Acute Kidney Injury Recovery Patterns in ST-Segment Elevation Myocardial Infarction Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definition of AKI and AKI Recovery
2.3. Statistical Analysis
3. Results
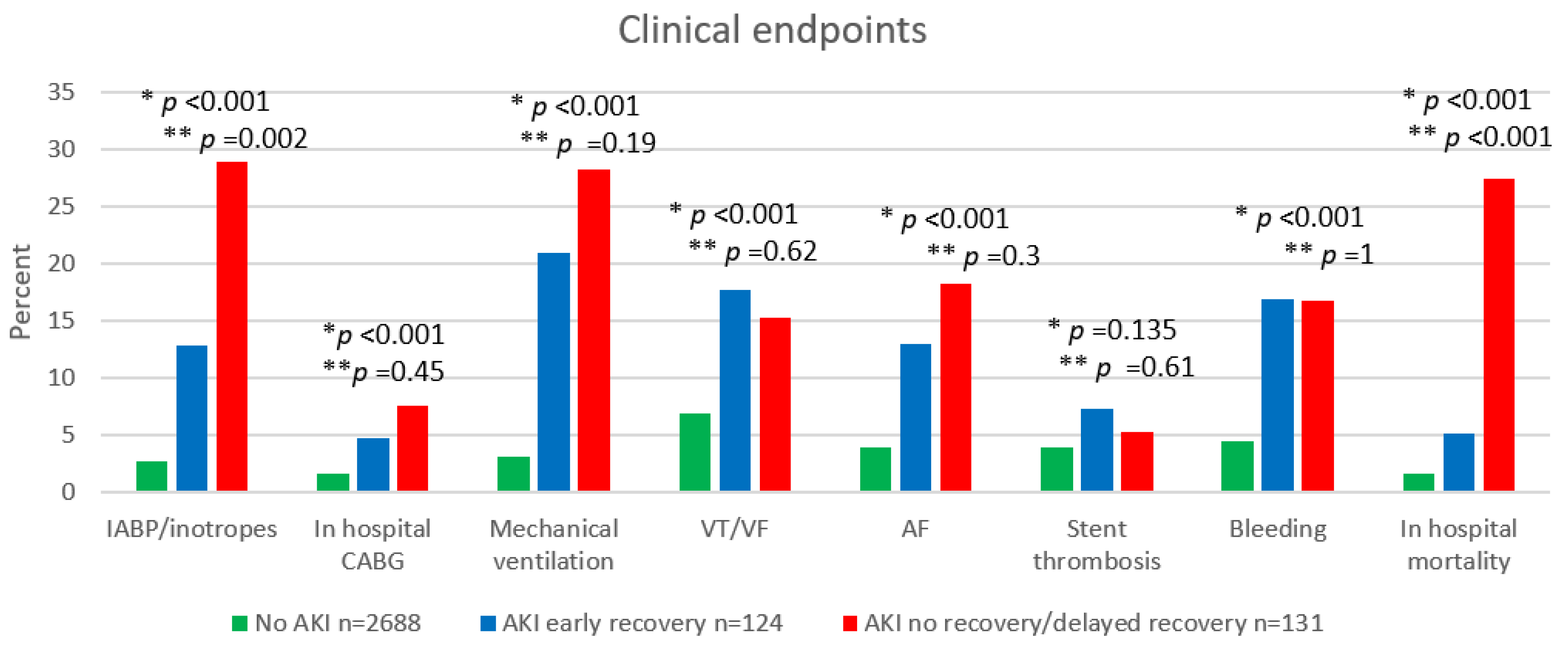
3.1. In-Hospital Outcome
3.2. Long Term Mortality
3.3. Long Term Renal Outcomes for Non-Recovered AKI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, L.S.; Amdur, R.L.; Shaw, A.D.; Faselis, C.; Palant, C.E.; Kimmel, P.L. Association between AKI and Long-Term Renal and Cardiovascular Outcomes in United States Veterans. Clin. J. Am. Soc. Nephrol. 2014, 9, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Messenger, J.C.; Rumsfeld, J.S.; et al. Contemporary Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Interventions. JACC Cardiovasc. Interv. 2014, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Forni, L.G.; Darmon, M.; Ostermann, M.; Oudemans-van Straaten, H.M.; Pettilä, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal Recovery after Acute Kidney Injury. Intensive Care Med. 2017, 43, 855–866. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute Kidney Disease and Renal Recovery: Consensus Report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.J.; Chawla, L.S. Recovery after Acute Kidney Injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A. How Can We Define Recovery after Acute Kidney Injury? Considerations from Epidemiology and Clinical Trial Design. Nephron Clin. Pract. 2014, 127, 81–88. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Zelnick, L.R.; Chinchilli, V.M.; Moledina, D.G.; Coca, S.G.; Parikh, C.R.; Garg, A.X.; Hsu, C.; Go, A.S.; Liu, K.D.; et al. Association between Early Recovery of Kidney Function after Acute Kidney Injury and Long-Term Clinical Outcomes. JAMA Netw. Open 2020, 3, e202682. [Google Scholar] [CrossRef]
- Poston, J.T.; Koyner, J.L. Sepsis Associated Acute Kidney Injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary. Circulation 2013, 127, 529–555. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017, 28, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Margolis, G.; Gal-Oz, A.; Letourneau-Shesaf, S.; Khoury, S.; Keren, G.; Shacham, Y. Acute Kidney Injury Based on the KDIGO Criteria among ST Elevation Myocardial Infarction Patients Treated by Primary Percutaneous Intervention. J. Nephrol. 2018, 31, 423–428. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Ghali, W.A.; Tonelli, M.; Faris, P.; Knudtson, M.L.; Pannu, N.; Klarenbach, S.W.; Manns, B.J.; Hemmelgarn, B.R. Acute Kidney Injury Following Coronary Angiography Is Associated with a Long-Term Decline in Kidney Function. Kidney Int. 2010, 78, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, A.; Kogan, E.; Hammerman, H.; Markiewicz, W.; Aronson, D. The Impact of Transient and Persistent Acute Kidney Injury on Long-Term Outcomes after Acute Myocardial Infarction. Kidney Int. 2009, 76, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Auer, J.; Verbrugge, F.H.; Lamm, G. Editor’s Choice—What Do Small Serum Creatinine Changes Tell Us about Outcomes after Acute Myocardial Infarction? Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Siew, E.D.; Abdel-Kader, K.; Perkins, A.M.; Greevy, R.A.; Parr, S.K.; Horner, J.; Vincz, A.J.; Denton, J.; Wilson, O.D.; Hung, A.M.; et al. Timing of Recovery from Moderate to Severe AKI and the Risk for Future Loss of Kidney Function. Am. J. Kidney Dis. 2020, 75, 204–213. [Google Scholar] [CrossRef]
- Göcze, I.; Wiesner, C.; Schlitt, H.J.; Bergler, T. Renal Recovery. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 403–414. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Chawla, L.; Ronco, C.; Kellum, J.A. Renal Recovery. Crit. Care 2014, 18, 301. [Google Scholar] [CrossRef] [Green Version]
- Parenica, J.; Kala, P.; Mebazaa, A.; Littnerova, S.; Benesova, K.; Tomandl, J.; Goldbergová Pavkova, M.; Jarkovský, J.; Spinar, J.; Tomandlova, M.; et al. Activation of the Nitric Oxide Pathway and Acute Myocardial Infarction Complicated by Acute Kidney Injury. Cardiorenal Med. 2020, 10, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Reinstadler, S.J.; Kronbichler, A.; Reindl, M.; Feistritzer, H.-J.; Innerhofer, V.; Mayr, A.; Klug, G.; Tiefenthaler, M.; Mayer, G.; Metzler, B. Acute Kidney Injury Is Associated with Microvascular Myocardial Damage following Myocardial Infarction. Kidney Int. 2017, 92, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Khoury, S.; Margolis, G.; Ravid, D.; Rozenbaum, Z.; Keren, G.; Shacham, Y. Outcomes of Early and Reversible Renal Impairment in Patients with ST Segment Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 684–689. [Google Scholar] [CrossRef] [PubMed]

| No AKI n = 2688 | AKI Early Recovery n = 124 | AKI No Recovery/ Delayed Recovery n = 131 | No AKI vs. AKI Early Recovery | No AKI vs. AKI No Recovery/ Delayed Recovery | AKI early Recovery vs. AKI No Recovery/ Delayed Recovery | p-Value | |
|---|---|---|---|---|---|---|---|
| Age, years | 60.9 ± 12.7 | 67.7 ± 12.2 | 75.7 ± 12.5 | <0.001 | <0.001 | 0.001 | <0.001 |
| Male gender | 2213 (82.3) | 91 (73.4) | 95 (72.5) | 0.016 | 0.007 | 0.889 | 0.001 |
| Past MI | 404 (15) | 24 (19.4) | 36 (27.5) | 0.2 | <0.001 | 0.141 | <0.001 |
| Hyperlipidemia | 1315 (48.9) | 70 (56.5) | 77 (58.8) | 0.12 | 0.031 | 0.8 | 0.027 |
| Hypertension | 1149 (42.8) | 81 (65.3) | 103 (78.6) | <0.001 | <0.001 | 0.025 | <0.001 |
| Diabetes | 617 (23) | 41 (33.1) | 50 (38.2) | 0.012 | <0.001 | 0.434 | <0.001 |
| Family history of IHD | 616 (23) | 19 (15.3) | 5 (3.8) | 0.048 | <0.001 | 0.002 | <0.001 |
