Concomitant Hepatectomy and Atrial Thrombectomy under Cardiopulmonary Bypass versus Staged Hepatectomy in the Treatment for Hepatocellular Carcinoma with Large Right Atrial Tumor Thrombi
Abstract
:1. Introduction
2. Materials and Methods
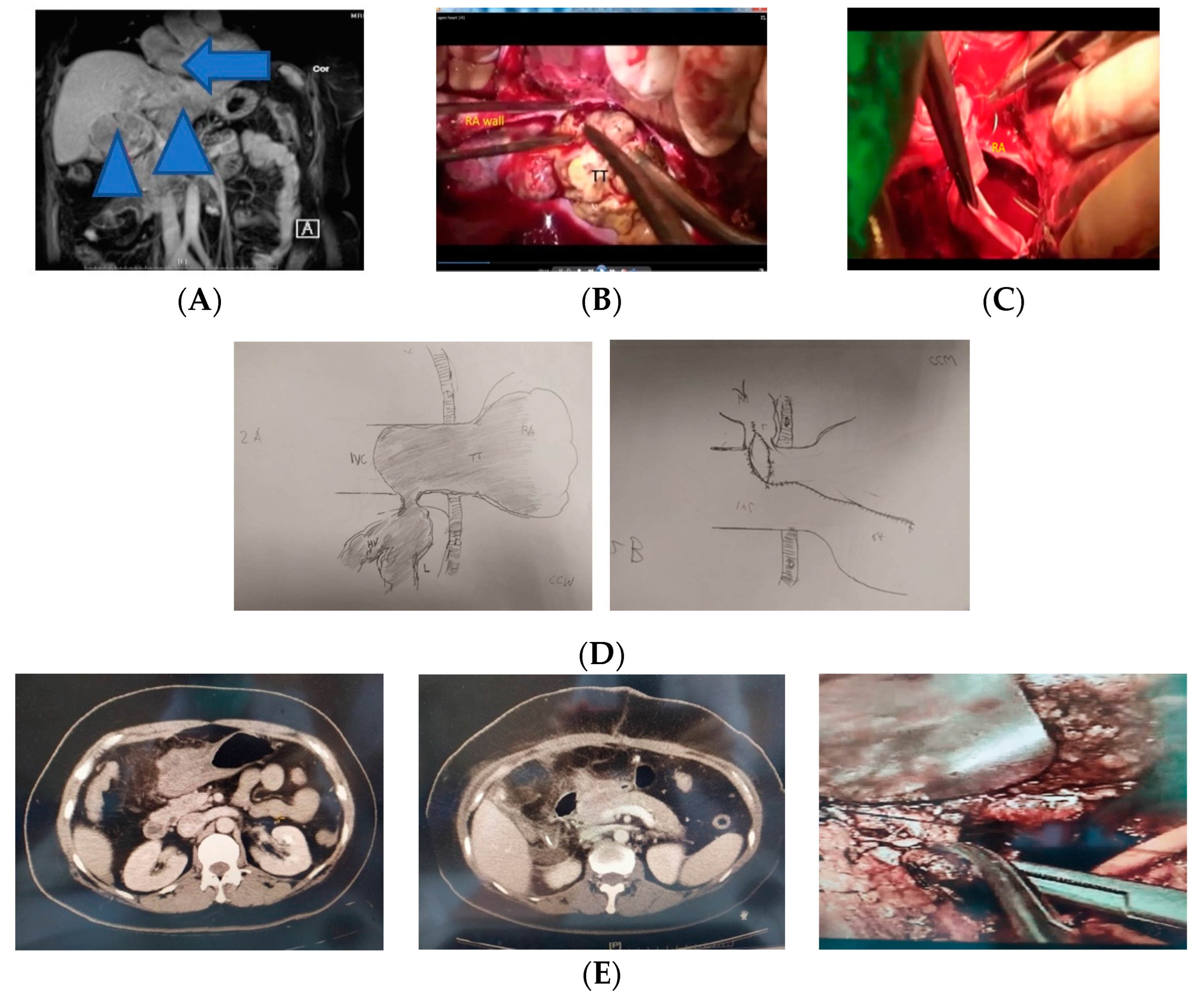
2.1. Preoperative Assessments for Hepatectomy of HCC with RATT
2.2. Intra- and Post-Operative Assessments of Patients (n = 7) in Period A (1998–2010)
2.3. Intra- and Post-Operative Assessments of Patients (n = 17) in Period B (2011 to 2018)
2.4. First Operation
2.5. Second Operation (Staged Hepatectomy)
2.6. Long-Term Follow-Up
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kokudo, T.; Hasegawa, K.; Yamamoto, S.; Shindoh, J.; Takemura, N.; Aoki, T.; Sakamoto, Y.; Makuuchi, M.; Sugawara, Y.; Kokudo, N. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J. Hepatol. 2014, 61, 583–588. [Google Scholar] [CrossRef]
- Kashima, Y.; Miyazaki, M.; Ito, H.; Kaiho, T.; Nakagawa, K.; Ambiru, S.; Shimizu, H.; Furuya, S.; Nakajima, N. Effective hepatic artery chemoembolization for advanced hepatocellular carcinoma with extensive tumour thrombus through the hepatic vein. J. Gastroenterol. Hepatol. 1999, 14, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.H.; Ko, Y.L.; Kuan, P.; Lien, W.P.; Chen, D.S. Metastasis of hepatocellular carcinoma to the heart: Unusual patterns in three cases with antemortem diagnosis. J. Formos. Med. Assoc. 1992, 91, 457–461. [Google Scholar] [PubMed]
- Lie, J.T.; Entman, M.L. “Hole-in-one” sudden death mitral stenosis and left atrial ball-thrombus. Am. Heart J. 1976, 91, 798–804. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nagano, H. Outcomes of surgery for hepatocellular carcinoma with tumor thrombus in the inferior vena cava or right atrium. Surg. Today 2018, 48, 819–824. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Chang, J.Y.; Ka, W.S.; Chao, T.Y.; Liu, T.W.; Chuang, T.R.; Chen, L.T. Hepatocellular carcinoma with intra-atrial tumor thrombi. A report of three cases responsive to thalidomide treatment and literature review. Oncology 2004, 67, 320–326. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, B.; Dong, S.; Zhang, B.; Chen, X. Hepatocellular Carcinoma with Tumor Thrombus Occupying the Right Atrium and Portal Vein: A Case Report and Literature Review. Medicine 2015, 94, e1049–e1053. [Google Scholar] [CrossRef]
- Komatsu, S.; Fukumoto, T.; Demizu, Y.; Miyawaki, D.; Terashima, K.; Niwa, Y.; Mima, M.; Fujii, O.; Sasaki, R.; Yamada, I.; et al. The effectiveness of particle radiotherapy for hepatocellular carcinoma associated with inferior vena tumor thrombus. J. Gastroenterol. 2011, 46, 913–920. [Google Scholar] [CrossRef]
- Chu, M.W.; Aboguddah, A.; Kraus, P.A.; Dewar, L.R. Urgent heart surgery for an atrial mass: Metastatic hepatocellular carcinoma. Ann. Thorac. Surg. 2001, 72, 931–933. [Google Scholar] [CrossRef]
- Tsai, T.P.; Yu, J.M. Minimally invasive cardiac surgery for resection of right atrial hepatic tumor in an octogenarian. J. Chin. Med. Assoc. 2002, 65, 345–347. [Google Scholar]
