An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis
Abstract
:1. Introduction
2. Indications for Therapy
| Drug | Action |
|---|---|
| Salicylates [16] | (1) Pro-apoptotic and anti-proliferative action that is triggered, at least in part, by the activation of the peroxisome proliferator-activated receptor (PPAR)-gamma and the modulation of PTEN and c-Myc (2) Have a role in the inhibition of mediators of lipoxygenase and cyclooxygenase, interleukin-1, interleukin-2 and TNF-alpha, and have an antioxidant and free-radical scavenger effect |
| Corticosteroids | Unclear, but it seems to involve the inhibition of cytokine release by inactivation of NFKβ and the consequent reduction in the lymphocyte recruitment, lower vascular permeability and inhibition of cytokine-mediated tissue necrosis |
| Calcineurin inhibitors Cyclosporine [17] Tacrolimus [17] | Inhibits the activation of T-cells and the production of IL-2 by T-helper lymphocytes, and blocks the production of IFN-xc and B-cell-activating factors A macrolide antibiotic with an anti-calcineurin action similar to CyA |
| Thiopurines Azathioprine Mercapropurine | Direct anti-inflammatory effect through the inhibition of cytotoxic T-cell and natural killer cells and their apoptosis |
| Infliximab [18] | Binds and blocks both soluble and transmembrane TNF-alpha receptors |
| Adalimumab [19] Golimumab [20] | Binds to both soluble and transmembrane-TNF, blocking the reaction with p55 and p 75 subunits of TNF receptors |
| Vedolizumab [21] | Binds the α4β7 integrin to block the gastrointestinal homing of T lymphocytes, thus reducing the chronic intestinal inflammation present in UC |
| Etrolizumab [22] | Specifically targets the β7 subunit of α4β7 and αEβ7 integrins |
| Ustekinumab [23] | Inhibits the activity of IL-12 and IL-23 by binding to the p40 subunit shared by both cytokines |
| Risankizumab | Binds to the p19 subunit of IL-23 |
| Mirikizumab | Binds to the p19 subunit of IL-23 |
| JAK inhibitors Tofacitinib | Intracellular action on a cascade of multiple pro-inflammatory cytokines |
3. Non-Biologic Therapies
3.1. Salicylates
3.1.1. Efficacy
3.1.2. Dosage
3.1.3. Safety
3.1.4. Controversies and Limitations
3.2. Corticosteroids
3.2.1. Efficacy
3.2.2. Dosage
3.2.3. Safety
3.2.4. Controversies and Limitations
3.3. Calcineurin Inhibitors
3.3.1. Cyclosporine
Efficacy
Dosage
Safety
3.3.2. Tacrolimus
Efficacy
Dosage
Safety
Controversies and Limitations
3.4. Immunomodulators
3.4.1. Thiopurines
Efficacy
Dosage
Safety
Controversies and Limitations
4. Biologic Therapies
4.1. Anti TNF-Alfa
4.1.1. Infliximab (IFX)
Efficacy
Dosage
Safety
4.1.2. Adalimumab, Golimumab
Efficacy
Dosage
Safety
4.2. Anti-Integrins
4.2.1. Vedolizumab
Efficacy
Dosage
Safety
4.3. Anti-IL23 Agents
4.3.1. Ustekinumab
Efficacy
Dosage
Safety
4.4. JAK-Inhibitors
4.4.1. Tofacitinib
Dosage
Efficacy
Safety
Controversies and Limitations
5. Other Therapies
5.1. Non-Pharmacological Therapies
5.2. Surgery
5.3. Future Perspectives
6. Conclusions
Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Cheifetz, A.S. Ulcerative colitis: Epidemiology, diagnosis, and management. Mayo Clin. Proc. 2014, 89, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targownik, L.E.; Singh, H.; Nugent, Z.; Bernstein, C.N. The epidemiology of colectomy in ulcerative colitis: Results from a population-based cohort. Am. J. Gastroenterol. 2012, 107, 1228–1235. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.R.; Colombel, J.F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef]
- Rocchi, A.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Feagan, B.; Panaccione, R.; Glasgow, K.W.; Fernandes, A.; Ghosh, S. Inflammatory bowel disease: A Canadian burden of illness review. Can. J. Gastroenterol. 2012, 26, 811–817. [Google Scholar] [CrossRef]
- Zallot, C.; Peyrin-Biroulet, L. Clinical risk factors for complicated disease: How reliable are they? Dig. Dis. 2012, 30 (Suppl. 3), 67–72. [Google Scholar] [CrossRef]
- Gumaste, V.; Sachar, D.B.; Greenstein, A.J. Benign and malignant colorectal strictures in ulcerative colitis. Gut 1992, 33, 938–941. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Billioud, V.; Sachar, D.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis as a progressive disease: The forgotten evidence. Inflamm. Bowel Dis. 2012, 18, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Romberg-Camps, M.J.; Dagnelie, P.C.; Kester, A.D.; Hesselink-van de Kruijs, M.A.; Cilissen, M.; Engels, L.G.; Van Deursen, C.; Hameeteman, W.H.; Wolters, F.L.; Russel, M.G.; et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am. J. Gastroenterol. 2009, 104, 371–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriyama, M.; Kato, J.; Fujimoto, T.; Nasu, J.; Miyaike, J.; Morita, T.; Okada, H.; Suzuki, S.; Shiode, J.; Yamamoto, H.; et al. Risk factors and indications for colectomy in ulcerative colitis patients are different according to patient’s clinical background. Dis. Colon Rectum 2006, 49, 1307–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez, J.M.; Louis, E. Can we predict the high-risk patient? Dig. Dis. 2014, 32, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hefti, M.M.; Chessin, D.B.; Harpaz, N.H.; Steinhagen, R.M.; Ullman, T.A. Severity of inflammation as a predictor of colectomy in patients with chronic ulcerative colitis. Dis. Colon Rectum 2009, 52, 193–197. [Google Scholar] [CrossRef]
- Iacucci, M.; de Silva, S.; Ghosh, S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Can. J. Gastroenterol. 2010, 24, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Loftus, C.G.; Egan, L.J.; Sandborn, W.J. Cyclosporine, tacrolimus, and mycophenolate mofetil in the treatment of inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2004, 33, 141–169. [Google Scholar] [CrossRef]
