Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carotid Distensibility and Compliance
2.2. Pulse Wave Velocity (PWV)
2.3. Pulse Wave Analysis and Central Blood Pressure Measurement
2.4. Statistical Analysis
2.5. Preliminary Study: Standardization of the Method for Measuring Vascular Distensibility
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutouyrie, P.; Tropeano, A.I.; Asmar, R.; Gautier, I.; Benetos, A.; Lacolley, P.; Laurent, S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 2002, 39, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Popele, N.M.; Mattace-Raso, F.U.; Vliegenthart, R.; Grobbee, D.E.; Asmar, R.; van der Kuip, D.A.; Hofman, A.; de Feijter, P.J.; Oudkerk, M.; Witteman, J.C. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: The Rotterdam Study. J. Hypertens. 2006, 24, 2371–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequi-Dominguez, I.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Nunez de Arenas-Arroyo, S.; Martinez-Vizcaino, V. Accuracy of Pulse Wave Velocity Predicting Cardiovascular and All-Cause Mortality. A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Thomas, F.; Joly, L.; Blacher, J.; Pannier, B.; Labat, C.; Salvi, P.; Smulyan, H.; Safar, M.E. Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J. Am. Coll. Cardiol. 2010, 55, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Safar, M.E.; Blacher, J.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Guyonvarch, P.M.; London, G.M. Central pulse pressure and mortality in end-stage renal disease. Hypertension 2002, 39, 735–738. [Google Scholar] [CrossRef] [Green Version]
- London, G.M.; Blacher, J.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Safar, M.E. Arterial wave reflections and survival in end-stage renal failure. Hypertension 2001, 38, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef] [Green Version]
- Pannier, B.; Guerin, A.P.; Marchais, S.J.; Safar, M.E.; London, G.M. Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension 2005, 45, 592–596. [Google Scholar] [CrossRef] [Green Version]
- Blacher, J.; Asmar, R.; Djane, S.; London, G.M.; Safar, M.E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999, 33, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Katsahian, S.; Fassot, C.; Tropeano, A.I.; Gautier, I.; Laloux, B.; Boutouyrie, P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003, 34, 1203–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meaume, S.; Benetos, A.; Henry, O.F.; Rudnichi, A.; Safar, M.E. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arter. Thromb. Vasc. Biol. 2001, 21, 2046–2050. [Google Scholar] [CrossRef]
