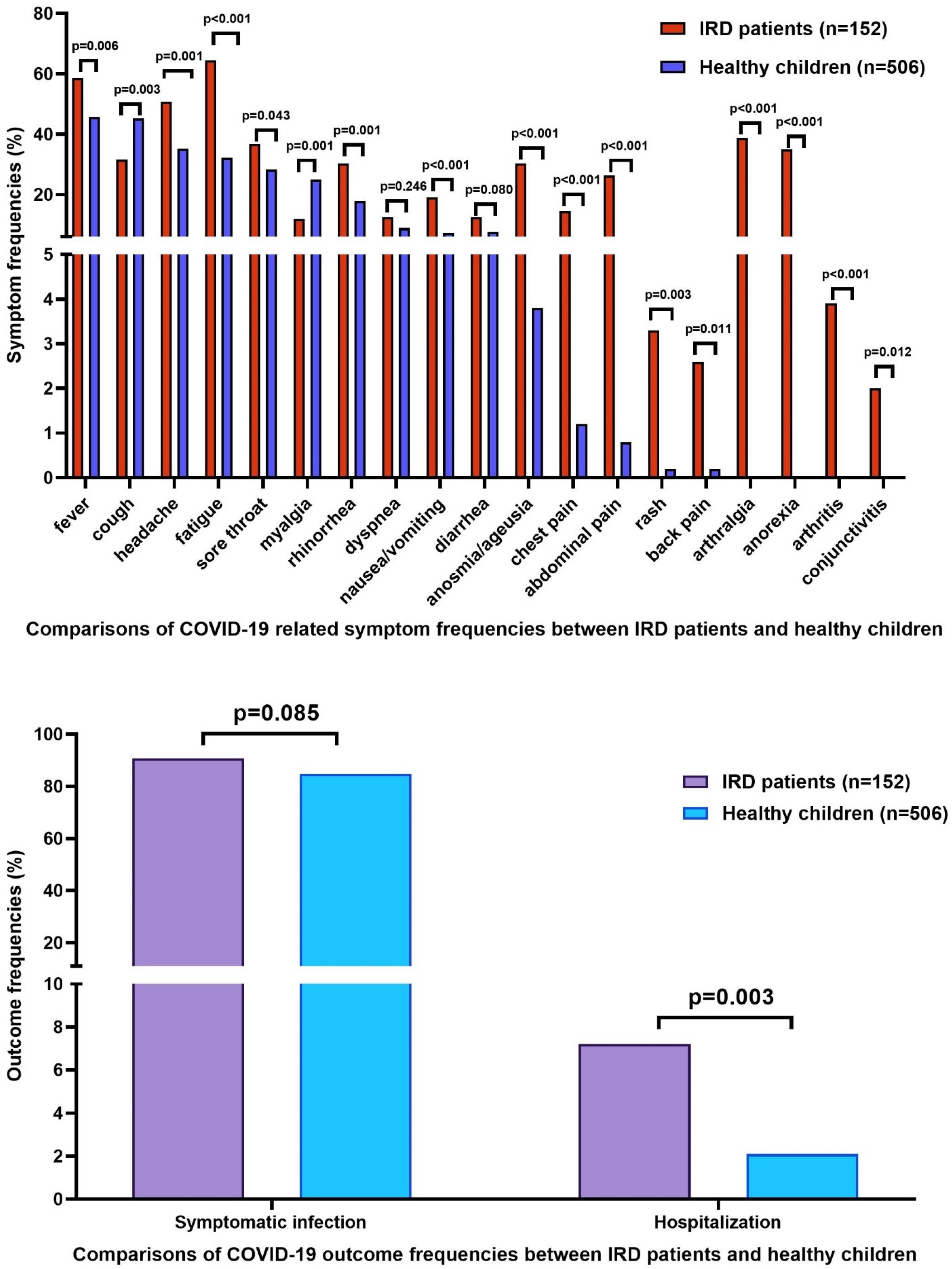
| Symptomatic infection (n, %) | 432 (85.4%) | 76 (93.8%) | 34 (87.2%) | 19 (86.4%) | 9 (90%) | 0.345 |
| Hospitalization (n, %) | 10 (2%) | 9 (11.1%) | - | 2 (9.1%) | - | <0.001 |
| Hospitalization duration ** (days) (median, min–max) | 5.5 (4–30) | 2 (1–21) | - | 6 (5–7) | - | 0.189 |
| COVID-19 treatment | ||||||
| HCQ (n, %) | - | 4 (4.9%) | 3 (7.7%) | 9 (40.9%) | 1 (10%) | |
| Antibiotic (n, %) | 10 (2%) | 5 (6.2%) | 4 (10.3%) | - | 1 (10%) | |
| Antiviral (n, %) | 7 (1.4%) | 19 (23.5%) | 9 (23.1%) | 8 (36.4%) | 5 (50%) | |
| Anticoagulant (n, %) | 3 (0.6%) | 4 (4.9%) | - | 2 (9.1%) | 1 (10%) |
| Symptomatic Infection | Hospitalization | |||||
|---|---|---|---|---|---|---|
| Asymptomatic Group (n = 88) | Symptomatic Group (n = 570) | p | Hospitalized Group (n = 21) | Non-Hospitalized Group (n = 637) | p | |
| Age (years) (median, min–max) | 11.5 (0.33–20.47) | 13.63 (0.4–20.95) | 0.005 | 13 (0.5–19.68) | 13.07 (0.33–20.95) | 0.911 |
| Gender | 0.401 | 0.763 | ||||
| Female (n, %) | 49 (55.7%) | 290 (50.9%) | 12 (57.1%) | 327 (51.3%) | ||
| Male (n, %) | 39 (44.3%) | 280 (49.1%) | 9 (42.9%) | 310 (48.7%) | ||
| Disease | 0.085 | 0.003 | ||||
| Healthy children (n, %) | 74 (84.1%) | 432 (75.8%) | 11 (52.4%) | 496 (77.9%) | ||
| Patients with IRD (n, %) | 14 (15.9%) | 138 (24.2%) | 10 (47.6%) | 141 (22.1%) | ||
| AIDs (n) | 5 | 76 | 9 | 72 | ||
| JIA (n) | 5 | 34 | - | 39 | ||
| CTD (n) | 3 | 19 | 2 | 20 | ||
| Vasculitis (n) | 1 | 9 | - | 10 | ||
| Follow-up duration * (months) (median, min–max) | 54.5 (18–127) | 64 (6–204) | 0.063 | 65 (11–157) | 62 (6–204) | 0.825 |
| Ongoing immunosuppressive treatments | ||||||
| bDMARDs (n, %) | 6 (6.8%) | 39 (6.8%) | 1 | 1 (4.8%) | 44 (6.9%) | 1 |
| cDMARDs (n, %) | 6 (6.8%) | 32 (5.6%) | 0.837 | 2 (9.5%) | 36 (5.7%) | 0.345 |
| Non-rheumatic disease (n, %) | 1 (1.1%) | 19 (3.3%) | 0.5 | 1 (4.8%) | 19 (3%) | 0.483 |
| Family contact history of COVID-19 (n, %) | 88 (100%) | 469 (82.3%) | <0.001 | 19 (90.5%) | 538 (84.5%) | 0.757 |
| Chest CT features of COVID-19 (n, %) | - | 18 (3.2%) | 0.152 | 9 (42.9%) | 9 (1.4%) | <0.001 |
