1-Year Fixed-Regimen Bevacizumab Treatment in DME-Vascular Network Image Analysis in Optical Coherence Tomography Angiography Study
Abstract
:1. Introduction
2. Materials and Methods
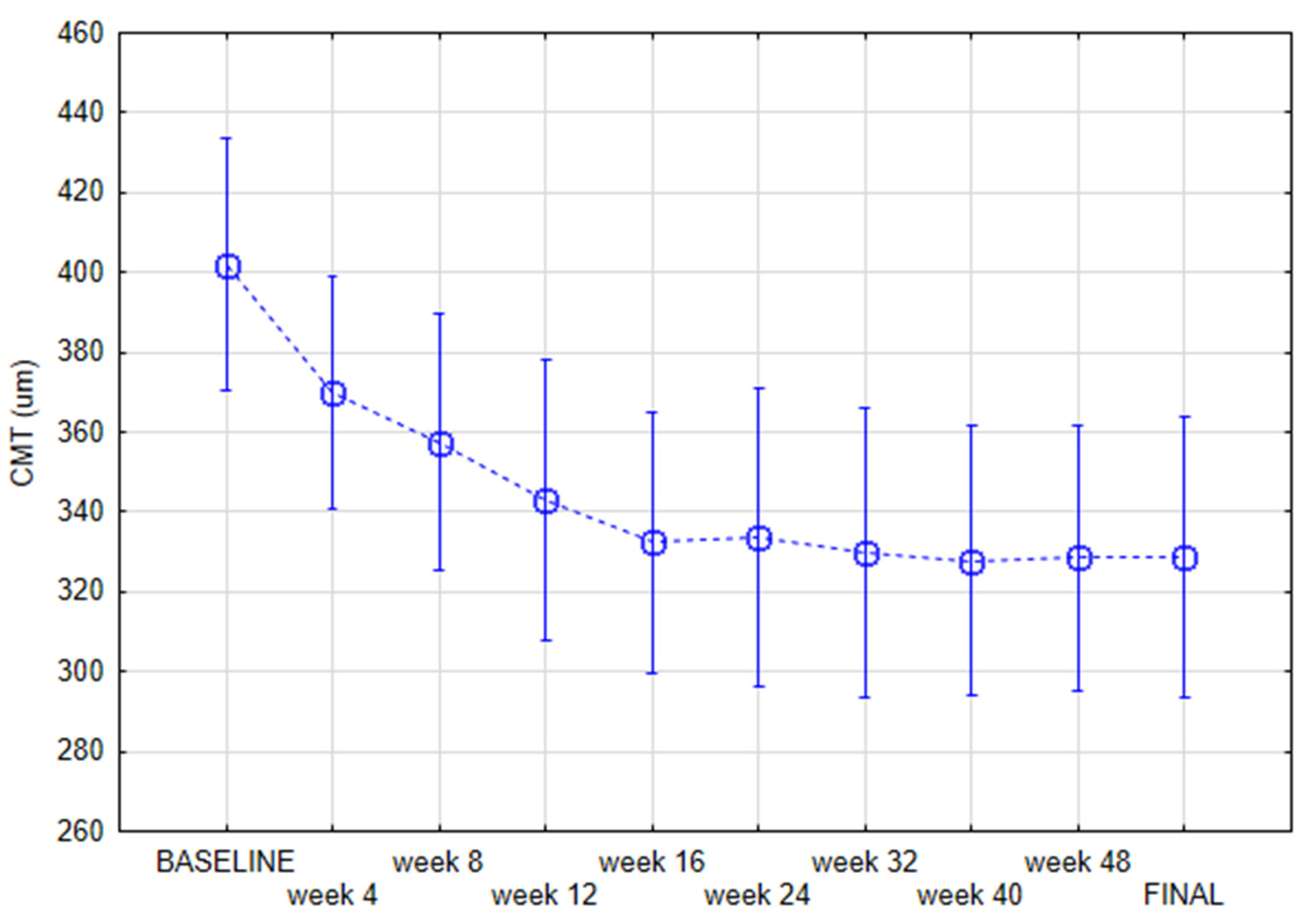
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, F.Y.; Chan, E.O.; Sun, Z.; Wong, R.; Lok, J.; Szeto, S.; Chan, J.C.; Lam, A.; Tham, C.C.; Ng, D.S.; et al. Clinically relevant factors associated with quantitative optical coherence tomography angiography metrics in deep capillary plexus in patients with diabetes. Eye Vis. 2020, 7, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Toma, C.; Villani, E.; Gatti, V.; Brambilla, M.; Muraca, A.; Ponziani, M.C.; Aimaretti, G.; Nuzzo, A.; Nucci, P.; et al. Early Detection of Mi-crovascular Changes in Patients with Diabetes Mellitus without and with Diabetic Retinopathy: Comparison between Different Swept-Source OCT-A Instruments. J. Diabetes Res. 2019, 2019, 2547216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wen, X.; Zeng, P.; Liao, Y.; Fan, S.; Zhang, Y.; Li, Y.; Xiao, J.; Lan, Y. Do microvascular changes occur preceding neural impairment in early-stage diabetic retinopathy? Evidence based on the optic nerve head using optical coherence tomography angiography. Geol. Rundsch. 2019, 56, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, D.; Sedova, A.; Datlinger, F.; Hafner, J.; Steiner, I.; Kriechbaum, K.; Scholda, C.; Sacu, S.; Schmidt-Erfurth, U.; Pollreisz, A. Association of macular perfusion status with microvascular parameters up to the far periphery in diabetic retinopathy using multimodal imaging. Int. J. Retin. Vitr. 2020, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parrulli, S.; Corvi, F.; Cozzi, M.; Monteduro, D.; Zicarelli, F.; Staurenghi, G. Microaneurysms visualisation using five different optical coherence tomography angiography devices compared to fluorescein angiography. Br. J. Ophthalmol. 2021, 105, 526–530. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [Green Version]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef]
- Eastline, M.; Munk, M.R.; Wolf, S.; Schaal, K.B.; Ebneter, A.; Tian, M.; Giannakaki-Zimmermann, H.; Zinkernagel, M.S. Repeatability of Wide-field Optical Coherence Tomography Angiography in Normal Retina. Transl. Vis. Sci. Technol. 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Zeydanli, E.O.; Gurelik, G. Angiography Markers Associated. Eur. J. Ophthalmol. 2020, 1–9. [Google Scholar]
- Sun, M.T.; Huang, S.; Chan, W.; Craig, J.E.; Knight, L.S.; Sanders, P.; Newland, H.; Casson, R.; Selva, D.; Wong, C.X. Impact of cardiometabolic factors on retinal vasculature: Angiography study. Clin. Exp. Ophthalmol. 