- Benetos, A.; Gautier, S.; Labat, C.; Salvi, P.; Valbusa, F.; Marino, F.; Toulza, O.; Agnoletti, D.; Zamboni, M.; Dubail, D.; et al. Mortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: The PARTAGE (Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized Very Aged Population) study. J. Am. Coll. Cardiol. 2012, 60, 1503–1511. [Google Scholar]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Lassnig, E.; Lamm, G.; Stark, N.; Rammer, M.; Eber, B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur. Heart J. 2005, 26, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Zambrano, J.P.; Chakko, S.; Veerani, A.; Schob, A.; Willens, H.J.; Perez, G.; Mendez, A.J. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension 2005, 45, 980–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattace-Raso, F.U.; van der Cammen, T.J.; Hofman, A.; van Popele, N.M.; Bos, M.L.; Schalekamp, M.A.; Asmar, R.; Reneman, R.S.; Hoeks, A.P.; Breteler, M.M.; et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation 2006, 113, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [Green Version]
- Salvi, P. Pulse Waves. How Vascular Hemodynamics Affects Blood Pressure, 2nd ed.; Springer Nature: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Laurent, S.; Caviezel, B.; Beck, L.; Girerd, X.; Billaud, E.; Boutouyrie, P.; Hoeks, A.; Safar, M. Carotid artery distensibility and distending pressure in hypertensive humans. Hypertension 1994, 23, 878–883. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, M.F.; Gallagher, D.E. Pulse wave analysis. J. Hypertens. Suppl. 1996, 14, S147–S157. [Google Scholar] [CrossRef]
- Avolio, A.P.; Van Bortel, L.M.; Boutouyrie, P.; Cockcroft, J.R.; McEniery, C.M.; Protogerou, A.D.; Roman, M.J.; Safar, M.E.; Segers, P.; Smulyan, H. Role of pulse pressure amplification in arterial hypertension: Experts’ opinion and review of the data. Hypertension 2009, 54, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, W.; O’Rourke, M.; Vlachopoulos, C. McDonald’s Blood Flow in Arteries. Theoretical, Experimental and Clinical Principles, 6th ed.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Namasivayam, M.; McDonnell, B.J.; McEniery, C.M.; O’Rourke, M.F. Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension 2009, 53, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEniery, C.M.; Yasmin, N.; Hall, I.R.; Qasem, A.; Wilkinson, I.B.; Cockcroft, J.R. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT). J. Am. Coll. Cardiol. 2005, 46, 1753–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, M.F.; Seward, J.B. Central arterial pressure and arterial pressure pulse: New views entering the second century after Korotkov. Mayo Clin. Proc. 2006, 81, 1057–1068. [Google Scholar] [CrossRef] [Green Version]
- Segers, P.; Rietzschel, E.R.; De Buyzere, M.L.; Vermeersch, S.J.; De Bacquer, D.; Van Bortel, L.M.; De Backer, G.; Gillebert, T.C.; Verdonck, P.R. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension 2007, 49, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- El Assaad, M.A.; Topouchian, J.A.; Asmar, R.G. Evaluation of two devices for self-measurement of blood pressure according to the international protocol: The Omron M5-I and the Omron 705IT. Blood Press. Monit. 2003, 8, 127–133. [Google Scholar] [CrossRef]
- Giannattasio, C.; Salvi, P.; Valbusa, F.; Kearney-Schwartz, A.; Capra, A.; Amigoni, M.; Failla, M.; Boffi, L.; Madotto, F.; Benetos, A.; et al. Simultaneous measurement of beat-to-beat carotid diameter and pressure changes to assess arterial mechanical properties. Hypertension 2008, 52, 896–902. [Google Scholar] [CrossRef] [Green Version]
- van Sloten, T.T.; Schram, M.T.; van den Hurk, K.; Dekker, J.M.; Nijpels, G.; Henry, R.M.; Stehouwer, C.D. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: The Hoorn study. J. Am. Coll. Cardiol. 2014, 63, 1739–1747. [Google Scholar] [CrossRef] [Green Version]
- Hoeks, A.P.; Brands, P.J.; Smeets, F.A.; Reneman, R.S. Assessment of the distensibility of superficial arteries. Ultrasound Med. Biol. 1990, 16, 121–128. [Google Scholar] [CrossRef]
- Kool, M.J.; van Merode, T.; Reneman, R.S.; Hoeks, A.P.; Struyker Boudier, H.A.; Van Bortel, L.M. Evaluation of reproducibility of a vessel wall movement detector system for assessment of large artery properties. Cardiovasc. Res. 1994, 28, 610–614. [Google Scholar] [CrossRef]
