Glycated Albumin and Glycated Albumin/HbA1c Predict the Progression of Coronavirus Disease 2019 from Mild to Severe Disease in Korean Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Medical Data
2.3. Laboratory Assay and Calculation of Estimated Glomerular Filtration Rate (eGFR)
- Scr = serum creatinine in mg/dL;
- κ = 0.7 (females) or 0.9 (males);
- α = −0.241 (female) or −0.302 (male);
- min (Scr/κ, 1) is the minimum of Scr/κ or 1.0;
- max (Scr/κ, 1) is the maximum of Scr/κ or 1.0; and
- Age (years).
2.4. Statistical Analysis
2.5. Ethical Approval Statement
3. Results
3.1. Baseline Characteristics of Study Subjects
3.2. Differences between Patients with Mild and Severe Disease
3.3. Glucose Control and the Status of COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int (accessed on 8 January 2022).
- COVID-19. Available online: http://ncov.mohw.go.kr (accessed on 8 January 2022).
- Her, M. How is COVID-19 affecting South Korea? What is our current strategy? Disaster Med. Public Health Prep. 2020, 14, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Local Government < Notice < COVID-19. Available online: http://ncov.mohw.go.kr/duBoardList.do?brdId=2&brdGubun=28 (accessed on 13 January 2022).
- Kim, S.; Kim, S.; Lee, J. No Ambulance Even after Barely Finding a Hospital to Admit. Waiting for Tens of Hours, and Worsening Symptoms. 15 December 2021. Available online: https://www.donga.com/news/Society/article/all/20211215/110789045/1 (accessed on 13 January 2022).
- Chung, S.M.; Ahn, J.H.; Moon, J.S. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: A retrospective cohort study. Diabetes Metab. J. 2020, 44, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Cao, Y. What is the role of admission HbA1c in managing COVID-19 patients? J. Diabetes 2021, 13, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Klek, S.P.; Peragallo-Dittko, V.; Goldstein, M.; Burdge, E.; Nadile, V.; Ramadhar, J.; Islam, S.; Rothberger, G.D. Correlation of hemoglobin A1C and outcomes in patients hospitalized with COVID-19. Endocr. Pract. 2021, 27, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, R.; Wang, J.; Cheng, Q.; Zhang, R.; Zhang, S.; Le, Y.; Wang, H.; Xiao, W.; Gao, H.; et al. Diabetes, even newly defined by HbA1c testing, is associated with an increased risk of in-hospital death in adults with COVID-19. BMC Endocr. Disord. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, S.; Rabiee, M.; Tahriri, M.; Abdolrahim, M.; Rajab, A.; Jazayeri, H.E.; Tayebi, L. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: A comprehensive review. Crit. Rev. Clin. Lab. Sci. 2017, 54, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, B.W. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab. J. 2012, 36, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, K.J.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Lee, H.C. The Glycated Albumin to Glycated Hemoglobin Ratio Might Not Be Associated with Carotid Atherosclerosis in Patients with Type 1 Diabetes. Diabetes Metab. J. 2014, 38, 456–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 13 January 2022).