- Danese, S. Mechanisms of action of infliximab in inflammatory bowel disease: An anti-inflammatory multitasker. Dig. Liver Dis. 2008, 40 (Suppl. 2), S225–S228. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95, quiz e14–e85. [Google Scholar] [CrossRef]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. J. Crohns Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanich, E.G.; Danilenko, D.M.; Wang, H.; O’Byrne, S.; Erickson, R.; Gelzleichter, T.; Hiraragi, H.; Chiu, H.; Ivelja, S.; Jeet, S.; et al. A humanized monoclonal antibody targeting the β7 integrin selectively blocks intestinal homing of T lymphocytes. Br. J. Pharmacol. 2011, 162, 1855–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, 5, CD000544. [Google Scholar] [CrossRef]
- Van Bodegraven, A.A.; Boer, R.O.; Lourens, J.; Tuynman, H.A.; Sindram, J.W. Distribution of mesalazine enemas in active and quiescent ulcerative colitis. Aliment. Pharmacol. Ther. 1996, 10, 327–332. [Google Scholar] [CrossRef]
- Marshall, J.K.; Thabane, M.; Steinhart, A.H.; Newman, J.R.; Anand, A.; Irvine, E.J. Rectal 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012, 11, CD004118. [Google Scholar] [CrossRef]
- Hu, M.Y.; Peppercorn, M.A. MMX mesalamine: A novel high-dose, once-daily 5-aminosalicylate formulation for the treatment of ulcerative colitis. Expert Opin. Pharmacother. 2008, 9, 1049–1058. [Google Scholar] [CrossRef]
- Levine, D.S.; Riff, D.S.; Pruitt, R.; Wruble, L.; Koval, G.; Sales, D.; Bell, J.K.; Johnson, L.K. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am. J. Gastroenterol. 2002, 97, 1398–1407. [Google Scholar] [CrossRef]
- Pruitt, R.; Hanson, J.; Safdi, M.; Wruble, L.; Hardi, R.; Johanson, J.; Koval, G.; Riff, D.; Winston, B.; Cross, A.; et al. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis. Am. J. Gastroenterol. 2002, 97, 3078–3086. [Google Scholar] [CrossRef]
- Marshall, J.K.; Irvine, E.J. Rectal corticosteroids versus alternative treatments in ulcerative colitis: A meta-analysis. Gut 1997, 40, 775–781. [Google Scholar] [CrossRef]
- Regueiro, M.; Loftus, E.V.; Steinhart, A.H.; Cohen, R.D. Medical management of left-sided ulcerative colitis and ulcerative proctitis: Critical evaluation of therapeutic trials. Inflamm. Bowel Dis. 2006, 12, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.J.; Fockens, P.; Meijer, J.W.; van der Heide, H.; Wiltink, E.H.; Tytgat, G.N. Beclomethasone dipropionate (3 mg) versus 5-aminosalicylic acid (2 g) versus the combination of both (3 mg/2 g) as retention enemas in active ulcerative proctitis. Eur. J. Gastroenterol. Hepatol. 1996, 8, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Ramsey, D.; Rubin, D.T. Randomised clinical trial: Delayed-release oral mesalazine 4.8 g/day vs. 2.4 g/day in endoscopic mucosal healing—ASCEND I and II combined analysis. Aliment. Pharmacol. Ther. 2011, 33, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Ma, J.; Wang, K.; Zhang, H. Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: A systematic review with meta-analysis. Oncotarget 2017, 8, 1031–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonovas, S.; Fiorino, G.; Lytras, T.; Nikolopoulos, G.; Peyrin-Biroulet, L.; Danese, S. Systematic review with meta-analysis: Use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1179–1192. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.W.; Singh, S.; Feuerstein, J.D.; Falck-Ytter, C.; Falck-Ytter, Y.; Cross, R.K.; on behalf of the American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019, 156, 748–764. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.; Nguyen, T.M.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 8, CD000544. [Google Scholar] [CrossRef]
- Gisbert, J.P.; González-Lama, Y.; Maté, J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm. Bowel Dis. 2007, 13, 629–638. [Google Scholar] [CrossRef]
- Kane, S.; Huo, D.; Aikens, J.; Hanauer, S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am. J. Med. 2003, 114, 39–43. [Google Scholar] [CrossRef]
- Moody, G.A.; Jayanthi, V.; Probert, C.S.; Mac Kay, H.; Mayberry, J.F. Long-term therapy with sulphasalazine protects against colorectal cancer in ulcerative colitis: A retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur. J. Gastroenterol. Hepatol. 1996, 8, 1179–1183. [Google Scholar] [CrossRef]
- Mitra, D.; Hodgkins, P.; Yen, L.; Davis, K.L.; Cohen, R.D. Association between oral 5-ASA adherence and health care utilization and costs among patients with active ulcerative colitis. BMC Gastroenterol. 2012, 12, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shale, M.J.; Riley, S.A. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 18, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.V. Systematic review: Adherence issues in the treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 577–585. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R.; Sandborn, W.J.; Zou, G.; Stitt, L.W.; Rutgeerts, P.J.; Gilgen, D.; Jairath, V.; Hindryckx, P.; Shackelton, L.M.; Vandervoort, M.K.; et al. Randomised non-inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Flourié, B.; Hagège, H.; Tucat, G.; Maetz, D.; Hébuterne, X.; Kuyvenhoven, J.P.; Tan, T.G.; Pierik, M.J.; Masclee, A.A.; Dewit, O.; et al. Randomised clinical trial: Once- vs. twice-daily prolonged-release mesalazine for active ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 37, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Korzenik, J.; Lashner, B.; Leighton, J.A.; Mahadevan, U.; Marion, J.F.; Safdi, M.; Sninsky, C.A.; Patel, R.M.; Friedenberg, K.A.; et al. Once-daily dosing of delayed-release oral mesalamine (400-mg tablet) is as effective as twice-daily dosing for maintenance of remission of ulcerative colitis. Gastroenterology 2010, 138, 1286–1296.e3. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Feuerstein, J.D.; Binion, D.G.; Tremaine, W.J. AGA Technical Review on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019, 156, 769–808.e29. [Google Scholar] [CrossRef] [Green Version]
- D’Incà, R.; Bertomoro, P.; Mazzocco, K.; Vettorato, M.G.; Rumiati, R.; Sturniolo, G.C. Risk factors for non-adherence to medication in inflammatory bowel disease patients. Aliment. Pharmacol. Ther. 2008, 27, 166–172. [Google Scholar] [CrossRef]