- Salvi, P.; Lio, G.; Labat, C.; Ricci, E.; Pannier, B.; Benetos, A. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: The PulsePen device. J. Hypertens. 2004, 22, 2285–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, P.; Scalise, F.; Rovina, M.; Moretti, F.; Salvi, L.; Grillo, A.; Gao, L.; Baldi, C.; Faini, A.; Furlanis, G.; et al. Noninvasive Estimation of Aortic Stiffness Through Different Approaches. Hypertension 2019, 74, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, L.; Perret-Guillaume, C.; Kearney-Schwartz, A.; Salvi, P.; Mandry, D.; Marie, P.Y.; Karcher, G.; Rossignol, P.; Zannad, F.; Benetos, A. Pulse wave velocity assessment by external noninvasive devices and phase-contrast magnetic resonance imaging in the obese. Hypertension 2009, 54, 421–426. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Staessen, J.A.; Vlachopoulos, C.; Duprez, D.; Plante, G.E. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 2002, 15, 426–444. [Google Scholar] [CrossRef]
- Mackenzie, I.S.; Wilkinson, I.B.; Cockcroft, J.R. Assessment of arterial stiffness in clinical practice. QJM 2002, 95, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Van Bortel, L.M.; Duprez, D.; Starmans-Kool, M.J.; Safar, M.E.; Giannattasio, C.; Cockcroft, J.; Kaiser, D.R.; Thuillez, C. Clinical applications of arterial stiffness, Task Force III: Recommendations for user procedures. Am. J. Hypertens. 2002, 15, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Bramwell, J.C.; Hill, A.V. Velocity of transmission of the pulse-wave and elasticity of the arteries. Lancet 1922, 1, 891–892. [Google Scholar] [CrossRef] [Green Version]
- Mackay, R.S.; Marg, E.; Oechsli, R. Automatic tonometer with exact theory: Various biological applications. Science 1960, 131, 1668–1669. [Google Scholar] [CrossRef]
- Pressman, G.L.; Newgard, P.M. A Transducer for the Continuous External Measurement of Arterial Blood Pressure. IEEE Trans. Biomed. Eng. 1963, 10, 73–81. [Google Scholar] [CrossRef]
- Matthys, K.; Verdonck, P. Development and modelling of arterial applanation tonometry: A review. Technol. Health Care 2002, 10, 65–76. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Grillo, A.; Simon, G.; Salvi, P.; Rovina, M.; Baldi, C.; Prearo, I.; Bernardi, S.; Fabris, B.; Faini, A.; Parati, G.; et al. Influence of carotid atherosclerotic plaques on pulse wave assessment with arterial tonometry. J. Hypertens. 2017, 35, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetos, A.; Laurent, S.; Hoeks, A.P.; Boutouyrie, P.H.; Safar, M.E. Arterial alterations with aging and high blood pressure. A noninvasive study of carotid and femoral arteries. Arter. Thromb. 1993, 13, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Benetos, A.; Asmar, R.; Gautier, S.; Salvi, P.; Safar, M. Heterogeneity of the arterial tree in essential hypertension: A noninvasive study of the terminal aorta and the common carotid artery. J. Hum. Hypertens. 1994, 8, 501–507. [Google Scholar]