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.L.; Somayaji, R.; Hinds, T.R.; Pier, M.; Droguett, K.; Rios, M.; Skerrett, S.J.; Villalon, M. Glycated albumin triggers an inflammatory response in the human airway epithelium and causes an increase in ciliary beat frequency. Front. Physiol. 2021, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Zoppini, G.; Fedeli, U.; Schievano, E.; Dauriz, M.; Targher, G.; Bonora, E.; Corti, M.C. Mortality from infectious diseases in diabetes. Nutr. Metab. Cardiovasc Dis. 2018, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020, 43, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Luo, S.; Zheng, X.; Cai, G.; Weng, J. Elevated fasting blood glucose within the first week of hospitalization was associated with progression to severe illness of COVID-19 in patients with preexisting diabetes: A multicenter observational study. J. Diabetes 2021, 13, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Song, Y.; Zhuang, Z.; Han, L.; Zhao, S.; Cao, P.; Geng, Y.; Xu, L.; Qin, J.; He, D.; et al. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens. Res. 2020, 43, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Caillon, A.; Zhao, K.; Klein, K.O.; Greenwood, C.M.; Lu, Z.; Paradis, P.; Schiffrin, E.L. High systolic blood pressure at hospital admission is an important risk factor in models predicting outcome of COVID-19 patients. Am. J. Hypertens. 2021, 34, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Finer, N.; Garnett, S.P.; Bruun, J.M. COVID-19 and obesity. Clin. Obes. 2020, 10, e12365. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Wallet, F.; Laville, M.; Disse, E. Obesity is associated with severe forms of COVID-19. Obesity 2020. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Cho, Y.; Choi, H.J.; Lee, H.; Lim, T.H.; Kanga, H.; Ko, B.S.; Oh, J. The effect of BMI on COVID-19 outcomes among older patients in South Korea: A nationwide retrospective cohort study. Ann. Med. 2021, 53, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
| Total | Mild Disease (n = 70) | Severe Disease (n = 59) | p-Value | |
|---|---|---|---|---|
| Age (years) | 63 (53, 70) | 68 (57, 74) | 58 (50, 65) | 0.0011 |
| Sex (male%) | 79 (61.2%) | 45 (64.3%) | 34 (57.6%) | 0.4395 |
| Time to admission from onset of COVID-19-related symptoms (days) | 4 (2, 6) | 3 (2, 4) | 5 (2, 7) | 0.0624 |