- Dassopoulos, T.; Cohen, R.D.; Scherl, E.J.; Schwartz, R.M.; Kosinski, L.; Regueiro, M.D. Ulcerative Colitis Care Pathway. Gastroenterology 2015, 149, 238–245. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Travis, S.; Moro, L.; Jones, R.; Gautille, T.; Bagin, R.; Huang, M.; Yeung, P.; Ballard, E.D. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE I study. Gastroenterology 2012, 143, 1218–1226.e12. [Google Scholar] [CrossRef] [Green Version]
- Travis, S.P.; Danese, S.; Kupcinskas, L.; Alexeeva, O.; D’Haens, G.; Gibson, P.R.; Moro, L.; Jones, R.; Ballard, E.D.; Masure, J.; et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: Results from the randomised CORE II study. Gut 2014, 63, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, M.E.; MacDonald, J.K.; Griffiths, A.M.; Steinhart, A.H.; Seow, C.H. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2015, 10, CD007698. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Gionchetti, P.; D’Arienzo, A.; Manguso, F.; Di Matteo, G.; Annese, V.; Valpiani, D.; Casetti, T.; Adamo, S.; Prada, A.; et al. Oral beclometasone dipropionate in the treatment of active ulcerative colitis: A double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2002, 16, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Campieri, M.; Adamo, S.; Valpiani, D.; D’Arienzo, A.; D’Albasio, G.; Pitzalis, M.; Cesari, P.; Casetti, T.; Castiglione, G.N.; Rizzello, F.; et al. Oral beclometasone dipropionate in the treatment of extensive and left-sided active ulcerative colitis: A multicentre randomised study. Aliment. Pharmacol. Ther. 2003, 17, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to corticosteroids in severe ulcerative colitis: A systematic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Manguso, F.; Zibellini, M.; Nuño, J.L.C.; Goldis, A.; Tkachenko, E.; Varoli, G.; Kleczkowski, D.; Annese, V.; D’Heygere, F.; et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: Results from a double-blind, randomized, parallel group study. Am. J. Gastroenterol. 2015, 110, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Cohen, R.D.; Sandborn, W.J.; Lichtenstein, G.R.; Axler, J.; Riddell, R.H.; Zhu, C.; Barrett, A.C.; Bortey, E.; Forbes, W.P. Budesonide Multimatrix Is Efficacious for Mesalamine-refractory, Mild to Moderate Ulcerative Colitis: A Randomised, Placebo-controlled Trial. J. Crohns Colitis 2017, 11, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Truelove, S.C.; Jewell, D.P. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet 1974, 1, 1067–1070. [Google Scholar] [CrossRef]
- Gionchetti, P.; Rizzello, F.; Annese, V.; Armuzzi, A.; Biancone, L.; Castiglione, F.; Comberlato, M.; Cottone, M.; Danese, S.; Daperno, M.; et al. Use of corticosteroids and immunosuppressive drugs in inflammatory bowel disease: Clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease. Dig. Liver Dis. 2017, 49, 604–617. [Google Scholar] [CrossRef] [Green Version]
- De Cassan, C.; Fiorino, G.; Danese, S. Second-generation corticosteroids for the treatment of Crohn’s disease and ulcerative colitis: More effective and less side effects? Dig. Dis. 2012, 30, 368–375. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.D.; Scott, F.I.; Brensinger, C.M.; Roy, J.A.; Osterman, M.T.; Mamtani, R.; Bewtra, M.; Chen, L.; Yun, H.; Xie, F.; et al. Increased Mortality Rates with Prolonged Corticosteroid Therapy When Compared with Antitumor Necrosis Factor-α-Directed Therapy for Inflammatory Bowel Disease. Am. J. Gastroenterol. 2018, 113, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selinger, C.P.; Parkes, G.C.; Bassi, A.; Limdi, J.K.; Ludlow, H.; Patel, P.; Smith, M.; Saluke, S.; Ndlovu, Z.; George, B.; et al. Assessment of steroid use as a key performance indicator in inflammatory bowel disease-analysis of data from 2385 UK patients. Aliment. Pharmacol. Ther. 2019, 50, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, V.; Saxena, S.; Cecil, E.; Subramanian, V.; Curcin, V.; Majeed, A.; Pollok, R.C. Steroid dependency and trends in prescribing for inflammatory bowel disease—A 20-year national population-based study. Aliment. Pharmacol. Ther. 2016, 44, 482–494. [Google Scholar] [CrossRef] [Green Version]
- Roblin, X.; Pillet, S.; Oussalah, A.; Berthelot, P.; Del Tedesco, E.; Phelip, J.M.; Chambonniere, M.L.; Garraud, O.; Peyrin-Biroulet, L.; Pozzetto, B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am. J. Gastroenterol. 2011, 106, 2001–2008. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- D’Haens, G.; Lemmens, L.; Geboes, K.; Vandeputte, L.; Van Acker, F.; Mortelmans, L.; Peeters, M.; Vermeire, S.; Penninckx, F.; Nevens, F.; et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 2001, 120, 1323–1329. [Google Scholar] [CrossRef]
- Jia, X.; Guo, R.; Hu, Z.; Liu, J.; Li, B.; Yang, Q.; He, J. Efficacy of infliximab, cyclosporine and tacrolimus on ulcerative colitis: A meta-analysis. Medicine 2020, 99, e22894. [Google Scholar] [CrossRef]
- Ogata, H.; Matsui, T.; Nakamura, M.; Iida, M.; Takazoe, M.; Suzuki, Y.; Hibi, T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006, 55, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, D.C.; Pintoffl, J.P.; Sturm, A.; Wiedenmann, B.; Dignass, A.U. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease—A long-term follow-up. Am. J. Gastroenterol. 2006, 101, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, M.; Walch, A.; Meshkat, M.; Gustavsson, A.; Järnerot, G.; Vogelsang, H.; Hertervig, E.; Novacek, G.; Friis-Liby, I.; Blomquist, L.; et al. Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: A retrospective observational study. Inflamm. Bowel Dis. 2012, 18, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Croft, A.; Walsh, A.; Doecke, J.; Cooley, R.; Howlett, M.; Radford-Smith, G. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: Ciclosporin vs. infliximab. Aliment. Pharmacol. Ther. 2013, 38, 294–302. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Linares, P.M.; McNicholl, A.G.; Maté, J.; Gomollón, F. Meta-analysis: The efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment. Pharmacol. Ther. 2009, 30, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Maconi, G.; Russo, A.; Imbesi, V.; Colombo, E.; Bianchi Porro, G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 2006, 55, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, C.; Rundquist, S.; Cao, Y.; Montgomery, S.; Halfvarson, J. Impact of thiopurines on the natural history and surgical outcome of ulcerative colitis: A cohort study. Gut 2019, 68, 623–632. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Yang, H.; Hassard, P.V.; Seidman, E.G.; Kam, L.Y.; Abreu, M.T.; Targan, S.R.; Vasiliauskas, E.A. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 2002, 122, 904–915. [Google Scholar] [CrossRef]
