Neurological Music Therapy Rebuilds Structural Connectome after Traumatic Brain Injury: Secondary Analysis from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
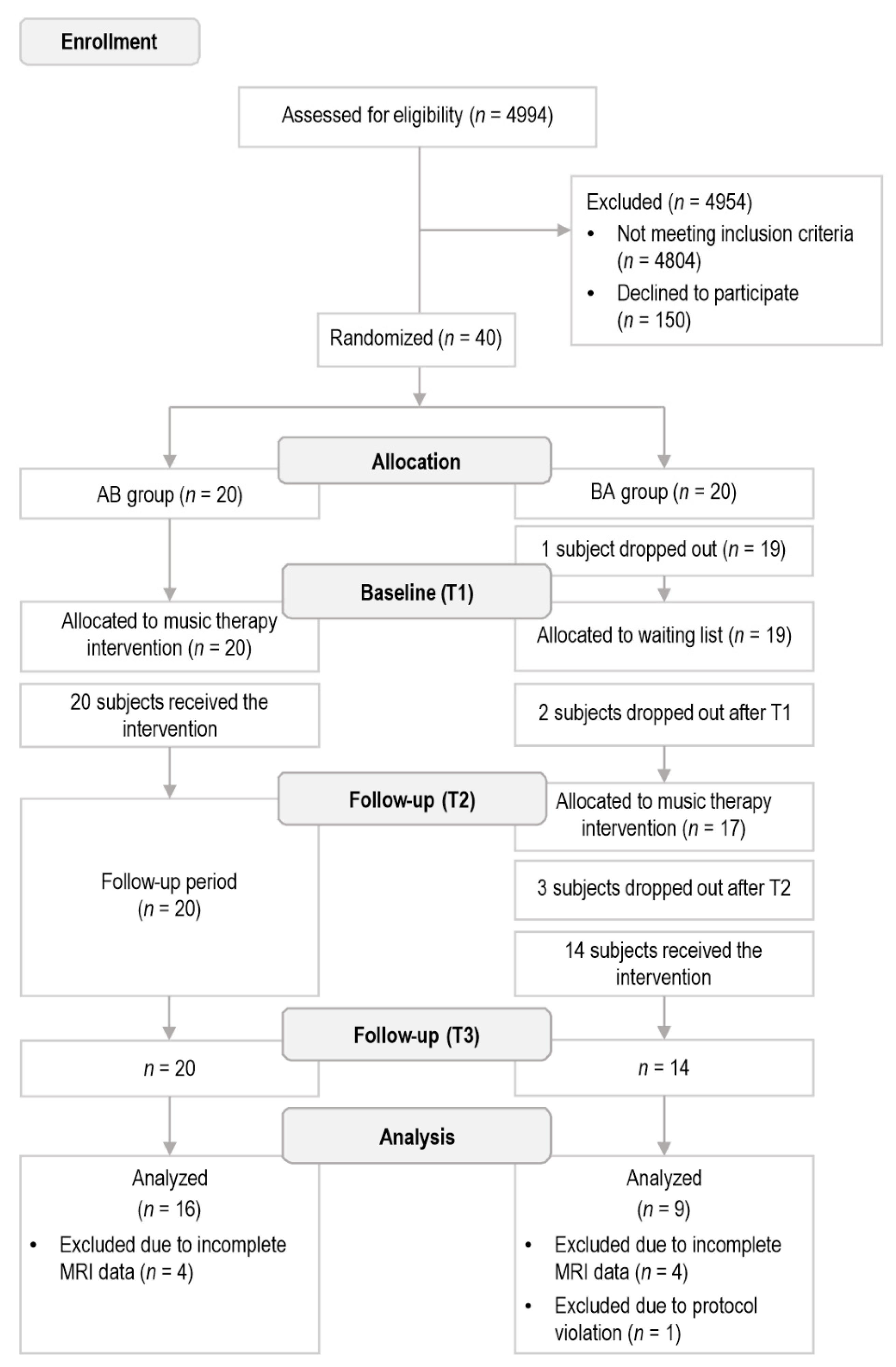
2.1. Subjects and Study Design
2.2. Intervention
2.3. Neuropsychological Assessment
2.4. MRI Data Acquisition and Reconstruction
2.5. Regions of Interest
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; David Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Colantonio, A.; Ratcliff, G.; Chase, S.; Kelsey, S.; Escobar, M.; Vernich, L. Long term outcomes after moderate to severe traumatic brain injury. Disabil. Rehabil. 2004, 26, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Strich, S.J. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J. Neurol. Neurosurg. Psychiatry 1956, 19, 163–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peerless, S.J.; Rewcastle, N.B. Shear injuries of the brain. Can. Med. Assoc. J. 1967, 96, 577–582. [Google Scholar]
- Hoofien, D.; Gilboa, A.; Vakil, E.; Donovick, P.J. Traumatic brain injury (TBI) 10–20 years later: A comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 2001, 15, 189–209. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Dikmen, S.S.; Corrigan, J.D.; Levin, H.S.; MacHamer, J.; Stiers, W.; Weisskopf, M.G. Cognitive outcome following traumatic brain injury. J. Head Trauma Rehabil. 2009, 24, 430–438. [Google Scholar] [CrossRef]
- McDonald, B.C.; Flashman, L.A.; Saykin, A.J. Executive dysfunction following traumatic brain injury: Neural substrates and treatment strategies. NeuroRehabilitation 2002, 17, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Kinnunen, K.M.; Greenwood, R.; Powell, J.H.; Leech, R.; Hawkins, P.C.; Bonnelle, V.; Patel, M.C.; Counsell, S.J.; Sharp, D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011, 134, 449–463. [Google Scholar] [CrossRef]
- Sharp, D.J.; Scott, G.; Leech, R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014, 10, 156–166. [Google Scholar] [CrossRef]
- Scheid, R.; Walther, K.; Guthke, T.; Preul, C.; Von Cramon, D.Y. Cognitive sequelae of diffuse axonal injury. Arch. Neurol. 2006, 63, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Brandstack, N.; Kurki, T.; Tenovuo, O. Quantitative diffusion-tensor tractography of long association tracts in patients with traumatic brain injury without associated findings at routine MR imaging. Radiology 2013, 267, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, E.M.; Owen, J.P.; Yuh, E.L.; Wang, M.B.; Vassar, M.J.; Ferguson, A.R.; Diaz-Arrastia, R.; Giacino, J.T.; Okonkwo, D.O.; Robertson, C.S.; et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci. Adv. 2020, 6, eaaz6892. [Google Scholar] [CrossRef]