| Time to developed hypoxia from onset of COVID-19-related symptoms (days) | 7.0 (5.0, 8.0) | |||
| Smoking status | 0.1939 | |||
| Non-smokers | 100 (77.5%) | 50 (71.4%) | 50 (84.8%) | |
| Ex-smokers | 7 (5.4%) | 5 (7.1%) | 2 (3.4%) | |
| Current smokers | 22 (17.1%) | 15 (21.4%) | 7 (11.9%) | |
| Past history | ||||
| Diabetes mellitus | 108 (83.7%) | 61 (87.1%) | 47 (79.7%) | 0.2515 |
| DM duration (years) | 4.0 (1.0, 10.0) | 5.0 (1.0, 13.0) | 3.0 (0.5, 10.0) | 0.0850 |
| Newly diagnosed DM | 20 (15.5%) | 10 (14.3%) | 10 (16.95%) | 0.6771 |
| Hypertension | 75 (58.1%) | 42 (60.0%) | 33 (55.9%) | 0.6408 |
| Dyslipidemia | 67 (51.9%) | 39 (55.7%) | 28 (47.5%) | 0.3498 |
| Ischemic heart disease | 9 (7.0%) | 9 (12.9%) | 0 (0%) | 0.0038 * |
| Stroke | 7 (5.4%) | 5 (7.1%) | 2 (3.4%) | 0.4525 * |
| Cancer survivor | 10 (7.8%) | 12 (17.1%) | 2 (3.4%) | 0.0124 |
| BMI (kg/m2) | 25.5 (24.0, 28.5) | 24.9 (23.5, 26.7) | 26.2 (24.2, 30.2) | 0.0145 |
| BMI ≥ 25 kg/m2 | 74 (57.4%) | 34 (48.6%) | 40 (67.8%) | 0.0278 |
| BMI ≥ 30 kg/m2 | 24 (18.8%) | 9 (12.9%) | 15 (25.9%) | 0.0677 |
| On admission | ||||
| SpO2 (%) | 97 (96, 97) | 97 (96, 98) | 96 (94, 97) | <0.0001 |
| Body temperature (°C) | 37.3 (36.9, 37.7) | 37.2 (36.8, 37.5) | 37.6 (37.0, 38.3) | 0.0005 |
| SBP (mmHg) | 137.6 ± 19.8 | 141.9 ± 19.7 | 132.5 ± 19.0 | 0.0244 |
| DBP (mmHg) | 82.6 ± 11.0 | 83.9 ± 11.7 | 81.0 ± 9.9 | 0.2370 |
| Glucose (mg/dL) | 190.0 (146.0, 262.0) | 189 (132, 257) | 192 (148, 275) | <0.0001 |
| Albumin (mg/dL) | 3.80 ± 0.34 | 3.92 ± 0.28 | 3.64 ± 0.35 | <0.0001 |
| AST (IU/L) | 32 (25, 45) | 29 (22, 41) | 34 (28, 54) | 0.0197 |
| ALT (IU/L) | 28 (30, 59) | 27 (18, 44) | 31 (21, 41) | 0.5466 |
| Creatinine (mg/dL) | 0.96 (0.84,1.12) | 0.96 (0.87, 1.12) | 0.97 (0.81, 1.12) | 0.9313 |
| eGFR (mL/min/1.73 m2) | 76.05 ± 17.48 | 75.61 ± 17.27 | 76.63 ± 17.86 | 0.5859 |
| Ferritin (ng/mL) | 286.6 (162.5, 722.4) | 212.2 (101.9, 382.5) | 334.1 (221.6, 1017.1) | 0.0063 |
| D-dimer (μg/mL) | 0.54 (0.38, 0.81) | 0.47 (0.34, 0.61) | 0.62 (0.43, 0.97) | 0.0055 |
| TC (mg/dL) | 142 (123, 164) | 142.6 ± 30.8 | 148.2 ± 33.5 | 0.2815 |
| TG (mg/dL) | 127 (106, 165) | 128 (115, 151) | 124.0 (92.5, 170.5) | 0.4181 |
| HDL-C (mg/dL) | 41 (37, 48) | 43.0 (36.0, 50.0) | 40.0 (37.0, 45.5) | 0.2011 |
| LDL-C (mg/dL) | 75 (63, 92) | 78.4 ± 22.4 | 76.7 ± 27.8 | 0.7208 |
| Worst laboratory value during admission | ||||