- Prefontaine, E.; Sutherland, L.R.; Macdonald, J.K.; Cepoiu, M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2009, 1, CD000067. [Google Scholar] [CrossRef]
- Luan, Z.J.; Li, Y.; Zhao, X.Y.; Wang, L.; Sun, Y.H.; Wang, S.Y.; Qian, J.M. Treatment efficacy and safety of low-dose azathioprine in chronic active ulcerative colitis patients: A meta-analysis and systemic review. J. Dig. Dis. 2016, 17, 652–659. [Google Scholar] [CrossRef]
- Yang, S.K.; Hong, M.; Baek, J.; Choi, H.; Zhao, W.; Jung, Y.; Haritunians, T.; Ye, B.D.; Kim, K.J.; Park, S.H.; et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat. Genet. 2014, 46, 1017–1020. [Google Scholar] [CrossRef]
- Zabala, W.; Cruz, R.; Barreiro-de Acosta, M.; Chaparro, M.; Panes, J.; Echarri, A.; Esteve, M.; Carpio, D.; Andreu, M.; García-Planella, E.; et al. New genetic associations in thiopurine-related bone marrow toxicity among inflammatory bowel disease patients. Pharmacogenomics 2013, 14, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Ordás, I.; Cabré, E.; Garcia-Sanchez, V.; Bastida, G.; Peñalva, M.; Gomollón, F.; García-Planella, E.; Merino, O.; Gutiérrez, A.; et al. Safety of thiopurine therapy in inflammatory bowel disease: Long-term follow-up study of 3931 patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; González-Lama, Y.; Maté, J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: A systematic review. Am. J. Gastroenterol. 2007, 102, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Moreau, B.; Clement, P.; Theoret, Y.; Seidman, E.G. Allopurinol in combination with thiopurine induces mucosal healing and improves clinical and metabolic outcomes in IBD. Ther. Adv. Gastroenterol. 2017, 10, 819–827. [Google Scholar] [CrossRef]
- Kotlyar, D.S.; Lewis, J.D.; Beaugerie, L.; Tierney, A.; Brensinger, C.M.; Gisbert, J.P.; Loftus, E.V.; Peyrin-Biroulet, L.; Blonski, W.C.; Van Domselaar, M.; et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: A meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 847–858.e4, quiz e848–e850. [Google Scholar] [CrossRef]
- Dayharsh, G.A.; Loftus, E.V.; Sandborn, W.J.; Tremaine, W.J.; Zinsmeister, A.R.; Witzig, T.E.; Macon, W.R.; Burgart, L.J. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 2002, 122, 72–77. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Demers, A.A.; Bernstein, C.N. Increased risk of nonmelanoma skin cancers among individuals with inflammatory bowel disease. Gastroenterology 2011, 141, 1612–1620. [Google Scholar] [CrossRef]
- Hazenberg, H.M.J.L.; de Boer, N.K.H.; Mulder, C.J.J.; Mom, S.H.; van Bodegraven, A.A.; Tack, G.J. Neoplasia and Precursor Lesions of the Female Genital Tract in IBD: Epidemiology, Role of Immunosuppressants, and Clinical Implications. Inflamm. Bowel Dis. 2018, 24, 510–531. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, D.V.; Brynskov, J.; Ainsworth, M.A.; Nersting, J.; Schmiegelow, K.; Steenholdt, C. A Role for Thiopurine Metabolites in the Synergism Between Thiopurines and Infliximab in Inflammatory Bowel Disease. J. Crohns Colitis 2018, 12, 298–305. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kubiliun, M.J.; Czul, F.; Sussman, D.A.; Quintero, M.A.; Jain, A.; Drake, K.A.; Hauenstein, S.I.; Lockton, S.; Deshpande, A.R.; et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin. Gastroenterol. Hepatol. 2015, 13, 1118–1124.e1113. [Google Scholar] [CrossRef]
- Chalhoub, J.M.; Rimmani, H.H.; Gumaste, V.V.; Sharara, A.I. Systematic Review and Meta-analysis: Adalimumab Monotherapy Versus Combination Therapy with Immunomodulators for Induction and Maintenance of Remission and Response in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Márquez, J.R.; Scott, B.B.; Flint, L.; van Hoogstraten, H.J.; Chen, A.C.; Zheng, H.; Danese, S.; et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014, 146, 392–400.e393. [Google Scholar] [CrossRef] [Green Version]
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Nachury, M.; Moreau, J.; et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet 2012, 380, 1909–1915. [Google Scholar] [CrossRef] [Green Version]
- Seagrove, A.C.; Alam, M.F.; Alrubaiy, L.; Cheung, W.Y.; Clement, C.; Cohen, D.; Grey, M.; Hilton, M.; Hutchings, H.; Morgan, J.; et al. Randomised controlled trial. Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: Trial design and protocol (CONSTRUCT). BMJ Open 2014, 4, e005091. [Google Scholar] [CrossRef] [Green Version]
- Fiorino, G.; Caprioli, F.; Daperno, M.; Mocciaro, F.; Principi, M.; Viscido, A.; Fantini, M.C.; Orlando, A.; Papi, C.; Annese, V.; et al. Use of biosimilars in inflammatory bowel disease: A position update of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig. Liver Dis. 2019, 51, 632–639. [Google Scholar] [CrossRef]
- Guerra Veloz, M.F.; Belvis Jiménez, M.; Valdes Delgado, T.; Castro Laria, L.; Maldonado Pérez, B.; Perea Amarillo, R.; Merino Bohórquez, V.; Caunedo Álvarez, Á.; Vilches Arenas, Á.; Argüelles-Arias, F. Long-term follow up after switching from original infliximab to an infliximab biosimilar: Real-world data. Ther. Adv. Gastroenterol. 2019, 12, 1756284819858052. [Google Scholar] [CrossRef]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef]
- REMICADE, ® (Infliximab) [Package Insert]; Janssen Pharmaceuticals, Inc.: Horsham, PA, USA, 2013.