- Kurahashi, S.; Sano, T.; Natsume, S.; Senda, Y.; Yamaura, H.; Inaba, Y.; Shimizu, Y. Surgical treatment after hepatic arterial infusion chemotherapy for hepatocellular carcinoma extending into the right atrium. Surg. Case Rep. 2015, 1, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgen, M.; Regimbeau, J.M.; Kianmanesh, R.; Marty, J.; Farges, O.; Belghiti, J. Removal of hepatocellular carcinoma extending in the right atrium without extracorporeal bypass. J. Am. Coll. Surg. 2002, 195, 892–894. [Google Scholar] [CrossRef]
- Li, A.J.; Yuan, H.; Yin, L.; Che, Q.; Lang, X.L.; Wu, M.C. Cavoatrial thrombectomy in hepatocellular carcinoma with tumor thrombus in the vena cava and atrium without the use of cardiopulmonary bypass. Ann. Vasc. Surg. 2014, 28, 1565.e5–1565.e8. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, M.; Kurihara, E.; Kikuchi, K.; Nishikawa, K.; Uematsu, Y. Hepatocellular carcinoma with tumor thrombus extending into the right atrium: Report of a successful resection with the use of cardiopulmonary bypass. Surgery 1991, 109, 214–219. [Google Scholar]
- Masaki, N.; Hayashi, S.; Maruyama, T.; Okabe, H.; Matsukawa, M.; Unno, J.; Maekawa, S.; Oka, T.; Tani, M.; Matsueda, K.; et al. Marked clinical improvement in patients with hepatocellular carcinoma by surgical removal of extended tumor mass in right atrium and pulmonary arteries. Cancer Chemother. Pharmacol. 1994, 33, S7–S11. [Google Scholar] [CrossRef]
- Tanaka, A.; Morimoto, T.; Ozaki, N.; Ikai, I.; Yamamoto, Y.; Tsunekawa, S.; Kitai, T.; Yamaoka, Y. Extension of surgical indication for advanced hepatocellular carcinoma: Is it possible to prolong life span or improve quality of life? Hepato-gastroenterology 1996, 43, 1172–1181. [Google Scholar]
- Iemura, J.; Aoshima, M.; Ishigami, N.; Kaneda, T.; Oba, N. Surgery for hepatocellular carcinoma with tumor thrombi in the right atrium. Hepatogastroenterology 1997, 44, 824–825. [Google Scholar]
- Wu, C.C.; Hseih, S.; Ho, W.M.; Tang, J.S.; Liu, T.J.; P’Eng, F.K. Surgical treatment for recurrent hepatocellular carcinoma with tumor thrombi in right atrium: Using cardiopulmonary bypass and deep hypothermic circulatory arrest. J. Surg. Oncol. 2000, 74, 227–231. [Google Scholar] [CrossRef]
- Saïsse, J.; Hardwigsen, J.; Castellani, P.; Caus, T.; Treut, Y.P.L. Budd-Chiari syndrome secondary to intracardiac extension of hepatocellular carcinoma. Two cases treated by radical resection. Hepato-gastroenterology 2001, 48, 836–839. [Google Scholar]
- Wang, Y.; Yuan, L.; Ge, R.L.; Sun, Y.; Wei, G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: Results of a retrospective cohort study. Ann. Surg. Oncol. 2013, 20, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Florman, S.; Weaver, M.; Primeaux, P.; Killackey, M.; Sierra, R.; Gomez, S.; Haque, S.; Regenstein, F.; Balart, L. Aggressive resection of hepatocellular carcinoma with right atrial involvement. Am. Surg. 2009, 75, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hayashi, M.; Katsumata, T.; Shibayama, Y.; Tanigawa, N. Hepatocellular carcinoma with right atrial tumor thrombus: Report of a case. Surg. Today 2011, 41, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Jian, L.L.; Gang, P.B.; Ando, K.M.; Ming, L.D. Removal of hepatocellular carcinoma extending into the right atrium with extracorporeal circulation. Hepato-gastroenterology 2012, 59, 1591–1593. [Google Scholar]