- Douglas, D.B.; Iv, M.; Douglas, P.K.; Anderson, A.; Vos, S.B.; Bammer, R.; Zeineh, M.; Wintermark, M. Diffusion tensor imaging of TBI: Potentials and challenges. Top. Magn. Reson. Imaging 2015, 24, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Farbota, K.D.; Bendlin, B.B.; Alexander, A.L.; Rowley, H.A.; Dempsey, R.J.; Johnson, S.C. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 2012, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Hellstrøm, T.; Westlye, L.T.; Kaufmann, T.; Trung Doan, N.; Søberg, H.L.; Sigurdardottir, S.; Nordhøy, W.; Helseth, E.; Andreassen, O.A.; Andelic, N. White matter microstructure is associated with functional, cognitive and emotional symptoms 12 months after mild traumatic brain injury. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Braeckman, K.; Descamps, B.; Caeyenberghs, K.; Vanhove, C. Longitudinal DTI changes following cognitive training therapy in a mild traumatic brain injury rat model. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Cernich, A.N.; Kurtz, S.M.; Mordecai, K.L.; Ryan, P.B. Cognitive rehabilitation in traumatic brain injury. Curr. Treat. Options Neurol. 2010, 12, 412–423. [Google Scholar] [CrossRef]
- Cicerone, K.; Levin, H.; Malec, J.; Stuss, D.; Whyte, J.; Edwards, E. Cognitive rehabilitation interventions for executive function: Moving from bench to bedside in patients with traumatic brain injury. J. Cogn. Neurosci. 2006, 18, 1212–1222. [Google Scholar] [CrossRef]
- Sihvonen, A.J.; Särkämö, T.; Leo, V.; Tervaniemi, M.; Altenmüller, E.; Soinila, S. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017, 16, 648–660. [Google Scholar] [CrossRef] [Green Version]
- Särkämö, T.; Tervaniemi, M.; Laitinen, S.; Forsblom, A.; Soinila, S.; Mikkonen, M.; Autti, T.; Silvennoinen, H.M.; Erkkilä, J.; Laine, M.; et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 2008, 131, 866–876. [Google Scholar] [CrossRef] [Green Version]