| Symptomatic Infection | Hospitalization | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.046 | 1.003–1.091 | 0.037 | 1.046 | 1.003–1.092 | 0.035 | 0.977 | 0.897–1.063 | 0.585 |
| Gender | 0.817 | 0.517–1.291 | 0.387 | 0.818 | 0.517–1.294 | 0.391 | 1.128 | 0.458–2.778 | 0.794 |
| bDMARD | 0.307 | 0.176–1.730 | 0.307 | 0.569 | 0.179–1.911 | 0.361 | 0.230 | 0.028–1.863 | 0.168 |
| cDMARD | 0.159 | 0.139–1.380 | 0.159 | 0.545 | 0.109–2.735 | 0.461 | 0.704 | 0.141–3.522 | 0.669 |
| IRD | 2.452 | 1.014–5.929 | 0.047 | - | - | 5.785 | 2.179–15.363 | <0.001 | |
| None | 1 | 1 | 1 | - | |||||
| AIDs | - | - | 2.656 | 0.976–7.226 | 0.056 | - | - | ||
| JIA | - | - | 2.062 | 0.434–9.789 | 0.362 | - | - | ||
| CTD | - | - | 1.814 | 0.248–13.245 | 0.557 | - | - | ||
| Vasculitis | - | - | 1.988 | 0.210–18.777 | 0.549 | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haslak, F.; Varol, S.E.; Gunalp, A.; Kaynar, O.; Yildiz, M.; Adrovic, A.; Sahin, S.; Kes, G.; Ayzit-Kilinc, A.; Akdeniz, B.; et al. Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children. J. Clin. Med. 2022, 11, 2102. https://doi.org/10.3390/jcm11082102
Haslak F, Varol SE, Gunalp A, Kaynar O, Yildiz M, Adrovic A, Sahin S, Kes G, Ayzit-Kilinc A, Akdeniz B, et al. Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children. Journal of Clinical Medicine. 2022; 11(8):2102. https://doi.org/10.3390/jcm11082102
Chicago/Turabian StyleHaslak, Fatih, Sevki Erdem Varol, Aybuke Gunalp, Ozge Kaynar, Mehmet Yildiz, Amra Adrovic, Sezgin Sahin, Gulsen Kes, Ayse Ayzit-Kilinc, Beste Akdeniz, and et al. 2022. "Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children" Journal of Clinical Medicine 11, no. 8: 2102. https://doi.org/10.3390/jcm11082102
APA StyleHaslak, F., Varol, S. E., Gunalp, A., Kaynar, O., Yildiz, M., Adrovic, A., Sahin, S., Kes, G., Ayzit-Kilinc, A., Akdeniz, B., Onal, P., Apaydin, G., Aygun, D., Arslan, H., Kilic-Baskan, A., Hepkaya, E., Meral, O., Barut, K., Cokugras, H. C., & Kasapcopur, O. (2022). Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children. Journal of Clinical Medicine, 11(8), 2102. https://doi.org/10.3390/jcm11082102