| Smoker | 1365 (51.3) | 50 (41) | 38 (29.2) | 0.026 | <0.001 | 0.064 | <0.001 |
| Time to ER | 120 [60–311] | 200 [82–720] | 180 [60–885] | <0.001 | 0.002 | 0.593 | <0.001 |
| Door to balloon | 45 [30–60] | 45 [30–60] | 45 [30–60] | 0.227 | 0.483 | 0.674 | 0.39 |
| Time to reperfusion, min | 175 [105–445] | 270 [120–765] | 240 [128–1060] | <0.001 | 0.002 | 0.861 | <0.001 |
| Coronary artery vessel disease | 0.006 | <0.001 | 0.451 | <0.001 | |||
| 1 | 1130 (42.3) | 43 (34.7) | 36 (28.8) | ||||
| 2 | 828 (31) | 31 (25) | 29 (23.2) | ||||
| 3 | 700 (26.2) | 50 (40.3) | 60 (48) | ||||
| eGFR | 78.42 ± 23.7 | 66.43 ± 24.4 | 50.53 ± 22.5 | <0.001 | <0.001 | <0.001 | <0.001 |
| Creatinine admission | 1.04 [0.9–1.19] | 1.11 [0.97–1.29] | 1.32 [1.06–1.71] | <0.001 | <0.001 | <0.001 | <0.001 |
| Creatinine peak | 1.03 [0.92–1.18] | 1.55 [1.38–1.98] | 2.12 [1.65–2.96] | <0.001 | <0.001 | <0.001 | <0.001 |
| Troponin-I admission | 0.91 [0.06–27.1] | 0.73 [0.05–16.3] | 2.95 [0.53–32.76] | 0.457 | 0.002 | 0.003 | 0.005 |
| Troponin-I peak | 43.96 [6.92–384.95] | 33.54 [2.65–126.25] | 63.59 [7.35–348.79] | 0.059 | 0.371 | 0.039 | 0.102 |
| HR | 95% CI | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.063 | 1.044 | 1.082 | <0.001 |
| Family history of IHD | 0.327 | 0.125 | 0.853 | 0.022 |
| HTN | 2.827 | 1.733 | 4.612 | <0.001 |
| CAD | 1.304 | 1.031 | 1.650 | 0.027 |
| EF | 0.913 | 0.891 | 0.935 | <0.001 |
| HR | 95% CI | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.071 | 1.050 | 1.093 | <0.001 |
| No AKI | Reference | |||
| AKI early recovery | 2.091 | 0.933 | 4.687 | 0.073 |
| AKI no recovery/delayed recovery | 7.763 | 4.686 | 12.859 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itach, T.; Banai, A.; Paran, Y.; Zahler, D.; Merdler, I.; Eliashiv, D.; Banai, S.; Shacham, Y. Acute Kidney Injury Recovery Patterns in ST-Segment Elevation Myocardial Infarction Patients. J. Clin. Med. 2022, 11, 2169. https://doi.org/10.3390/jcm11082169
Itach T, Banai A, Paran Y, Zahler D, Merdler I, Eliashiv D, Banai S, Shacham Y. Acute Kidney Injury Recovery Patterns in ST-Segment Elevation Myocardial Infarction Patients. Journal of Clinical Medicine. 2022; 11(8):2169. https://doi.org/10.3390/jcm11082169
Chicago/Turabian StyleItach, Tamar, Ariel Banai, Yael Paran, David Zahler, Ilan Merdler, David Eliashiv, Shmuel Banai, and Yacov Shacham. 2022. "Acute Kidney Injury Recovery Patterns in ST-Segment Elevation Myocardial Infarction Patients" Journal of Clinical Medicine 11, no. 8: 2169. https://doi.org/10.3390/jcm11082169
APA StyleItach, T., Banai, A., Paran, Y., Zahler, D., Merdler, I., Eliashiv, D., Banai, S., & Shacham, Y. (2022). Acute Kidney Injury Recovery Patterns in ST-Segment Elevation Myocardial Infarction Patients. Journal of Clinical Medicine, 11(8), 2169. https://doi.org/10.3390/jcm11082169






