Gardnerella vaginalis in Recurrent Urinary Tract Infection Is Associated with Dysbiosis of the Bladder Microbiome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. DNA Extraction and 16S rDNA Sequencing
2.3. Bioinformatics Analysis and Data Processing
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Gardnerella Positive Detection Rate in the NC and rUTI Groups
3.3. Microbiome Diversity of the NC and rUTI Groups
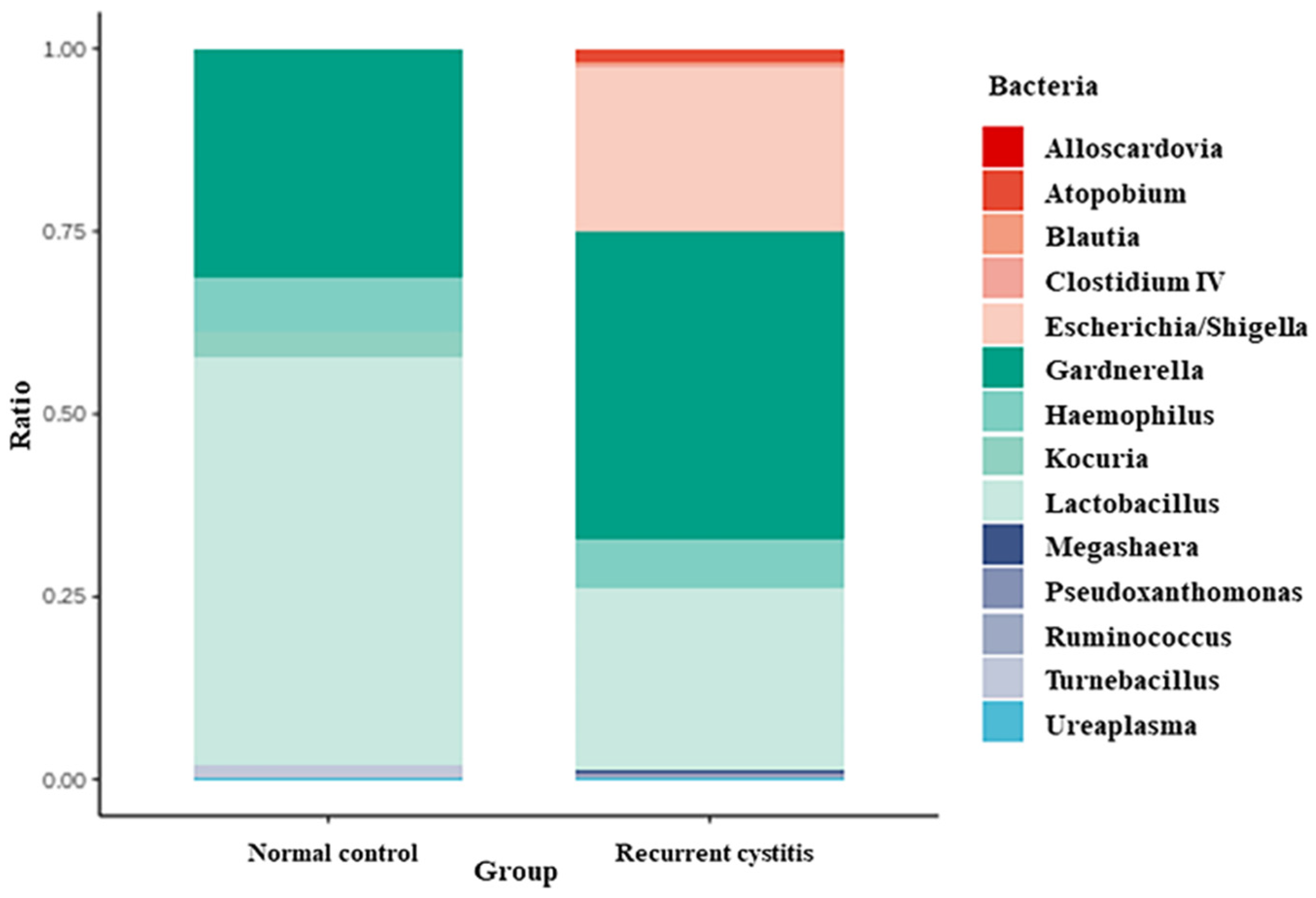
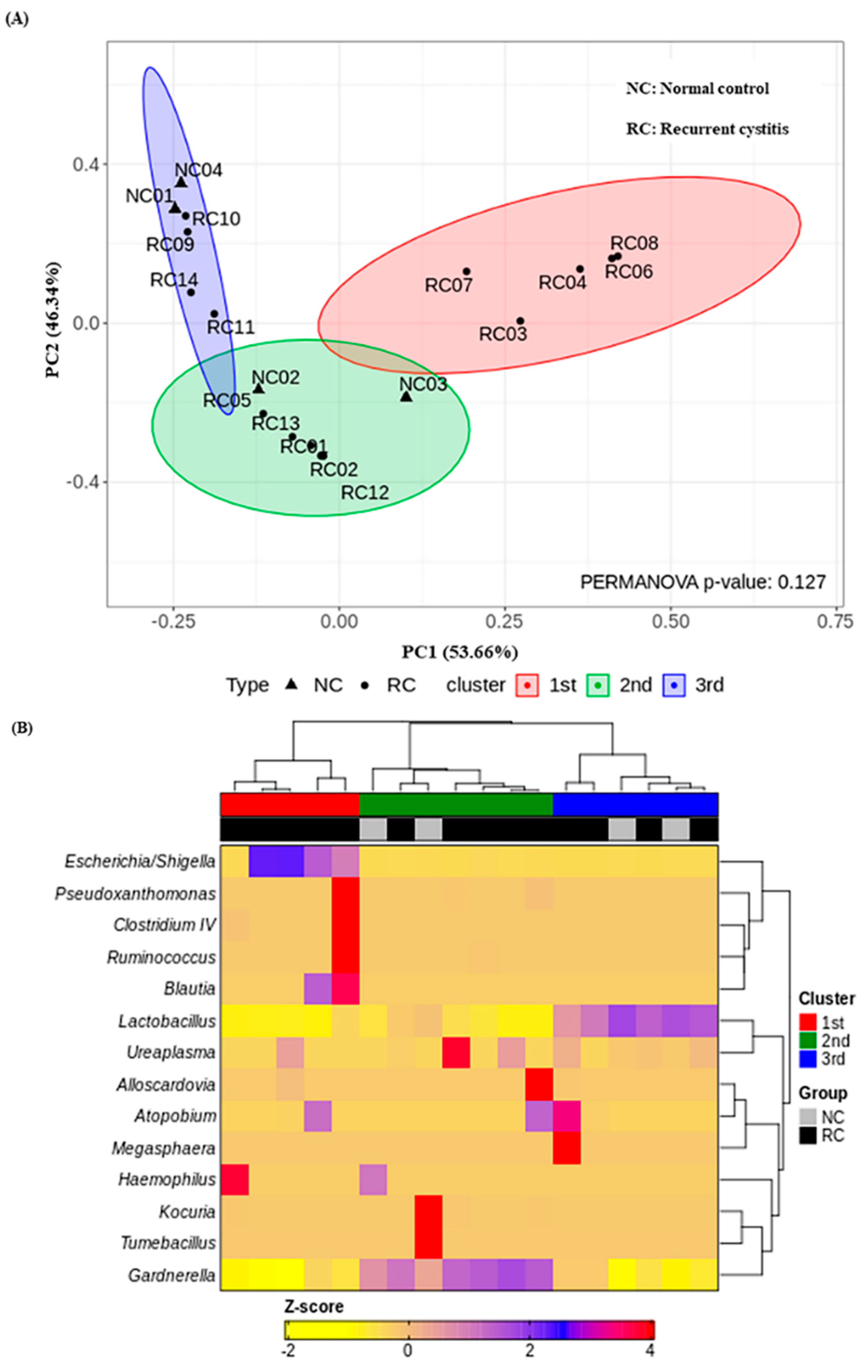
3.4. Three Urotypes of Bladder Microbiome Associated with Gardnerella
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, C.E.; Lacy, S.S.; Hinman, F., Jr. The urethra and its relationship to urinary tract infection. II. The urethral flora of the female with recurrent urinary infection. J. Urol. 1968, 99, 632–638. [Google Scholar] [CrossRef]
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women: A review. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113 (Suppl. S1), 5S–13S. [Google Scholar] [CrossRef]
- Kalra, O.P.; Raizada, A. Approach to a patient with urosepsis. J. Glob. Infect. Dis. 2009, 1, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Ballarini, S.; Mascarenhas, T.; Zahran, M.; Quimper, E.; -Choucair, J.; Iselin, C.E. Recurrent Lower Urinary Tract Infections Have a Detrimental Effect on Patient Quality of Life: A Prospective, Observational Study. Infect. Dis. Ther. 2014, 4, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephs-Spaulding, J.; Krogh, T.J.; Rettig, H.C.; Lyng, M.; Chkonia, M.; Waschina, S.; Graspeuntner, S.; Rupp, J.; Moller-Jensen, J.; Kaleta, C. Recurrent Urinary Tract Infections: Unraveling the Complicated Environment of Uncomplicated rUTIs. Front. Cell. Infect. Microbiol. 2021, 11, 562525. [Google Scholar] [CrossRef]
- Mestrovic, T.; Matijasic, M.; Peric, M.; Cipcic Paljetak, H.; Baresic, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef]
- Stapleton, A.E. The Vaginal Microbiota and Urinary Tract Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Lam, M.H.; Birch, D.F.; Fairley, K.F. Prevalence of Gardnerella vaginalis in the urinary tract. J. Clin. Microbiol. 1988, 26, 1130–1133. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Gasiorek, M.; Hsieh, M.H.; Forster, C.S. Utility of DNA Next-Generation Sequencing and Expanded Quantitative Urine Culture in Diagnosis and Management of Chronic or Persistent Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2019, 58, e00204-19. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.B.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Lewis, A.L.; Gilbert, N.M. Roles of the vagina and the vaginal microbiota in urinary tract infection: Evidence from clinical correlations and experimental models. GMS Infect. Dis. 2020, 8, Doc02. [Google Scholar] [CrossRef]
- Komesu, Y.M.; Dinwiddie, D.L.; Richter, H.E.; Lukacz, E.S.; Sung, V.W.; Siddiqui, N.Y.; Zyczynski, H.M.; Ridgeway, B.; Rogers, R.G.; Arya, L.A.; et al. Defining the relationship between vaginal and urinary microbiomes. Am. J. Obstet. Gynecol. 2020, 222, 154.e1–154.e10. [Google Scholar] [CrossRef]
- Gilbert, N.M.; O’Brien, V.P.; Lewis, A.L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 2017, 13, e1006238. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [Green Version]
- Kaehler, B.D.; Bokulich, N.A.; McDonald, D.; Knight, R.; Caporaso, J.G.; Huttley, G.A. Species abundance information improves sequence taxonomy classification accuracy. Nat. Commun. 2019, 10, 4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKnight, D.T.; Huerlimann, R.; Bower, D.S.; Schwarzkopf, L.; Alford, R.A.; Zenger, K.R. microDecon: A highly accurate read-subtraction tool for the post-sequencing removal of contamination in metabarcoding studies. Environ. DNA 2019, 1, 14–25. [Google Scholar] [CrossRef]
- Tapiainen, T.; Paalanne, N.; Tejesvi, M.V.; Koivusaari, P.; Korpela, K.; Pokka, T.; Salo, J.; Kaukola, T.; Pirttila, A.M.; Uhari, M.; et al. Maternal influence on the fetal microbiome in a population-based study of the first-pass meconium. Pediatr. Res. 2018, 84, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, H.; Younas, S.; Casteels, I.; Joossens, M. Current knowledge on the human eye microbiome: A systematic review of available amplicon and metagenomic sequencing data. Acta Ophthalmol. 2021, 99, 16–25. [Google Scholar] [CrossRef]
- Park, H.-S.; Jun, C.-H. A simple and fast algorithm for K-medoids clustering. Expert Syst. Appl. 2009, 36, 3336–3341. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Schauberger, C.W.; Merkitch, K.W.; Prell, A.M. Acute cystitis in women: Experience with a telephone-based algorithm. WMJ Off. Publ. State Med Soc. Wis. 2007, 106, 326–329. [Google Scholar]