- Sihvonen, A.J.; Leo, V.; Ripollés, P.; Lehtovaara, T.; Ylönen, A.; Rajanaro, P.; Laitinen, S.; Forsblom, A.; Saunavaara, J.; Autti, T.; et al. Vocal music enhances memory and language recovery after stroke: Pooled results from two RCTs. Ann. Clin. Transl. Neurol. 2020, 7, 2272–2287. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, A.J.; Ripollés, P.; Leo, V.; Saunavaara, J.; Parkkola, R.; Rodriguez-Fornells, A.; Soinila, S.; Särkämö, T.; Rodríguez-Fornells, A.; Soinila, S.; et al. Vocal music listening enhances post-stroke language network reorganization. eNeuro 2021, 8, 34140351. [Google Scholar] [CrossRef] [PubMed]
- Särkämö, T.; Ripollés, P.; Vepsäläinen, H.; Autti, T.; Silvennoinen, H.M.; Salli, E.; Laitinen, S.; Forsblom, A.; Soinila, S.; Rodríguez-Fornells, A. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: A voxel-based morphometry study. Front. Hum. Neurosci. 2014, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Siponkoski, S.T.; Martínez-Molina, N.; Kuusela, L.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Melkas, S.; Pekkola, J.; et al. Music Therapy Enhances Executive Functions and Prefrontal Structural Neuroplasticity after Traumatic Brain Injury: Evidence from a Randomized Controlled Trial. J. Neurotrauma 2020, 37, 618–634. [Google Scholar] [CrossRef]
- Martínez-Molina, N.; Siponkoski, S.T.; Kuusela, L.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Melkas, S.; Pekkola, J.; et al. Resting-State Network Plasticity Induced by Music Therapy after Traumatic Brain Injury. Neural Plast. 2021, 2021, 6682471. [Google Scholar] [CrossRef]
- Yeh, F.C.; Badre, D.; Verstynen, T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. Neuroimage 2016, 125, 162–171. [Google Scholar] [CrossRef]
- Hula, W.D.; Panesar, S.; Gravier, M.L.; Yeh, F.C.; Dresang, H.C.; Dickey, M.W.; Fernandez-Miranda, J.C. Structural white matter connectometry of word production in aphasia: An observational study. Brain 2020, 143, 2532–2544. [Google Scholar] [CrossRef]
- Dresang, H.C.; Hula, W.D.; Yeh, F.-C.; Warren, T.; Dickey, M.W. White-Matter Neuroanatomical Predictors of Aphasic Verb Retrieval. Brain Connect. 2021, 11, 319–330. [Google Scholar] [CrossRef]
- Traumatic Brain Injury. Current Care Guidelines. Working group Set up by the Finnish Medical Society Duodecim, Finnish Neurological Society, Societas Medicinae Physicalis et Rehabilitationis Fenniae, Finnish Neurosurgical Society, Finnish Neuropsychological Society and Assocication of Finnish Insurance Medicine Doctors, Helsinki. Helsinki: The Finnish Medical Society Duodecim, 2021 (Referred 6.4.2022). Available online: www.kaypahoito.fi (accessed on 6 April 2022).