| Glucose (mg/dL) | 204 (171, 290) | 193 (152, 257) | 243 (180, 326) | 0.0117 |
| Cr (mg/dL) | 0.96 (0.84, 1.12) | 0.96 (0.87, 1.12) | 0.97 (0.81, 1.12) | 0.9313 |
| eGFR (mL/min/1.73 m2) | 73.88 ± 17.68 | 73.58 ± 17.80 | 74.24 ± 17.68 | 0.8326 |
| CRP (mg/dL) | 4.54 (1.43, 9.68) | 2.23 (0.72, 4.42) | 9.80 (5.90, 12.23) | <0.0001 |
| Ferritin (ng/mL) | 286.6 (162.5, 722.4) | 255.0 (118.6, 425.1) | 459.3 (221.6, 1185.7) | 0.0040 |
| D-dimer (μg/mL) | 0.55 (0.38, 0.81) | 0.47 (0.34, 0.61) | 0.695 (0.500, 1.025) | 0.0005 |
| Sugar control status | ||||
| HbA1c (%) | 7.1 (6.5, 7.9) | 6.95 (6.40, 7.50) | 7.30 (6.80, 8.30) | 0.0344 |
| HbA1c ≥ 6.5% | 101 (78.3%) | 50 (71.4%) | 51 (86.4%) | 0.0364 |
| HbA1c ≥ 7% | 76 (58.9%) | 35 (50.0%) | 41 (69.5%) | 0.0250 |
| Glycated albumin (GA) (%) | 18.2 (16.5, 21.9) | 18.4 (15.5, 19.9) | 20.95 (17.4, 24.4) | 0.0013 |
| GA ≥ 20% | 44 (34.11%) | 17 (24.3%) | 27 (45.8%) | 0.0104 |
| GA ≥ 26% | 16 (12.4%) | 5 (7.1%) | 11 (18.6%) | 0.0473 |
| GA/HbA1c | 2.57 (2.39, 2.76) | 2.55 (2.32, 2.76) | 2.68 (2.46, 2.76) | 0.0145 |
| GA/HbA1c ≥ 2.7 | 40 (31.0%) | 21 (30.0%) | 19 (32.2%) | 0.7875 |
| FBS (mg/dL) | 143 (112, 183) | 122 (106, 152) | 164 (136, 268) | <0.0001 |
| Blood pressure on discharge | ||||
| SBP (mmHg) | 126.4 ± 17.6 | 125.5 ± 19.0 | 127.4 ± 15.8 | 0.5580 |
| DBP (mmHg) | 93.0 ± 9.0 | 92.4 ± 9.3 | 93.7 ± 8.6 | 0.4213 |
| Completion of Vaccination | 0.0140 | |||
| Only 1st dose of vaccination | 36 (27.9%) | 19 (27.1%) | 17 (28.8%) | 0.2849 |
| Both doses of vaccination | 42 (32.6%) | 30 (42.9%) | 12 (20.3%) | 0.0035 |
| Breakthrough infection | 4 (7.4%) | 2 (7.4%) | 2 (7.4%) | 1.0000 * |
| Treatment of COVID-19 | ||||
| Regdanvimab | 77 (59.7%) | 43 (61.4%) | 34 (57.6%) | 0.6610 |
| Remdesivir | 55 (42.3%) | 6 (8.57%) | 49 (83.1%) | <0.0001 |
| Dexamethasone | 63 (48.8%) | 11 (15.7%) | 52 (88.1%) | <0.0001 |
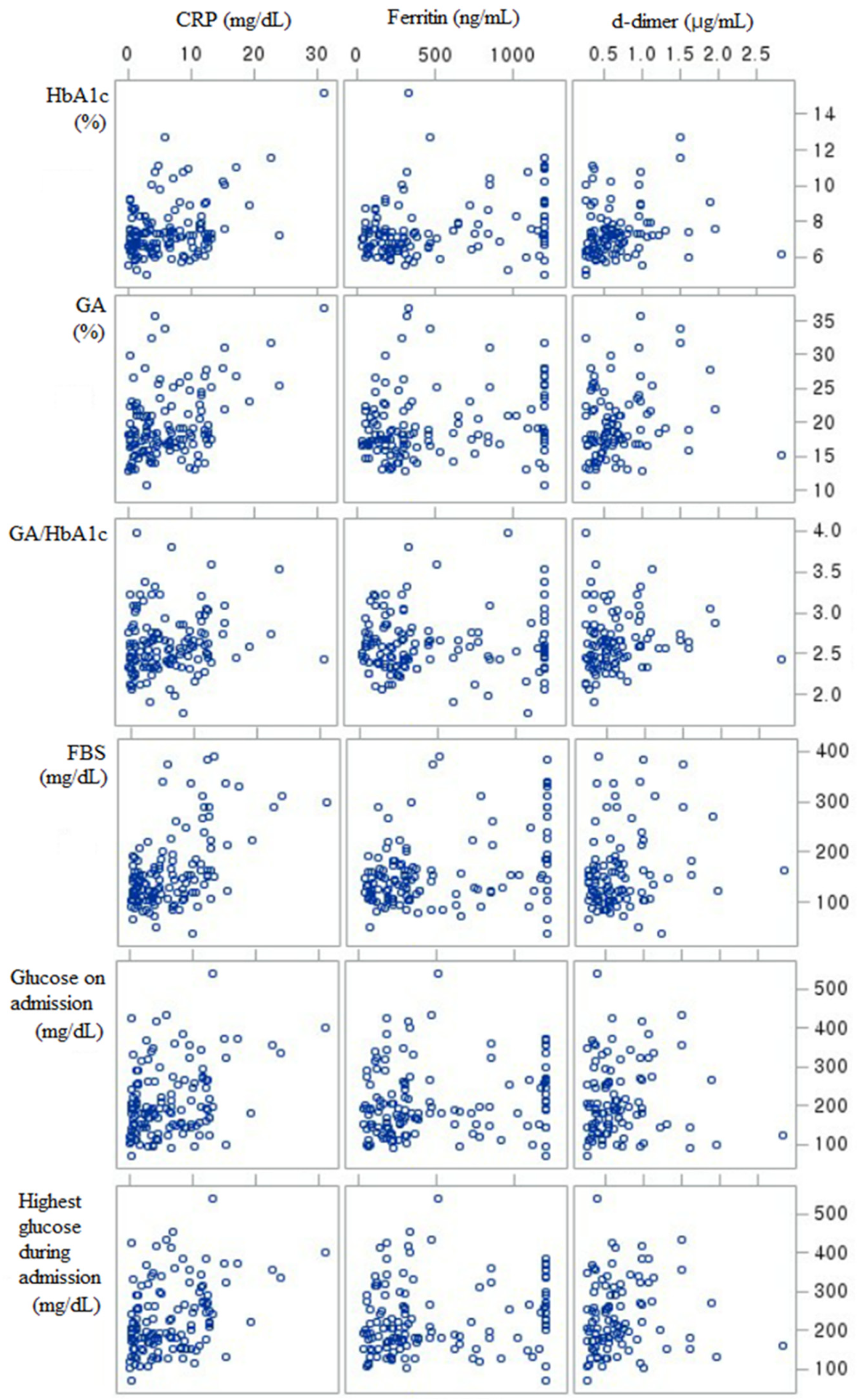
| HbA1c | GA | GA/HbA1c | FBS | Glucose on Admission | Highest Glucose | Worst CRP | Worst Ferritin | Worst D-Dimer | |
|---|---|---|---|---|---|---|---|---|---|
| HbA1c | 1 | ||||||||
| GA | 0.6118 † | 1 | |||||||
| GA/HbA1c | 0.1280 * | 0.5261 † | 1 | ||||||
| FBS | 0.2593 † | 0.3158 † | 0.1290 * | 1 | |||||
| Glucose on admission | 0.3397 † | 0.3920 † | 0.1748 * | 0.2551 † | 1 | ||||
| Highest glucose | 0.3715 † | 0.4488 † | 0.1922 * | 0.3840 † | 0.77605 † | 1 | |||