- Lee, D.S.; Lee, S.J.; Choe, H.S. Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. Biomed Res. Int. 2018, 2018, 7656752. [Google Scholar] [CrossRef] [Green Version]
- Muhamad Rizal, N.S.; Neoh, H.M.; Ramli, R.; PR, A.L.K.P.; Hanafiah, A.; Abdul Samat, M.N.; Tan, T.L.; Wong, K.K.; Nathan, S.; Chieng, S.; et al. Advantages and Limitations of 16S rRNA Next-Generation Sequencing for Pathogen Identification in the Diagnostic Microbiology Laboratory: Perspectives from a Middle-Income Country. Diagnostics 2020, 10, 816. [Google Scholar] [CrossRef]
- Poretsky, R.; Rodriguez, R.L.; Luo, C.; Tsementzi, D.; Konstantinidis, K.T. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS ONE 2014, 9, e93827. [Google Scholar] [CrossRef] [Green Version]
- Harmanli, O.H.; Cheng, G.Y.; Nyirjesy, P.; Chatwani, A.; Gaughan, J.P. Urinary tract infections in women with bacterial vaginosis. Obstet. Gynecol. 2000, 95, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Pastijn, A.; Gevers, R.; Murillo, D. Estrogen therapy in older patients with recurrent urinary tract infections: A review. Int. J. Fertil. Womens Med. 2004, 49, 71–74. [Google Scholar] [PubMed]
- Krause, M.; Wheeler, T.L., 2nd; Snyder, T.E.; Richter, H.E. Local Effects of Vaginally Administered Estrogen Therapy: A Review. J. Pelvic Med. Surg. 2009, 15, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Hawes, S.E.; Scholes, D.; Boyko, E.J.; Hughes, J.P.; Fihn, S.D. Sexual intercourse and risk of symptomatic urinary tract infection in post-menopausal women. J. Gen. Intern. Med. 2008, 23, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, A.J.; Brubaker, L. Urobiome updates: Advances in urinary microbiome research. Nat. Rev. Urol. 2019, 16, 73–74. [Google Scholar] [CrossRef]
- Paladine, H.L.; Desai, U.A. Vaginitis: Diagnosis and Treatment. Am. Fam. Physician 2018, 97, 321–329. [Google Scholar]
- Heinemann, C.; Reid, G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can. J. Microbiol. 2005, 51, 777–781. [Google Scholar] [CrossRef]
- Raz, R. Urinary tract infection in postmenopausal women. Korean J. Urol. 2011, 52, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Raz, R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J. Infect. Dis. 2001, 183, S74–S76. [Google Scholar] [CrossRef] [Green Version]