- Wilson, J.T.L.; Pettigrew, L.E.L.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; McCauley, S.R.; Kelly, T.M.; Weyand, A.M.; Pedroza, C.; Levin, H.S.; Clifton, G.L.; Schnelle, K.P.; Shah, M.V.; Moretti, P. The neurological outcome scale for traumatic brain injury (NOS-TBI): I. Construct validity. J. Neurotrauma 2010, 27, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A frontal assessment battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Yeh, F.C.; Tseng, W.Y.I. NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 2011, 58, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Wedeen, V.J.; Tseng, W.Y.I. Generalized q-sampling imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [CrossRef]
- Schilling, K.G.; Yeh, F.C.; Nath, V.; Hansen, C.; Williams, O.; Resnick, S.; Anderson, A.W.; Landman, B.A. A fiber coherence index for quality control of B-table orientation in diffusion MRI scans. Magn. Reson. Imaging 2019, 58, 82–89. [Google Scholar] [CrossRef]
- Yeh, F.C.; Liu, L.; Hitchens, T.K.; Wu, Y.L. Mapping immune cell infiltration using restricted diffusion MRI. Magn. Reson. Med. 2017, 77, 603–612. [Google Scholar] [CrossRef]
- Yeh, F.C.; Vettel, J.M.; Singh, A.; Poczos, B.; Grafton, S.T.; Erickson, K.I.; Tseng, W.Y.I.; Verstynen, T.D. Quantifying Differences and Similarities in Whole-Brain White Matter Architecture Using Local Connectome Fingerprints. PLoS Comput. Biol. 2016, 12, e1005203. [Google Scholar] [CrossRef]
- Yeh, F.C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.Y.I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef] [Green Version]
- Rolls, E.T.; Huang, C.C.; Lin, C.P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Yeh, F.C.; Panesar, S.; Barrios, J.; Fernandes, D.; Abhinav, K.; Meola, A.; Fernandez-Miranda, J.C. Automatic Removal of False Connections in Diffusion MRI Tractography Using Topology-Informed Pruning (TIP). Neurotherapeutics 2019, 16, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, N.P.; Miyake, A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex 2017, 86, 186–204. [Google Scholar] [CrossRef] [Green Version]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Shen, K.; Welton, T.; Lyon, M.; McCorkindale, A.N.; Sutherland, G.T.; Burnham, S.; Fripp, J.; Martins, R.; Grieve, S.M. Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum. Brain Mapp. 2020, 41, 1226–1236. [Google Scholar] [CrossRef]
- Sasson, E.; Doniger, G.M.; Pasternak, O.; Tarrasch, R.; Assaf, Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front. Neurosci. 2013, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallen, C.L.; Turner, G.R.; Adnan, A.; D’Esposito, M. Reconfiguration of brain network architecture to support executive control in aging. Neurobiol. Aging 2016, 44, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tian, L.; Zhang, L.; Cheng, R.; Wei, R.; He, F.; Li, J.; Luo, B.; Ye, X. Relationship between white matter integrity and post-traumatic cognitive deficits: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Filley, C.M. White matter: Organization and functional relevance. Neuropsychol. Rev. 2010, 20, 158–173. [Google Scholar] [CrossRef]
- Filley, C.M.; Kelly, J.P. White Matter and Cognition in Traumatic Brain Injury. J. Alzheimers. Dis. 2018, 65, 345–362. [Google Scholar] [CrossRef]
- Medana, I.M.; Esiri, M.M. Axonal damage: A key predictor of outcome in human CNS diseases. Brain 2003, 126, 515–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomassini, V.; Matthews, P.M.; Thompson, A.J.; Fuglo, D.; Geurts, J.J.; Johansen-Berg, H.; Jones, D.K.; Rocca, M.A.; Wise, R.G.; Barkhof, F.; et al. Neuroplasticity and functional recovery in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Fields, R.D. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef] [Green Version]
- Bettcher, B.M.; Mungas, D.; Patel, N.; Elofson, J.; Dutt, S.; Wynn, M.; Watson, C.L.; Stephens, M.; Walsh, C.M.; Kramer, J.H. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia 2016, 85, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Vaquero, L.; Rousseau, P.N.; Vozian, D.; Klein, D.; Penhune, V. What you learn & when you learn it: Impact of early bilingual & music experience on the structural characteristics of auditory-motor pathways. Neuroimage 2020, 213, 116689. [Google Scholar] [CrossRef]