- Michielan, A.; Martinato, M.; Favarin, A.; Zanotto, V.; Caccaro, R.; Caruso, A.; Sturniolo, G.C.; D’Incà, R. A nurse-led accelerated procedure for infliximab infusion is well tolerated and effective in patients with inflammatory bowel disease. Dig. Liver Dis. 2015, 47, 372–377. [Google Scholar] [CrossRef]
- Babouri, A.; Roblin, X.; Filippi, J.; Hébuterne, X.; Bigard, M.A.; Peyrin-Biroulet, L. Tolerability of one hour 10mg/kg infliximab infusions in inflammatory bowel diseases: A prospective multicenter cohort study. J. Crohns Colitis 2014, 8, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.W.; Singh, R.; Fedorak, R.N. A one-hour infusion of infliximab during maintenance therapy is safe and well tolerated: A prospective cohort study. Aliment. Pharmacol. Ther. 2011, 34, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Sands, B.E.; Anderson, F.H.; Bernstein, C.N.; Chey, W.Y.; Feagan, B.G.; Fedorak, R.N.; Kamm, M.A.; Korzenik, J.R.; Lashner, B.A.; Onken, J.E.; et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N. Engl. J. Med. 2004, 350, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rutgeerts, P.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Lu, J.; Horgan, K.; Rachmilewitz, D.; Hanauer, S.B.; et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009, 137, 1250–1260, quiz 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Horin, S.; Chowers, Y. Review article: Loss of response to anti-TNF treatments in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 33, 987–995. [Google Scholar] [CrossRef]
- Chaparro, M.; Guerra, I.; Muñoz-Linares, P.; Gisbert, J.P. Systematic review: Antibodies and anti-TNF-α levels in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 35, 971–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; De Sarro, C.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety profile of biologic drugs for psoriasis in clinical practice: An Italian prospective pharmacovigilance study. PLoS ONE 2020, 15, e0241575. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Ron, Y.; Kivity, S.; Ben-Horin, S.; Israeli, E.; Fraser, G.M.; Dotan, I.; Chowers, Y.; Confino-Cohen, R.; Weiss, B. Infliximab-Related Infusion Reactions: Systematic Review. J. Crohn’s Colitis 2015, 9, 806–815. [Google Scholar] [CrossRef] [Green Version]
- Bonovas, S.; Pantavou, K.; Evripidou, D.; Bastiampillai, A.J.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Safety of biological therapies in ulcerative colitis: An umbrella review of meta-analyses. Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 43–47. [Google Scholar] [CrossRef]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.Y.; Guo, B.; Lufumpa, E.; Li, X.M.; Chen, L.H.; Meng, X.; Li, B.Z. Comparative of the Effectiveness and Safety of Biological Agents, Tofacitinib, and Fecal Microbiota Transplantation in Ulcerative Colitis: Systematic Review and Network Meta-Analysis. Immunol. Investig. 2020, 50, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.; Van Assche, G.; Reinisch, W. Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results. Gastroenterol. Hepatol. (NY) 2013, 9, 317–320. [Google Scholar]
- Colombel, J.F.; Sandborn, W.J.; Ghosh, S.; Wolf, D.C.; Panaccione, R.; Feagan, B.; Reinisch, W.; Robinson, A.M.; Lazar, A.; Kron, M.; et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am. J. Gastroenterol. 2014, 109, 1771–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Elisei, W.; Faggiani, R.; Allegretta, L.; Valle, N.D.; Forti, G.; Franceschi, M.; Ferronato, A.; Gallina, S.; Larussa, T.; et al. Effectiveness and safety of adalimumab to treat outpatient ulcerative colitis: A real-life multicenter, observational study in primary inflammatory bowel disease centers. Medicine 2018, 97, e11897. [Google Scholar] [CrossRef]
- Lukas, M.; Malickova, K.; Kolar, M.; Bortlik, M.; Vasatko, M.; Machkova, N.; Hruba, V.; Duricova, D. Switching from Originator Adalimumab to the Biosimilar SB5 in Patients with Inflammatory Bowel Disease: Short-term Experience from a Single Tertiary Clinical Centre. J. Crohns Colitis 2020, 14, 915–919. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Sakuraba, A.; Wang, A.; Macaulay, D.; Reichmann, W.; Wang, S.; Chao, J.; Skup, M. Comparison of real-world outcomes of adalimumab and infliximab for patients with ulcerative colitis in the United States. Curr. Med. Res. Opin. 2016, 32, 1233–1241. [Google Scholar] [CrossRef]
- Barberio, B.; Zingone, F.; Frazzoni, L.; D’Incà, R.; Maccarone, M.C.; Ghisa, M.; Massimi, D.; Lorenzon, G.; Savarino, E. Real life comparison of different anti-TNF biologic therapies for ulcerative colitis treatment: A retrospective cohort study. Dig. Dis. 2020, 39, 16–24. [Google Scholar] [CrossRef]
- Kawalec, P.; Pilc, A. An indirect comparison of infliximab versus adalimumab or golimumab for active ulcerative colitis. Arch. Med. Sci. 2016, 12, 1097–1109. [Google Scholar] [CrossRef] [Green Version]
- HUMIRA, ® (Adalimumab) [Package Insert]; Abbott Laboratories: North Chicago, IL, USA, 2015.
- SIMPONI, ® (Golimumab) [Package Insert]; Janssen Biotech, Inc.: Horsham, PA, USA, 2015.
- Taxonera, C.; Iborra, M.; Bosca-Watts, M.M.; Rubio, S.; Nantes, Ó.; Higuera, R.; Bertoletti, F.; Martínez-Montiel, P.; Sierra-Ausin, M.; Manceñido, N.; et al. Early dose optimization of golimumab induces late response and long-term clinical benefit in moderately to severely active ulcerative colitis. Curr. Med. Res. Opin. 2019, 35, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Gordon, K.B.; Rosenbaum, J.T.; Arikan, D.; Lau, W.L.; Li, P.; Faccin, F.; Panaccione, R. Long-Term Safety of Adalimumab in 29,967 Adult Patients from Global Clinical Trials Across Multiple Indications: An Updated Analysis. Adv. Ther. 2020, 37, 364–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, C.; Sobell, J.M.; Leonardi, C.L.; Lynde, C.W.; Karunaratne, M.; Valdecantos, W.C.; Hendrickson, B.A. Safety of Adalimumab Dosed Every Week and Every Other Week: Focus on Patients with Hidradenitis Suppurativa or Psoriasis. Am. J. Clin. Dermatol. 2018, 19, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inman, R.D.; Davis, J.C.; Heijde, D.; Diekman, L.; Sieper, J.; Kim, S.I.; Mack, M.; Han, J.; Visvanathan, S.; Xu, Z.; et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: Results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008, 58, 3402–3412. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V.; Danese, S.; Colombel, J.F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Orlando, A.; Papi, C.; Festa, S.; Pugliese, D.; Bonovas, S.; Pansieri, C.; Piovani, D.; Fiorino, G.; Fantini, M.C.; et al. Use of biologics and small molecule drugs for the management of moderate to severe ulcerative colitis: IG-IBD clinical guidelines based on the GRADE methodology. Dig. Liver Dis. 2022, 54, 440–451. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Danese, S.; Argollo, M.; Pouillon, L.; Peppas, S.; Gonzalez-Lorenzo, M.; Lytras, T.; Bonovas, S. Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients with Crohn’s Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 838–846.e832. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e512. [Google Scholar] [CrossRef] [Green Version]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Meserve, J.; Aniwan, S.; Koliani-Pace, J.L.; Shashi, P.; Weiss, A.; Faleck, D.; Winters, A.; Chablaney, S.; Kochhar, G.; Boland, B.S.; et al. Retrospective Analysis of Safety of Vedolizumab in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1533–1540.e1532. [Google Scholar] [CrossRef] [Green Version]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: A prospective multi-centre cohort study. Aliment. Pharmacol. Ther. 2019, 50, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Card, T.; Ungaro, R.; Bhayat, F.; Blake, A.; Hantsbarger, G.; Travis, S. Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data. Aliment. Pharmacol. Ther. 2020, 51, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Ochsenkühn, T.; Tillack, C.; Szokodi, D.; Janelidze, S.; Schnitzler, F. Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis. United Eur. Gastroenterol. J. 2020, 8, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro, M.; Garre, A.; Iborra, M.; Sierra-Ausín, M.; Barreiro-de Acosta, M.; Fernández-Clotet, A.; de Castro, L.; Boscá-Watts, M.; Casanova, M.J.; López-García, A.; et al. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-world Evidence from the ENEIDA Registry. J. Crohn’s Colitis 2021, 15, 1846–1851. [Google Scholar] [CrossRef]
- Fumery, M.; Filippi, J.; Abitbol, V.; Biron, A.; Laharie, D.; Serrero, M.; Altwegg, R.; Bouhnik, Y.; Peyrin-Biroulet, L.; Gilletta, C.; et al. Effectiveness and safety of ustekinumab maintenance therapy in 103 patients with ulcerative colitis: A GETAID cohort study. Aliment. Pharmacol. Ther. 2021, 54, 944–951. [Google Scholar] [CrossRef]
- Wils, P.; Bouhnik, Y.; Michetti, P.; Flourie, B.; Brixi, H.; Bourrier, A.; Allez, M.; Duclos, B.; Serrero, M.; Buisson, A.; et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: A multicentre experience. Aliment. Pharmacol. Ther. 2018, 47, 588–595. [Google Scholar] [CrossRef]
- Xeljanz. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz#authorisation-details-section (accessed on 8 April 2022).