- Wakayama, K.; Kamiyama, T.; Yokoo, H.; Kakisaka, T.; Kamachi, H.; Tsuruga, Y.; Nakanishi, K.; Shimamura, T.; Todo, S.; Taketomi, A. Surgical management of hepatocellular carcinoma with tumor thrombi in the inferior vena cava or right atrium. World J. Surg. Oncol. 2013, 11, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Onitsuka, A.; Hirose, H.; Ozeki, Y.; Hino, A.; Mori, Y.; Murakawa, S.; Muto, Y. Hepatoma with Growth in Right Atrium: Report of Successful Resection. Dig. Surg. 1990, 7, 57–60. [Google Scholar] [CrossRef]
- Schadde, E.; Slankamenac, K.; Breitenstein, S.; Lesurtel, M.; De Oliveira, M.; Beck-Schimmer, B.; Dutkowski, P.; Clavien, P.A. Are two-stage hepatectomies associated with more complications than one-stage procedures? HPB 2013, 15, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, T.; Cloyd, J.M.; Omichi, K.; Chun, Y.S.; Conrad, C.; Tzeng, C.W.D.; Wei, S.H.; Aloia, T.A.; Vauthey, J.N. Two-Stage Hepatectomy vs One-stage major hepatectomy with contralateral resection or ablation for advanced bilobar colorectal liver metastases. J. Am. Coll. Surg. 2018, 226, 825–834. [Google Scholar] [CrossRef]
- Makuuchi, M.; Thai, B.L.; Takayasu, K.; Takayama, T.; Kosuge, T.; Gunven, P.; Yamazaki, S.; Hasegawa, H.; Ozaki, H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: A preliminary report. Surgery 1990, 107, 521–527. [Google Scholar]
- Moris, D.; Chakedis, J.; Sun, S.H.; Spolverato, G.; Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Spartalis, E.; Pawlik, T.M. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: A systematic review. J. Surg. Oncol. 2018, 117, 341–353. [Google Scholar] [CrossRef]
- Wu, C.C.; Ho, Y.Z.; Ho, W.L.; Wu, T.C.; Liu, T.J.; P’Eng, F.K. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: A reappraisal. Br. J. Surg. 1995, 82, 122–126. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsieh, S.R.; Chen, J.T.; Ho, W.L.; Lin, M.C.; Yeh, D.C.; Liu, T.J.; P’eng, F.K. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch. Surg. 2000, 135, 1273–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.C.; Yeh, D.C.; Ho, W.M.; Yu, C.L.; Cheng, S.B.; Liu, T.J.; P’eng, F.K. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients: A randomized comparison of hemihepatic and total hepatic occlusion techniques. Arch. Surg. 2002, 137, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.C.; Peng, C.M.; Cheng, S.B.; Yeh, D.C.; Lui, W.Y.; Liu, T.J.; P’eng, F.K. The necessity of hepatic vein reconstruction after resection of cranial part of the liver and major hepatic veins in cirrhotic patients. Surgery 2012, 151, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Ho, W.M.; Cheng, S.B.; Yeh, D.C.; Wen, M.C.; Liu, T.J.; P’eng, F.K. Perioperative