- van der Heijden-Spek, J.J.; Staessen, J.A.; Fagard, R.H.; Hoeks, A.P.; Boudier, H.A.; van Bortel, L.M. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: A population study. Hypertension 2000, 35, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Smulyan, H.; Vardan, S.; Griffiths, A.; Gribbin, B. Forearm arterial distensibility in systolic hypertension. J. Am. Coll. Cardiol. 1984, 3, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Girerd, X.; Mourad, J.J.; Lacolley, P.; Beck, L.; Boutouyrie, P.; Mignot, J.P.; Safar, M. Elastic modulus of the radial artery wall material is not increased in patients with essential hypertension. Arter. Thromb. 1994, 14, 1223–1231. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Hayoz, D.; Trazzi, S.; Boutouyrie, P.; Waeber, B.; Omboni, S.; Brunner, H.R.; Mancia, G.; Safar, M. Isobaric compliance of the radial artery is increased in patients with essential hypertension. J. Hypertens. 1993, 11, 89–98. [Google Scholar] [CrossRef]
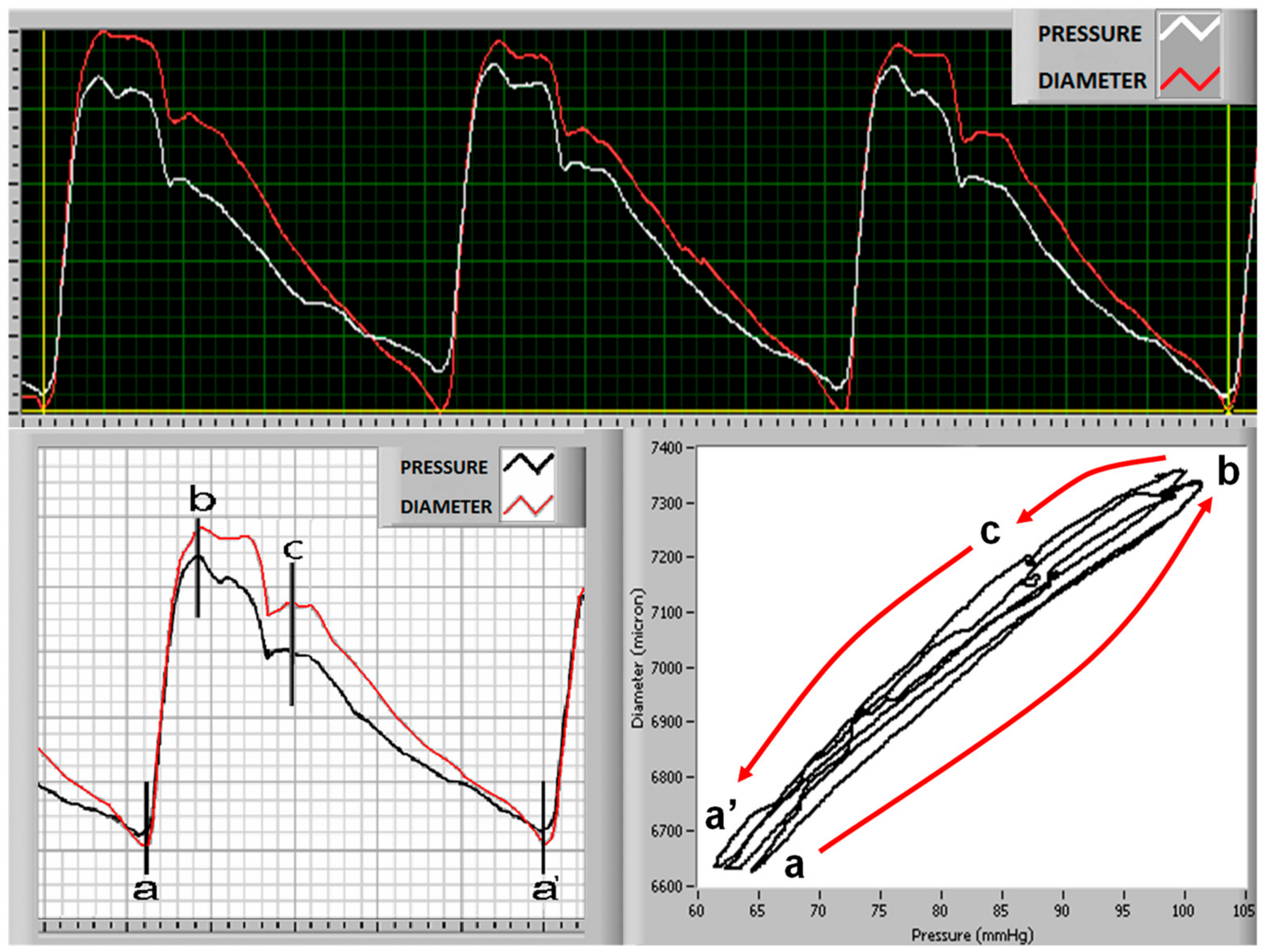



| Parameter | Pooled | Age Groups (years) | Trend | ||
|---|---|---|---|---|---|
| 20–45 | 46–70 | >70 | p | ||
| Subjects | 75 | 25 | 25 | 25 | |
| Gender, M/F | 25/50 | 9/16 | 8/17 | 8/17 | |
| BMI, kg/m2 | 24.4 ± 3.4 | 23.3 ± 3.6 | 24.9 ± 3.4 | 24.9 ± 3.3 | n.s. |
| Height, cm | 166.9 ± 9.3 | 170.3 ± 8.8 | 166.9 ± 9.0 | 163.0 ± 9.0 | 0.03 |
| Weight, kg | 68.0 ± 12.2 | 67.6 ± 10.4 | 69.7 ± 13.5 | 66.6 ± 12.8 | n.s. |
| Brachial Systolic BP, mmHg | 126.4 ± 20.0 | 112.6 ± 11.2 | 124.2 ± 13.5 | 142.2 ± 21.4 | <0.0001 |
| Central Systolic BP, mmHg | 114.3 ± 18.4 | 101.0 ± 11.2 | 113.0 ± 12.1 | 128.8 ± 19.2 | <0.0001 |
| Brachial PP, mmHg | 55.7 ± 17.0 | 47.1±7.9 | 49.6 ± 11.5 | 70.4 ± 18.7 | <0.0001 |
| Central PP, mmHg | 43.6 ± 15.5 | 35.4 ± 8.0 | 38.3 ± 10.6 | 57.0 ± 16.6 | <0.0001 |
| Mean BP, mmHg | 89.2 ± 11.7 | 81.3 ± 7.1 | 91.2 ± 8.6 | 95.3 ± 13.8 | <0.0001 |
| Diastolic BP, mmHg | 70.± 10.1 | 65.6 ± 5.8 | 74.6 ± 8.0 | 71.8 ± 13.2 | 0.005 |
| LVET, ms | 296.1 ± 28.9 | 294.8 ± 22.0 | 303.4 ± 24.7 | 290.1 ± 37.3 | n.s. |
| Diastolic Time, ms | 592.0 ± 116.6 | 594.0 ± 101.0 | 575.2 ± 131.2 | 606.9 ± 118.2 | n.s. |
| Heart Rate, bpm | 69.1 ± 10.3 | 68.6 ± 8.7 | 70.1 ± 11.9 | 68.4 ± 10.4 | n.s. |
| Amplification, mmHg | 12.1 ± 4.3 | 11.6 ± 2.6 | 11.2 ± 4.7 | 13.4 ± 5.0 | n.s. |