- Raz, R.; Stamm, W.E. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N. Engl. J. Med. 1993, 329, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Cauci, S.; Driussi, S.; De Santo, D.; Penacchioni, P.; Iannicelli, T.; Lanzafame, P.; De Seta, F.; Quadrifoglio, F.; de Aloysio, D.; Guaschino, S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 2002, 40, 2147–2152. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am. J. Obstet. Gynecol. 1999, 180, 1072–1079. [Google Scholar] [CrossRef]
- Pedraza-Aviles, A.G.; Zaragoza, M.C.; Mota-Vazquez, R.; Hernandez-Soto, C.; Ramirez-Santana, M.; Terrazas-Maldonado, M.L. Treatment of urinary tract infection by Gardnerella vaginalis: A comparison of oral metronidazole versus ampicillin. Rev. Latinoam. Microbiol. 2001, 43, 65–69. [Google Scholar] [PubMed]
- Hooton, T.M.; Scholes, D.; Gupta, K.; Stapleton, A.E.; Roberts, P.L.; Stamm, W.E. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: A randomized trial. JAMA 2005, 293, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschick, C.; Szafranski, S.P.; Kunze, B.; Sztajer, H.; Masur, C.; Abels, C.; Wagner-Dobler, I. Screening of Compounds against Gardnerella vaginalis Biofilms. PLoS ONE 2016, 11, e0154086. [Google Scholar] [CrossRef]



| Total (n = 96) | Normal Control (n = 18) | Recurrent Urinary Tract Infection (n = 78) | p | |
|---|---|---|---|---|
| Age (years) | 54.5 ± 14.9 | 47.1 ± 11.8 | 56.2 ± 15.1 | 0.291 |
| Female | 96 (100) | 18 (100) | 78 (100) | 0.999 |
| Menopause | 61 (63.5) | 8 (44.4) | 53 (67.9) | 0.062 |
| Diabetes | 15 (15.6) | 2 (11.1) | 13 (16.7) | 0.558 |
| Urinalysis | ||||
| Urine RBC (per HPF) | 0 (0–400) | 0 (0–400) | 0 (0–30) | 0.051 |
| Urine WBC (per HPF) | 0 (0–204) | 0 (0–29) | 0 (0–204) | 0.252 |
| Urine bacteria (per HPF) | 0 (0–3+) | 0 (0–3+) | 0 (0–3+) | 0.720 |
| Urine protein | 0 (0–2+) | 0 (0–1+) | 0 (0–2+) | 0.679 |
| Urine glucose | 0 (0–3+) | 0 (0–3+) | 0 (0–3+) | 0.710 |
| Number of Patients | Gardnerella-Positive Samples | p-Value | |
|---|---|---|---|
| Normal control | 18 | 4 (22.2%) | 0.677 |
| Recurrent UTI | 78 | 14 (18.0%) | |
| Total | 96 | 18 (18.8%) |
| Genera | Percent Contribution in G (+) Normal Control Group | Percent Contribution in G (+) Recurrent UTI Group | ||||
|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | |
| Lactobacillus | 55.67 | 8.19 | 96.00 | 24.87 | 0.00 | 86.38 |