parenteral tranexamic acid in liver tumor resection. Ann. Surg. 2006, 243, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Kang, S.M.; Ho, W.M.; Tang, J.S.; Yeh, D.C.; Liu, T.J.; P’eng, F.K. Prediction and limitation of hepatic tumor resection without blood transfusion in cirrhotic patients. Arch. Surg. 1998, 133, 1007–1010. [Google Scholar] [CrossRef] [Green Version]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complication: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Leo, F.; Rapisarda, F.; Pier, L.S.; Batignani, G. Cavo-atrial thrombectomy combined with left hemi- hepatectomy for vascular invasion from hepatocellular carcinoma on diseased liver under hypothermic cardio-circulatory arrest. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Pesi, B.; Giudici, F.; Moraldi, L.; Montesi, G.; Romagnoli, S.; Pinelli, F.; Stefano, P.; Batignani, G. Hepatoccellular carcinoma on cirrhosis complicated with tumoral thrombi extended to the right atrium: Results in three cases treated with major hepatectomy and thrombectomy under hypothermic cardiocirculatory arrest and literature review. World J. Surg. Oncol. 2016, 14, 83–88. [Google Scholar] [CrossRef] [Green Version]
- LaQuaglia, M.J.; Kim, H.B.; Fynn-Thompson, F.; Baird, C.; Vakili, K. Resection of hepatic tumors with central venous and right atrial extension using cardiopulmonary bypass. J. Pediatric. Surg. Case Rep. 2018, 30, 14–18. [Google Scholar] [CrossRef]
- Besser, M.W.; Klein, A.A. The coagulopathy of cardiopulmonary bypass. Crit. Rev. Clin. Lab. Sci. 2010, 47, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Shimada, M.; Tsujita, E.; Maehara, S.I.; Yamashita, Y.I.; Rikimaru, T.; Tanaka, S.; Sugimachi, K. Thrombectomy before hepatic resection for hepatocellular carcinoma with a tumor thrombus extending to the inferior vena cava. Int. Surg. 2001, 86, 141–143. [Google Scholar] [PubMed]
- Kunz, N.S.; Tieder, J.S.; Whitlock, K.; Jackson, J.C.; Avansino, J.R. Primary fascial closure versus staged closure with Silo in patients with gastroschisis: A meta-analysis. J. Pediatr. Surg. 2013, 48, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okusaka, T.; Ikeda, M. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. ESMO Open 2018, 3 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Wu, T.; Lan, Y.; Yang, W. Current progress of immune checkpoint inhibitors in the treatment of advanced hepatocellular carcinoma. Biosci. Rep. 2022, 42, BSR20212304. [Google Scholar] [CrossRef]
- Matsukuma, S.; Eguchi, H.; Wada, H.; Noda, T.; Shindo, Y.; Tokumitsu, Y.; Matsui, H.; Takahashi, H.; Kobayashi, S.; Nagano, H. Liver resection with thrombectomy for patients with hepatocellular carcinoma and tumour thrombus in the inferior vena cava or right atrium. BJS Open 2020, 4, 241–251. [Google Scholar] [CrossRef]


| Period A (n = 7) | Period B (n = 17) | p Value | |
|---|---|---|---|
| sex (M:F) | 6:1 | 14:3 | 1.000 |
| age (years) | 58 (50–66) | 59 (49–65) | 0.928 |