| PP Amplification, % | 30.1 ± 11.5 | 34.6 ± 10.6 | 31.1 ± 12.9 | 24.8 ± 8.8 | 0.008 |
| AIx, % | 12.8 ± 20.7 | −8.5 ± 11.7 | 20.1 ± 16.7 | 26.7 ± 13.1 | <0.0001 |
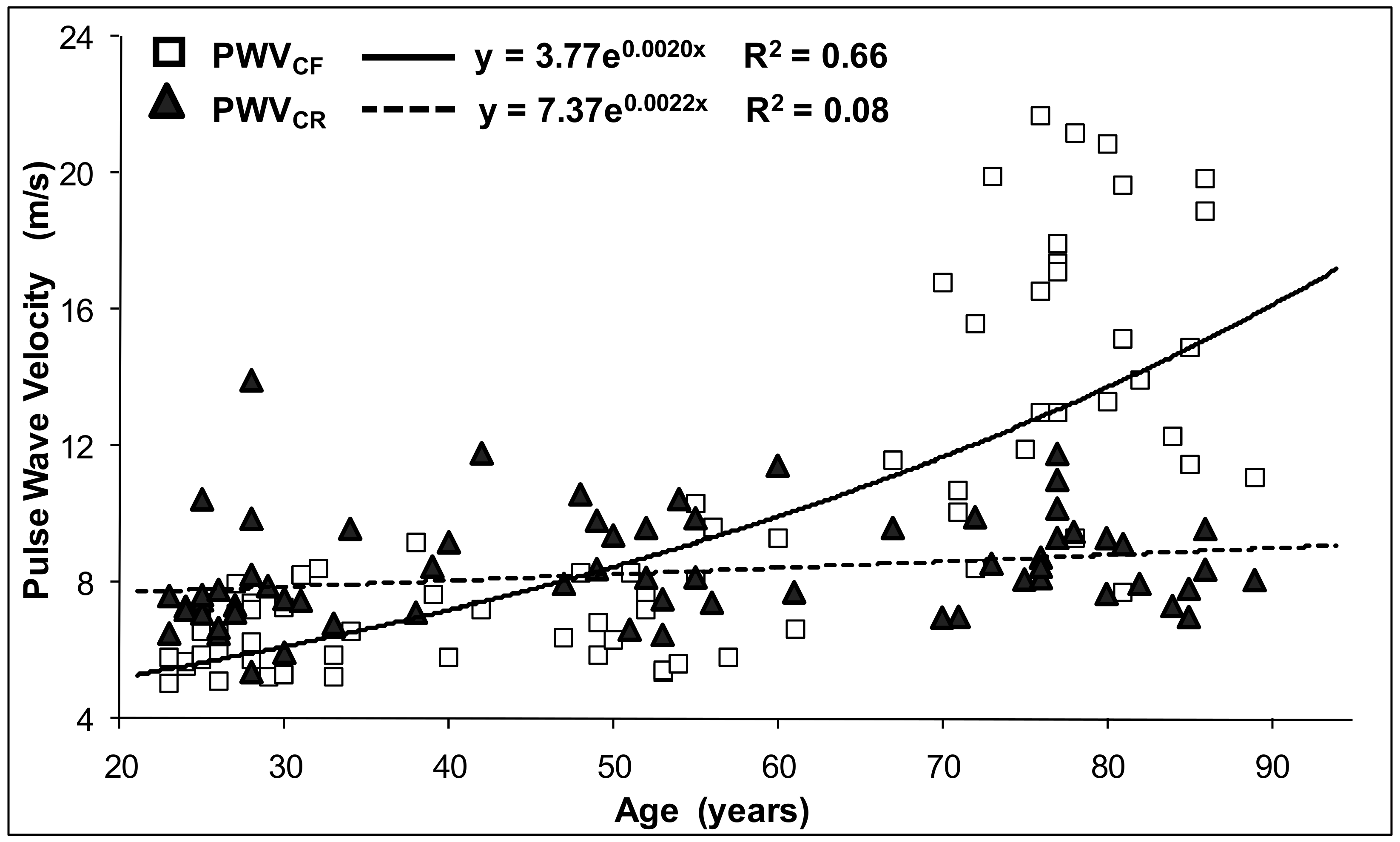
| cf-PWV, m/s | 9.90 ± 4.81 | 6.35 ± 0.99 | 8.03 ± 2.38 | 15.33 ± 4.13 | <0.0001 |
| cr-PWV, m/s | 8.41 ± 1.58 | 7.76 ± 1.75 | 8.82 ± 1.51 | 8.72 ± 1.24 | 0.04 |
| Carotid cross-sectional | |||||
| Compliance, μm/mmHg | 9.79 ± 4.89 | 15.28 ± 3.36 | 8.57 ± 2.22 | 5.54 ± 2.41 | <0.0001 |
| Distensibility, mmHg−1 | 1.50 ± 0.86 | 2.49 ± 0.55 | 1.27 ± 0.37 | 0.74 ± 0.38 | <0.0001 |
| Elastic Modulus, mmHg | 977 ± 663 | 427 ± 127 | 864 ± 276 | 1641 ± 691 | <0.0001 |
| Stiffness Index | 4.26 ± 2.49 | 2.17 ± 0.61 | 3.77 ± 1.07 | 6.84 ± 2.40 | <0.0001 |
| Carotid Global Slope | 9.4 ± 4.7 | 14.7 ± 3.3 | 8.2 ± 2.1 | 5.3 ± 2.2 | <0.0001 |
| Carotid Systolic Slope | 9.7 ± 4.9 | 15.1 ± 3.6 | 8.7 ± 2.3 | 5.3 ± 1.9 | <0.0001 |
| Carotid Diastolic Slope | 11.9 ± 5.9 | 18.4 ± 4.1 | 10.4 ± 3.3 | 6.9 ± 2.9 | <0.0001 |
| Parameter | PPA | AIx | cf-PWV | CCS Distensibility | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Sex | 0.05 | n.s. | 0.33 | 0.005 | −0.13 | n.s. | 0.02 | n.s. |
| Age | −0.47 | <0.001 | 0.76 | <0.001 | 0.79 | <0.001 | −0.84 | <0.001 |
| BMI | −0.18 | n.s. | 0.15 | n.s. | 0.38 | 0.001 | −0.40 | <0.001 |
| Height | 0.19 | n.s. | −0.53 | <0.001 | −0.14 | n.s. | 0.28 | 0.02 |
| Weight | −0.03 | n.s. | −0.26 | 0.03 | 0.17 | n.s. | −0.09 | n.s. |
| bSBP | −0.35 | 0.002 | 0.49 | <0.001 | 0.59 | <0.001 | −0.67 | <0.001 |
| cSBP | −0.47 | <0.001 | 0.52 | <0.001 | 0.58 | <0.001 | −0.66 | <0.001 |
| bPP | −0.48 | <0.001 | 0.41 | <0.001 | 0.54 | <0.001 | −0.59 | <0.001 |
| cPP | −0.67 | <0.001 | 0.46 | <0.001 | 0.53 | <0.001 | −0.59 | <0.001 |
| MAP | −0.13 | n.s. | 0.40 | <0.001 | 0.50 | <0.001 | −0.57 | <0.001 |
| DBP | 0.10 | n.s. | 0.24 | 0.04 | 0.29 | 0.01 | −0.35 | 0.002 |
| LVET | −0.25 | 0.03 | 0.31 | 0.007 | −0.24 | 0.04 | 0.16 | n.s. |
| DT | −0.35 | 0.002 | 0.22 | 0.05 | 0.02 | n.s. | 0.08 | n.s. |
| HR | 0.33 | 0.004 | −0.26 | 0.03 | 0.05 | n.s. | −0.11 | n.s. |
| Carotid Cross-Sectional Measurements | Peripheral PP | Central PP | PP Amplification | AIx | cf-PWV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Distensibility | −0.67 | <0.001 | −0.59 | <0.001 | 0.34 | 0.003 | −0.63 | <0.001 | −0.75 | <0.001 |
| Compliance | −0.65 | <0.001 | −0.59 | <0.001 | 0.34 | 0.003 | −0.65 | <0.001 | −0.72 | <0.001 |