| Gardnerella | 30.94 | 3.70 | 61.49 | 42.18 | 1.13 | 99.09 |
| Haemophilus | 7.58 | 0.00 | 30.33 | 6.52 | 0.00 | 91.33 |
| Kocuria | 3.60 | 0.00 | 14.38 | 0.01 | 0.00 | 0.13 |
| Tumebacillus | 1.91 | 0.00 | 7.65 | 0.00 | 0.00 | 0.00 |
| Escherichia | 0.23 | 0.00 | 0.60 | 22.57 | 0.00 | 96.93 |
| Ureaplasma | 0.06 | 0.00 | 0.12 | 0.32 | 0.00 | 2.56 |
| Atopobium | 0.00 | 0.00 | 0.00 | 1.74 | 0.00 | 12.73 |
| Pseudoxanthomonas | 0.00 | 0.00 | 0.00 | 0.40 | 0.00 | 5.46 |
| Megasphaera | 0.00 | 0.00 | 0.00 | 0.35 | 0.00 | 4.96 |
| Ruminococcus | 0.00 | 0.00 | 0.00 | 0.29 | 0 | 4.08 |
| Clostridium IV | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 3.48 |
| Blautia | 0.00 | 0.00 | 0.00 | 0.33 | 0.00 | 3.20 |
| Alloscardovia | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 2.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.-J.; Song, J.S.; Kim, W.B.; Yun, J.; Shin, H.B.; Jang, M.-A.; Ryu, C.B.; Kim, S.S.; Chung, J.C.; Kuk, J.C.; et al. Gardnerella vaginalis in Recurrent Urinary Tract Infection Is Associated with Dysbiosis of the Bladder Microbiome. J. Clin. Med. 2022, 11, 2295. https://doi.org/10.3390/jcm11092295
Yoo J-J, Song JS, Kim WB, Yun J, Shin HB, Jang M-A, Ryu CB, Kim SS, Chung JC, Kuk JC, et al. Gardnerella vaginalis in Recurrent Urinary Tract Infection Is Associated with Dysbiosis of the Bladder Microbiome. Journal of Clinical Medicine. 2022; 11(9):2295. https://doi.org/10.3390/jcm11092295
Chicago/Turabian StyleYoo, Jeong-Ju, Ju Sun Song, Woong Bin Kim, Jina Yun, Hee Bong Shin, Mi-Ae Jang, Chang Beom Ryu, Sung Shin Kim, Jun Chul Chung, Jung Cheol Kuk, and et al. 2022. "Gardnerella vaginalis in Recurrent Urinary Tract Infection Is Associated with Dysbiosis of the Bladder Microbiome" Journal of Clinical Medicine 11, no. 9: 2295. https://doi.org/10.3390/jcm11092295
APA StyleYoo, J.-J., Song, J. S., Kim, W. B., Yun, J., Shin, H. B., Jang, M.-A., Ryu, C. B., Kim, S. S., Chung, J. C., Kuk, J. C., Shin, E. J., Song, H.-Y., Yu, B. C., Lee, E.-S., Ryu, S., Kim, J. H., Jung, S. S., Kim, Y. H., & on behalf of the SMS (Soonchunhyang Microbiome Multi-Disciplinary Study Group). (2022). Gardnerella vaginalis in Recurrent Urinary Tract Infection Is Associated with Dysbiosis of the Bladder Microbiome. Journal of Clinical Medicine, 11(9), 2295. https://doi.org/10.3390/jcm11092295