| cirrhosis (yes:no) | 3:4 | 10:7 | 0.659 |
| newly diagnosed: recurrent | 5:2 | 13:4 | 0.878 |
| hepatitis status B:C:B+C | 6:1:0 | 12:4:1 | 0.682 |
| serum AFP (ng/mL) | 218 (5–11,200) | 371 (11–10,411) | 0.711 |
| ICGR15 (%) | 19.2 (8.0–62.5) | 18.6 (7.5–43.4) | 0.620 |
| Child-Pugh Grade A:B | 6:1 | 2:15 | 1.000 |
| main tumor number ≥2 | 2 | 5 | 1.000 |
| tumor size (cm) | 6.0(3–9) | 5.0 (4–9.5) | 0.855 |
| satellite nodule (yes:no) | 7:0 | 17:0 | 1.000 |
| tumor capsule formation | 6 | 14 | 1.000 |
| tumor differentiationmoderate:poor | 1:6 | 2:15 | 1.000 |
| Period A (n = 7) | Period B (n = 17) * | p Value | |
|---|---|---|---|
| liver transection time (min) | 26.3 (25.0–44.2) | 23.8 (11.5–48.2) | 0.892 |
| liver transection area (cm2) | 30.8 (29–47.6) | 28.5 (18.0–45.5) | 0.286 |
| CPB duration (min) | 544.5 (14.5–105) | 40.5 (12–102.8) | 0.372 |
| operation time (r) | 10.3 (9.3–12.3) | 9.5 (7.5–10.8) # | 0.114 |
| operative blood loss (mL) | 6750 (5600–12,800) | 1680 (910–8600) # | <0.001 # |
| blood transfusion (mL) | 5500 (2300–11,000) | 0 (0–7800) # | <0.001 # |
| postoperative ICU stay (days) | 7 (3–28) | 2 (1–12) | 0.035 |
| need blood transfusion | 7 | 6 | 0.015 |
| postoperative hospital stay (days) | 26 (22–61) | 25 (21–56) # | 0.242 |
| Complications | 4 | 4 | 0.356 |
| SACS | 2 | 2 | 1.000 |
| bile leakage | 1 | 0 | |
| intraabdominal hematoma | 2 | 2 | 1.000 |
| arrhythmia | 1 | 1 | 1.000 |
| Clavien–Dindo grade >3 | 1 | 2 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, W.-S.; Shen, C.-H.; Luo, S.-C.; Wu, F.-H.; Wei, H.-J.; Yu, C.-L.; Wu, C.-C.; Yen, Y.; P’eng, F.-K. Concomitant Hepatectomy and Atrial Thrombectomy under Cardiopulmonary Bypass versus Staged Hepatectomy in the Treatment for Hepatocellular Carcinoma with Large Right Atrial Tumor Thrombi. J. Clin. Med. 2022, 11, 2140. https://doi.org/10.3390/jcm11082140
Chao W-S, Shen C-H, Luo S-C, Wu F-H, Wei H-J, Yu C-L, Wu C-C, Yen Y, P’eng F-K. Concomitant Hepatectomy and Atrial Thrombectomy under Cardiopulmonary Bypass versus Staged Hepatectomy in the Treatment for Hepatocellular Carcinoma with Large Right Atrial Tumor Thrombi. Journal of Clinical Medicine. 2022; 11(8):2140. https://doi.org/10.3390/jcm11082140
Chicago/Turabian StyleChao, Wen-Shan, Ching-Hui Shen, Shao-Ciao Luo, Feng-Hsu Wu, Hao-Ji Wei, Chu-Leng Yu, Cheng-Chung Wu, Yun Yen, and Fang-Ku P’eng. 2022. "Concomitant Hepatectomy and Atrial Thrombectomy under Cardiopulmonary Bypass versus Staged Hepatectomy in the Treatment for Hepatocellular Carcinoma with Large Right Atrial Tumor Thrombi" Journal of Clinical Medicine 11, no. 8: 2140. https://doi.org/10.3390/jcm11082140
APA StyleChao, W.-S., Shen, C.-H., Luo, S.-C., Wu, F.-H., Wei, H.-J., Yu, C.-L., Wu, C.-C., Yen, Y., & P’eng, F.-K. (2022). Concomitant Hepatectomy and Atrial Thrombectomy under Cardiopulmonary Bypass versus Staged Hepatectomy in the Treatment for Hepatocellular Carcinoma with Large Right Atrial Tumor Thrombi. Journal of Clinical Medicine, 11(8), 2140. https://doi.org/10.3390/jcm11082140