2021, 49, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Told, R.; Reiter, G.; Schranz, M.; Reumueller, A.; Hacker, V.; Mittermueller, T.; Roberts, P.; Sacu, S.; Schmidt-Erfurth, U. Correlation of Retinal Thickness and Swept-Source Optical Coherence Tomography Angiography Derived Vascular Changes in Patients with Neovascular Age-Related Macular Degeneration. Curr. Eye Res. 2021, 46, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Told, R.; Reiter, G.S.; Mittermüller, T.J.; Schranz, M.; Reumueller, A.; Schlanitz, F.G.; Weigert, G.; Pollreisz, A.; Sacu, S.; Schmidt-Erfurth, U. Profiling neovascular age-related macular degeneration choroidal neovascularization lesion response to anti-vascular endothelial growth factor therapy using SSOCTA. Acta Ophthalmol. 2021, 99, e240–e246. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A Computational Tool for Quantitative Analysis of Vascular Networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [Green Version]
- Ripolles-Garcia, A.; Ruthel, G.; Ying, G.S.; Chen, Y.; Cuenca, N.; Aguirre, G.D.; Beltran, W.A. Characterization of the Canine Retinal Vasculature with Optical Coherence Tomography Angiography: Comparisons with Histology and Fluorescein Angiography. Front. Neuroanat. 2021, 15, 109. [Google Scholar] [CrossRef]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of Quantitative Optical Coherence Tomography Angiography Metrics in Patients with Diabetes. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Tang, F.; Wong, R.; Lok, J.; Szeto, S.K.; Chan, J.C.; Chan, C.K.; Tham, C.Y.C.; Ng, D.S.; Cheung, C.Y. OCT Angiography Metrics Predict Progression of Diabetic Retinopathy and Development of Diabetic Macular Edema. Ophthalmology 2019, 126, 1675–1684. [Google Scholar] [CrossRef]
- Ekinci, M.; Ceylan, E.; Çakıcı, Ö.; Tanyildiz, B.; Olcaysu, O.; Çağatay, H.H.; Cakici, O. Treatment of macular edema in diabetic retinopathy: Comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Rev. Ophthalmol. 2014, 9, 139–143. [Google Scholar] [CrossRef]
- Glassman, A.R.; Wells, J.A., III; Josic, K.; Maguire, M.G.; Antoszyk, A.N.; Baker, C.; Beaulieu, W.T.; Elman, M.J.; Jampol, L.M.; Sun, J.K.; et al. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology 2020, 127, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, A.B.; Takaki, E.; De Almeida, F.P.P.; Peroni, R.; Cardillo, J.A.; Siqueira, R.C.; Scott, I.U.; Messias, A.; Jorge, R. A Prospective Randomized Trial of Intravitreal Bevacizumab Versus Ranibizumab for the Management of Diabetic Macular Edema. Am. J. Ophthalmol. 2013, 156, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Luisi, J.; Liu, H.; Motamedi, M.; Zhang, W. OCT-angiography for non-invasive monitoring of neuronal and vascular structure in mouse retina: Implication for characterization of retinal neurovascular coupling. EC Ophthalmol. 2017, 5, 89–98. [Google Scholar] [PubMed]