- Panés, J.; Vermeire, S.; Lindsay, J.O.; Sands, B.E.; Su, C.; Friedman, G.; Zhang, H.; Yarlas, A.; Bayliss, M.; Maher, S.; et al. Tofacitinib in Patients with Ulcerative Colitis: Health-Related Quality of Life in Phase 3 Randomised Controlled Induction and Maintenance Studies. J. Crohns Colitis 2019, 13, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Sands, B.E.; Armuzzi, A.; Marshall, J.K.; Lindsay, J.O.; Sandborn, W.J.; Danese, S.; Panés, J.; Bressler, B.; Colombel, J.F.; Lawendy, N.; et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: Results from OCTAVE Open. Aliment. Pharmacol. Ther. 2020, 51, 271–280. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Quirk, D.; Wang, W.; Nduaka, C.I.; Mukherjee, A.; Su, C.; Sands, B.E. Efficacy and Safety of Extended Induction with Tofacitinib for the Treatment of Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W.; for the Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e1512. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Panés, J.; D’Haens, G.R.; Sands, B.E.; Su, C.; Moscariello, M.; Jones, T.; Pedersen, R.; Friedman, G.S.; Lawendy, N.; et al. Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data from Global Clinical Trials. Clin. Gastroenterol. Hepatol. 2019, 17, 1541–1550. [Google Scholar] [CrossRef] [Green Version]
- FDA Approves Boxed Warning About Increased Risk of Blood Clots and Death with Higher Dose of Arthritis and Ulcerative Colitis Medicine Tofacitinib (Xeljanz, Xeljanz XR). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (accessed on 23 November 2020).
- Shah, S.C.; Colombel, J.F.; Sands, B.E.; Narula, N. Mucosal Healing Is Associated with Improved Long-term Outcomes of Patients with Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1245–1255.e1248. [Google Scholar] [CrossRef] [Green Version]
- Cholapranee, A.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Vulliemoz, M.; Brand, S.; Juillerat, P.; Mottet, C.; Ben-Horin, S.; Michetti, P.; on behalf of Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. TNF-Alpha Blockers in Inflammatory Bowel Diseases: Practical Recommendations and a User’s Guide: An Update. Digestion 2020, 101 (Suppl. 1), 16–26. [Google Scholar] [CrossRef]
- Ma, C.; Beilman, C.L.; Huang, V.W.; Fedorak, D.K.; Wong, K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Similar Clinical and Surgical Outcomes Achieved with Early Compared to Late Anti-TNF Induction in Mild-to-Moderate Ulcerative Colitis: A Retrospective Cohort Study. Can. J. Gastroenterol. Hepatol. 2016, 2016, 2079582. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.K.; Greenberg, G.R.; Croitoru, K.; Nguyen, G.C.; Silverberg, M.S.; Steinhart, A.H. Extent of Early Clinical Response to Infliximab Predicts Long-term Treatment Success in Active Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 2090–2096. [Google Scholar] [CrossRef]
- Oussalah, A.; Evesque, L.; Laharie, D.; Roblin, X.; Boschetti, G.; Nancey, S.; Filippi, J.; Flourié, B.; Hebuterne, X.; Bigard, M.A.; et al. A multicenter experience with infliximab for ulcerative colitis: Outcomes and predictors of response, optimization, colectomy, and hospitalization. Am. J. Gastroenterol. 2010, 105, 2617–2625. [Google Scholar] [CrossRef]
- Mir, S.A.; Nagy-Szakal, D.; Smith, E.O.; Gilger, M.A.; Kellermayer, R. Duration of disease may predict response to infliximab in pediatric ulcerative colitis. J. Clin. Gastroenterol. 2014, 48, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Solberg, I.C.; Lygren, I.; Jahnsen, J.; Aadland, E.; Høie, O.; Cvancarova, M.; Bernklev, T.; Henriksen, M.; Sauar, J.; Vatn, M.H.; et al. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study). Scand. J. Gastroenterol. 2009, 44, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.; Katsanos, K.H.; Burisch, J.; Allez, M.; Papamichael, K.; Stallmach, A.; Mao, R.; Berset, I.P.; Gisbert, J.P.; Sebastian, S.; et al. European Crohn’s and Colitis Organisation Topical Review on Treatment Withdrawal [‘Exit Strategies’] in Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, F. Report: Economic implications of inflammatory bowel disease and its management. Am. J. Manag. Care 2016, 22, s51–s60. [Google Scholar] [PubMed]
- Severs, M.; Oldenburg, B.; van Bodegraven, A.A.; Siersema, P.D.; Mangen, M.J.; on behalf of the initiative of Crohn’s and Colitis. The Economic Impact of the Introduction of Biosimilars in Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Abraham, B.; Quigley, E.M.M. Antibiotics and probiotics in inflammatory bowel disease: When to use them? Frontline Gastroenterol. 2020, 11, 62–69. [Google Scholar] [CrossRef]
- Biancone, L.; Michetti, P.; Travis, S.; Escher, J.C.; Moser, G.; Forbes, A.; Hoffmann, J.C.; Dignass, A.; Gionchetti, P.; Jantschek, G.; et al. European evidence-based Consensus on the management of ulcerative colitis: Special situations. J. Crohns Colitis 2008, 2, 63–92. [Google Scholar] [CrossRef] [Green Version]
- Sáez-González, E.; Moret, I.; Alvarez-Sotomayor, D.; Díaz-Jaime, F.C.; Cerrillo, E.; Iborra, M.; Nos, P.; Beltrán, B. Immunological Mechanisms of Adsorptive Cytapheresis in Inflammatory Bowel Disease. Dig. Dis. Sci. 2017, 62, 1417–1425. [Google Scholar] [CrossRef]