- Halwani, G.F.; Loui, P.; Rüber, T.; Schlaug, G. Effects of practice and experience on the arcuate fasciculus: Comparing singers, instrumentalists, and non-musicians. Front. Psychol. 2011, 2, 156. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.; Hijmans, B.S.; Cerliani, L.; Bangert, M.; Nanetti, L.; Keller, P.E.; Keysers, C. Inter-individual differences in audio-motor learning of piano melodies and white matter fiber tract architecture. Hum. Brain Mapp. 2014, 35, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Loui, P.; Raine, L.B.; Chaddock-Heyman, L.; Kramer, A.F.; Hillman, C.H. Musical instrument practice predicts white matter microstructure and cognitive abilities in childhood. Front. Psychol. 2019, 10, 1198. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Damasio, A.; Ilari, B.; Veiga, R.; Joshi, A.A.; Leahy, R.M.; Haldar, J.P.; Varadarajan, D.; Bhushan, C.; Damasio, H. Childhood music training induces change in micro and macroscopic brain structure: Results from a longitudinal study. Cereb. Cortex 2018, 28, 4336–4347. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.L.; Nagy, Z.; Skare, S.; Forsman, L.; Forssberg, H.; Ullén, F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005, 8, 1148–1150. [Google Scholar] [CrossRef]
- Siponkoski, S.-T.; Koskinen, S.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Martínez-Molina, N.; Melkas, S.; Laine, M.; et al. Effects of neurological music therapy on behavioural and emotional recovery after traumatic brain injury: A randomized controlled cross-over trial. Neuropsychol. Rehabil. 2021, 1–33. [Google Scholar] [CrossRef]

| AB | BA | Difference between Groups (p) | |
|---|---|---|---|
| Demographic information | |||
| Age | 42.1 (14.9) | 40.8 (11.5) | 0.814 (t) |
| Gender (female/male) | 7/9 | 3/6 | 0.691 (X2) |
| Education in years | 14.3 (2.7) | 14.9 (2.1) | 0.535 (t) |
| Clinical information | |||
| TBI severity (moderate/severe) | 13/3 | 5/4 | 0.170 (X2) |
| GCS (severe/moderate/minor) a | 2/3/10 | 2/0/6 | 0.357 (X2) |
| PTA classification (mild/moderate/severe) b | 9/3/2 | 3/3/2 | 0.330 (X2) |
| Cause of injury (traffic-related/fall/other) | 6/9/1 | 2/3/4 | 0.072 (X2) |
| Time since injury (months) | 8.4 (6.0) | 7.1 (6.1) | 0.622 (t) |
| Lesion laterality c (left/right/both) | 3/1/11 | 3/0/6 | 0.603 (t) |
| DAI c (yes/no) | 6/9 | 6/3 | 0.400 (X2) |
| Hemorrhages, bleeds or ischemic injury c (yes/no) | 10/5 | 5/4 | 0.678 (X2) |
| GOSE d | 5.2 (1.5) | 5.6 (1.1) | 0.541 (t) |
| NOS-TBI e | 2.0 (2.1) | 1.8 (2.3) | 0.812 (t) |
| Musical background | |||
| Instrument playing (yes/no) | 11/6 | 5/3 | 1.000 (X2) |
| Years of playing | 5.2 (11.3) | 3.8 (5.8) | 0.783 (t) |
| Singing (yes/no) | 9/7 | 3/6 | 0.411 (X2) |
| Years of singing | 7.9 (13.7) | 1.3 (3.0) | 0.170 (t) |
| Dancing (yes/no) | 9/7 | 4/5 | 0.688 (X2) |
| Years of dancing | 5.4 (10.9) | 4.8 (9.8) | 0.889 (t) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sihvonen, A.J.; Siponkoski, S.-T.; Martínez-Molina, N.; Laitinen, S.; Holma, M.; Ahlfors, M.; Kuusela, L.; Pekkola, J.; Koskinen, S.; Särkämö, T. Neurological Music Therapy Rebuilds Structural Connectome after Traumatic Brain Injury: Secondary Analysis from a Randomized Controlled Trial. J. Clin. Med. 2022, 11, 2184. https://doi.org/10.3390/jcm11082184
Sihvonen AJ, Siponkoski S-T, Martínez-Molina N, Laitinen S, Holma M, Ahlfors M, Kuusela L, Pekkola J, Koskinen S, Särkämö T. Neurological Music Therapy Rebuilds Structural Connectome after Traumatic Brain Injury: Secondary Analysis from a Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(8):2184. https://doi.org/10.3390/jcm11082184
Chicago/Turabian StyleSihvonen, Aleksi J., Sini-Tuuli Siponkoski, Noelia Martínez-Molina, Sari Laitinen, Milla Holma, Mirja Ahlfors, Linda Kuusela, Johanna Pekkola, Sanna Koskinen, and Teppo Särkämö. 2022. "Neurological Music Therapy Rebuilds Structural Connectome after Traumatic Brain Injury: Secondary Analysis from a Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 8: 2184. https://doi.org/10.3390/jcm11082184
APA StyleSihvonen, A. J., Siponkoski, S.-T., Martínez-Molina, N., Laitinen, S., Holma, M., Ahlfors, M., Kuusela, L., Pekkola, J., Koskinen, S., & Särkämö, T. (2022). Neurological Music Therapy Rebuilds Structural Connectome after Traumatic Brain Injury: Secondary Analysis from a Randomized Controlled Trial. Journal of Clinical Medicine, 11(8), 2184. https://doi.org/10.3390/jcm11082184