| Elastic Modulus | 0.67 | <0.001 | 0.59 | <0.001 | −0.34 | 0.003 | 0.63 | <0.001 | 0.75 | <0.001 |
| Stiffness Index | 0.54 | <0.001 | 0.51 | <0.001 | −0.33 | 0.004 | 0.61 | <0.001 | 0.74 | <0.001 |
| Total slope | −0.65 | <0.001 | −0.59 | <0.001 | 0.36 | 0.002 | −0.65 | <0.001 | −0.71 | <0.001 |
| Systolic slope | −0.66 | <0.001 | −0.59 | <0.001 | 0.32 | 0.005 | −0.62 | <0.001 | −0.75 | <0.001 |
| Diastolic slope | −0.61 | <0.001 | −0.53 | <0.001 | 0.25 | 0.03 | −0.61 | <0.001 | −0.68 | <0.001 |
| Dependent Variable | r2 | Independent Variable | Regression Coefficient | SE | Β | p | r2 Change(%) |
|---|---|---|---|---|---|---|---|
| SBP Amplification | 0.29 | MAP | 0.134 | 0.045 | 0.37 | 0.004 | 15.3 |
| mmHg | HR | 0.120 | 0.046 | 0.29 | 0.01 | 7.4 | |
| PP Amplification | 0.31 | HR | 0.365 | 0.118 | 0.33 | 0.003 | 10.2 |
| Age | −0.154 | 0.067 | −0.30 | 0.02 | 14.9 | ||
| AIx | 0.72 | Age | 0.534 | 0.077 | 0.59 | <0.001 | 52.8 |
| HR | −0.530 | 0.136 | −0.27 | <0.001 | 6.6 | ||
| Sex | 14.435 | 4.235 | 0.35 | 0.001 | 10.1 | ||
| MAP | 0.381 | 0.133 | 0.22 | 0.006 | 1.6 | ||
| cf-PWV | 0.66 | Age | 0.169 | 0.020 | 0.79 | <0.001 | 61.4 |
| CCS Distensibility | 0.79 | Age | −0.030 | 0.003 | −0.76 | <0.001 | 70.8 |
| Weight | −0.017 | 0.006 | −0.24 | 0.003 | 3.9 | ||
| MAP | −0.012 | 0.005 | −0.16 | 0.02 | 3.0 | ||
| HR | −0.011 | 0.005 | −0.13 | 0.04 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvi, P.; Valbusa, F.; Kearney-Schwartz, A.; Labat, C.; Grillo, A.; Parati, G.; Benetos, A. Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods. J. Clin. Med. 2022, 11, 2225. https://doi.org/10.3390/jcm11082225
Salvi P, Valbusa F, Kearney-Schwartz A, Labat C, Grillo A, Parati G, Benetos A. Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods. Journal of Clinical Medicine. 2022; 11(8):2225. https://doi.org/10.3390/jcm11082225
Chicago/Turabian StyleSalvi, Paolo, Filippo Valbusa, Anna Kearney-Schwartz, Carlos Labat, Andrea Grillo, Gianfranco Parati, and Athanase Benetos. 2022. "Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods" Journal of Clinical Medicine 11, no. 8: 2225. https://doi.org/10.3390/jcm11082225
APA StyleSalvi, P., Valbusa, F., Kearney-Schwartz, A., Labat, C., Grillo, A., Parati, G., & Benetos, A. (2022). Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods. Journal of Clinical Medicine, 11(8), 2225. https://doi.org/10.3390/jcm11082225