- Hibi, T.; Sameshima, Y.; Sekiguchi, Y.; Hisatome, Y.; Maruyama, F.; Moriwaki, K.; Shima, C.; Saniabadi, A.R.; Matsumoto, T. Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: Clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan. Dig. Liver Dis. 2009, 41, 570–577. [Google Scholar] [CrossRef]
- Kruis, W.; Nguyen, P.; Morgenstern, J. Granulocyte/Monocyte Adsorptive Apheresis in Moderate to Severe Ulcerative Colitis—Effective or Not? Digestion 2015, 92, 39–44. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Matsuoka, K.; Kobayashi, T.; Sawada, K.; Fujiyoshi, T.; Ando, T.; Ohnishi, Y.; Ishida, T.; Oka, M.; Yamada, M.; et al. A large-scale, prospective, observational study of leukocytapheresis for ulcerative colitis: Treatment outcomes of 847 patients in clinical practice. J. Crohns Colitis 2014, 8, 981–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Matsuoka, K.; Yokoyama, Y.; Nakamura, T.; Ino, T.; Numata, T.; Shibata, H.; Aoki, H.; Matsuno, Y.; Hibi, T. A multicenter, retrospective, observational study of the clinical outcomes and risk factors for relapse of ulcerative colitis at 1 year after leukocytapheresis. J. Gastroenterol. 2018, 53, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohns Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Øresland, T.; Bemelman, W.A.; Sampietro, G.M.; Spinelli, A.; Windsor, A.; Ferrante, M.; Marteau, P.; Zmora, O.; Kotze, P.G.; Espin-Basany, E.; et al. European evidence based consensus on surgery for ulcerative colitis. J. Crohns Colitis 2015, 9, 4–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.R.; Al Bakir, I.; Ding, N.J.; Lee, G.H.; Askari, A.; Warusavitarne, J.; Moorghen, M.; Humphries, A.; Ignjatovic-Wilson, A.; Thomas-Gibson, S.; et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: A large single-centre study. Gut 2019, 68, 414–422. [Google Scholar] [CrossRef]
- Lopez, A.; Ford, A.C.; Colombel, J.F.; Reinisch, W.; Sandborn, W.J.; Peyrin-Biroulet, L. Efficacy of tumour necrosis factor antagonists on remission, colectomy and hospitalisations in ulcerative colitis: Meta-analysis of placebo-controlled trials. Dig. Liver Dis. 2015, 47, 356–364. [Google Scholar] [CrossRef]
- Barnes, E.L.; Jiang, Y.; Kappelman, M.D.; Long, M.D.; Sandler, R.S.; Kinlaw, A.C.; Herfarth, H.H. Decreasing Colectomy Rate for Ulcerative Colitis in the United States Between 2007 and 2016: A Time Trend Analysis. Inflamm. Bowel Dis. 2020, 26, 1225–1231. [Google Scholar] [CrossRef]
- Rutgeerts, P.J.; Fedorak, R.N.; Hommes, D.W.; Sturm, A.; Baumgart, D.C.; Bressler, B.; Schreiber, S.; Mansfield, J.C.; Williams, M.; Tang, M.; et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013, 62, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Vermeire, S.; O’Byrne, S.; Keir, M.; Williams, M.; Lu, T.T.; Mansfield, J.C.; Lamb, C.A.; Feagan, B.G.; Panes, J.; Salas, A.; et al. Etrolizumab as induction therapy for ulcerative colitis: A randomised, controlled, phase 2 trial. Lancet 2014, 384, 309–318. [Google Scholar] [CrossRef] [Green Version]
- clinicaltrials.gov. A Study of the Efficacy and Safety of Etrolizumab Treatment in Maintenance of Disease Remission in Ulcerative Colitis (UC) Participants Who Are Naive to Tumor Necrosis Factor (TNF) Inhibitors (LAUREL). Available online: https://clinicaltrials.gov/ct2/show/NCT02165215 (accessed on 8 April 2022).
- Danese, S.; Colombel, J.F.; Lukas, M.; Gisbert, J.P.; D’Haens, G.; Hayee, B.; Panaccione, R.; Kim, H.S.; Reinisch, W.; Tyrrell, H.; et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): A randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 118–127. [Google Scholar] [CrossRef]
- Feagan, B.G.; Panes, J.; Ferrante, M.; Kaser, A.; D’Haens, G.R.; Sandborn, W.J.; Louis, E.; Neurath, M.F.; Franchimont, D.; Dewit, O.; et al. Risankizumab in patients with moderate to severe Crohn’s disease: An open-label extension study. Lancet Gastroenterol. Hepatol. 2018, 3, 671–680. [Google Scholar] [CrossRef]
- clinicaltrials.gov. A Study to Assess the Efficacy and Safety of Risankizumab in Participants with Ulcerative Colitis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03398135 (accessed on 8 April 2022).
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Feagan, B.G.; Hibi, T.; Tuttle, J.L.; Klekotka, P.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 537–549.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- clinicaltrials.gov. A Study to Evaluate the Long-Term Efficacy and Safety of Mirikizumab in Participants with Moderately to Severely Active Ulcerative Colitis (LUCENT 3). Available online: https://clinicaltrials.gov/ct2/show/NCT03519945 (accessed on 8 April 2022).
- Feagan, B.G.; Danese, S.; Loftus, E.V., Jr.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempinski, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Schreiber, S.; D’Haens, G.; Tanida, S.; Siffledeen, J.; Enejosa, J.; Zhou, W.; Othman, A.A.; et al. Efficacy of Upadacitinib in a Randomized Trial of Patients with Active Ulcerative Colitis. Gastroenterology 2020, 158, 2139–2149.e14. [Google Scholar] [CrossRef] [PubMed]
- clinicaltrials.gov. A Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants with Moderately to Severely Active Ulcerative Colitis (U-Accomplish). Available online: https://clinicaltrials.gov/ct2/show/NCT03653026 (accessed on 8 April 2022).