- Lee, T.H.; Bin Lim, H.; Nam, K.Y.; Kim, K.; Kim, J.Y. Factors Affecting Repeatability of Assessment of the Retinal Microvasculature Using Optical Coherence Tomography Angiography in Healthy Subjects. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Tang, F.Y.; Huang, H.; Cheung, C.; Chen, H. Repeatability, interocular correlation and agreement of quantitative swept-source optical coherence tomography angiography macular metrics in healthy subjects. Br. J. Ophthalmol. 2018, 103, 415–420. [Google Scholar] [CrossRef]


| Variable | Mean | Minimum | Maximum | SD. |
|---|---|---|---|---|
| Age | 68.00 | 48.00 | 84.00 | 8.60 |
| Body mass index | 26.71 | 19.38 | 39.79 | 4.33 |
| Time of diabetes | 12.69 | 4.00 | 31.00 | 8.01 |
| HbA1c% | 7.36 | 5.70 | 9.40 | 1.09 |
| Axial length | 23.29 | 21.53 | 24.96 | 0.74 |
| Spherical equivalent | 0.81 | −1.50 | 2.50 | 1.14 |
| Cylindrical value | −0.42 | −1.50 | 0.00 | 0.50 |
| IOP | 15.79 | 12.00 | 21.00 | 2.04 |
| number | % | |||
| Gender | Male | 11 | 41 | |
| Female | 16 | 59 | ||
| Chronic kidney disease | 3 | 11 | ||
| Hypertension | 18 | 67 | ||
| Myocardial ischemic disease | 6 | 22 | ||
| Myocardial infarction | 4 | 15 | ||
| Brain stroke | 2 | 7 | ||
| Lens status (pseudophakia) | 10 | 34 | ||
| Tablets | 16 | 59 | ||
| Insulin | 6 | 22 | ||
| Combined therapy | 7 | 26 |
| Variable | Mean | SD | Mean | SD | Repeated Measures ANOVA p-Value | |
|---|---|---|---|---|---|---|
| Explant area | 15 | 260,506.1 | 1058.212 | 282,082.8 | 73,277.40 | 0.7 |
| Vessels area | 15 | 106,547.1 | 7182.383 | 118,541.1 | 29,471.49 | 0.6 |
| Vessels percentage area | 15 | 40.9 | 2.716 | 42.1 | 1.73 | 0.4 |
| Total number of junctions | 15 | 556.0 | 61.582 | 594.8 | 153.88 | 0.4 |
| Total vessels length | 15 | 16,906.3 | 934.952 | 18,537.3 | 4783.51 | 0.3 |
| Average vessels length | 15 | 214.2 | 61.409 | 224.9 | 46.03 | 0.6 |
| Total number of end points | 15 | 457.3 | 43.225 | 469.4 | 112.43 | 0.7 |
| Mean lacunarity | 15 | 0.05 | 0.01 | 0.05 | 0.01 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, M.; Wylęgała, A.; Wylęgała, E.; Teper, S. 1-Year Fixed-Regimen Bevacizumab Treatment in DME-Vascular Network Image Analysis in Optical Coherence Tomography Angiography Study. J. Clin. Med. 2022, 11, 2125. https://doi.org/10.3390/jcm11082125
Hunt M, Wylęgała A, Wylęgała E, Teper S. 1-Year Fixed-Regimen Bevacizumab Treatment in DME-Vascular Network Image Analysis in Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine. 2022; 11(8):2125. https://doi.org/10.3390/jcm11082125
Chicago/Turabian StyleHunt, Magdalena, Adam Wylęgała, Edward Wylęgała, and Sławomir Teper. 2022. "1-Year Fixed-Regimen Bevacizumab Treatment in DME-Vascular Network Image Analysis in Optical Coherence Tomography Angiography Study" Journal of Clinical Medicine 11, no. 8: 2125. https://doi.org/10.3390/jcm11082125
APA StyleHunt, M., Wylęgała, A., Wylęgała, E., & Teper, S. (2022). 1-Year Fixed-Regimen Bevacizumab Treatment in DME-Vascular Network Image Analysis in Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine, 11(8), 2125. https://doi.org/10.3390/jcm11082125