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Vermeire, S.; Chiorean, M.; Panes, J.; Peyrin-Biroulet, L.; Zhang, J.; Sands, B.E.; Lazin, K.; Klassen, P.; Naik, S.U.; Cabell, C.H.; et al. Long-term Safety and Efficacy of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study. J. Crohn’s Colitis 2021, 15, 950–959. [Google Scholar] [CrossRef]
- Herfarth, H.; Vavricka, S.R. 5-Aminosalicylic Acid Chemoprevention in Inflammatory Bowel Diseases: Is It Necessary in the Age of Biologics and Small Molecules? Inflamm. Intest. Dis. 2022, 7, 28–35. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Scharl, M.; Gubler, M.; Rogler, G. Biologics for extraintestinal manifestations of IBD. Curr. Drug Targets 2014, 15, 1064–1073. [Google Scholar] [CrossRef]
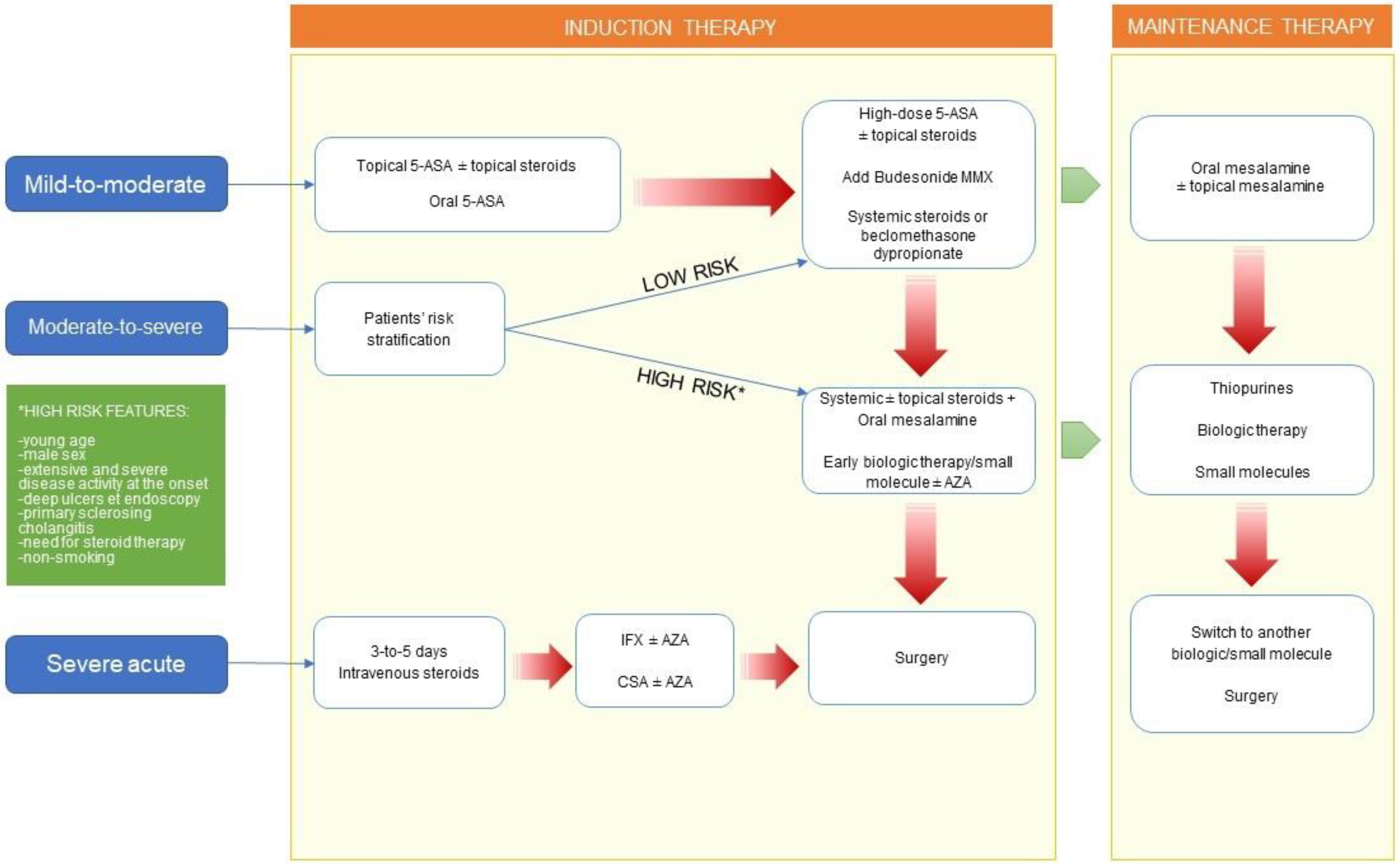
| Mild-to-Moderate Disease | Moderate-to-Severe Disease | ||||||
|---|---|---|---|---|---|---|---|
| Localization | First-Line Options | Second-Line Options | First-Line Options | Refractory | |||
| Induction | Maintenance | Induction | Maintenance | Induction | Maintenance | ||
| Proctitis | Topical mesalamine (suppositories/foam) ± combination with topical steroids or oral mesalamine | Oral mesalamine ± topical mesalamine | Systemic steroids Biologics | Immunomodulators Biologics | Systemic steroids ± topical steroids Biologics | Immunomodulators Biologics ± topical mesalamine | Surgery |
| Left-sided UC | Topical mesalamine (enema) ± combination with topical steroids or oral mesalamine Budesonide MMX | Oral mesalamine ± topical mesalamine | Systemic steroids Biologics | Immunomodulators Biologics | Systemic steroids Biologics | Immunomodulators Biologics | Surgery |
| Extensive UC | Topical mesalamine in combination with oral mesalamine Systemic steroids or beclomethasone dipropionate | Oral mesalamine ± topical mesalamine | Biologics | Immunomodulators Biologics | Systemic steroids Biologics | Immunomodulators Biologics | Surgery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferretti, F.; Cannatelli, R.; Monico, M.C.; Maconi, G.; Ardizzone, S. An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis. J. Clin. Med. 2022, 11, 2302. https://doi.org/10.3390/jcm11092302
Ferretti F, Cannatelli R, Monico MC, Maconi G, Ardizzone S. An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis. Journal of Clinical Medicine. 2022; 11(9):2302. https://doi.org/10.3390/jcm11092302
Chicago/Turabian StyleFerretti, Francesca, Rosanna Cannatelli, Maria Camilla Monico, Giovanni Maconi, and Sandro Ardizzone. 2022. "An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis" Journal of Clinical Medicine 11, no. 9: 2302. https://doi.org/10.3390/jcm11092302