| Worst CRP | 0.1554 * | 0.2004 * | 0.1257 * | 0.2907 † | 0.16908 * | 0.2323 † | 1 | ||
| Worst ferritin | 0.1627 * | 0.1463 * | −0.0065 | 0.1850 * | 0.11939 * | 0.1359 * | 0.2454 † | 1 | |
| Worst d-dimer | 0.1530 * | 0.1431 * | 0.1187 | 0.1202 | 0.0659 | 0.1254 | 0.2973 † | 0.0633 | 1 |
| Variable | OR | 95% CI |
|---|---|---|
| Age (years) | 0.959 | (0.930, 0.988) |
| Male Sex | 0.756 | (0.391, 1.538) |
| BMI (kg/m2) | 1.093 | (1.003, 1.191) |
| BMI ≥ 25 vs. BMI < 25 | 2.229 | (1.085, 4.578) |
| BMI ≥ 30 vs. BMI < 30 | 2.311 | (0.927, 5.756) |
| Time to admission from onset of COVID-19-related symptoms (days) | 1.162 | (0.992, 1.361) |
| SBP on admission (mmHg) | 0.975 | (0.956, 0.994) |
| Albumin on admission (mg/dL) | 0.065 | (0.018, 0.239) |
| AST on admission (IU/L) | 1.013 | (0.997, 1.029) |
| HbA1c (%) | 1.319 | (1.026, 1.695) |
| HbA1c ≥ 6.5% vs. HbA1c < 6.5% | 2.550 | (1.029, 6.322) |
| HbA1c ≥ 7.0% vs. HbA1c < 7.0% | 2.278 | (1.102, 4.706) |
| GA (%) | 1.116 | (1.032, 1.207) |
| GA ≥ 20% vs. GA < 20% | 2.630 | (1.244, 5.562) |
| GA ≥ 26% vs. GA < 26% | 2.979 | (0.971, 9.138) |
| GA/HbA1c | 2.571 | (0.947, 6.977) |
| GA/HbA1c ≥ 2.7 vs. GA/HbA1c < 2.7 | 1.108 | (0.525, 2.342) |
| Glucose (mg/dL) | ||
| Glucose on admission | 1.002 | (0.998. 1.006) |
| Worst glucose during admission | 1.006 | (1.002, 1.011) |
| FBS | 1.017 | (1.009, 1.024) |
| Completion of Vaccination | ||
| Only 1st dose of vaccination vs. never | 0.626 | (0.265, 1.480) |
| Both doses of vaccination vs. never | 0.280 | (0.117, 0.669) |
| Variable | OR | 95% CI |
|---|---|---|
| HbA1c (%) | 1.169 | (0.865, 1.579) |
| HbA1c ≥ 6.5% vs. HbA1c < 6.5% | 2.171 | (0.688, 6.849) |
| HbA1c ≥ 7.0% vs. HbA1c < 7.0% | 1.589 | (0.642, 3.932) |
| GA (%) | 1.151 | (1.024, 1.294) |
| GA ≥ 20% vs. GA < 20% | 4.030 | (1.407, 11.540) |
| GA ≥ 26% vs. GA < 26% | 4.655 | (0.848, 25.568) |
| GA/HbA1c | 8.330 | (1.786, 38.842) |
| GA/HbA1c ≥ 2.7 vs. GA/HbA1c < 2.7 | 2.204 | (0.792, 6.134) |
| Glucose (mg/dL) | ||
| Glucose on admission | 1.004 | (0.998, 1.010) |
| Worst glucose during admission * | 1.006 | (0.988, 1.024) |
| FBS * | 1.003 | (0.978, 1.028) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Choi, Y.; Park, S.A.; Seo, J.Y.; Ahn, C.W.; Han, J. Glycated Albumin and Glycated Albumin/HbA1c Predict the Progression of Coronavirus Disease 2019 from Mild to Severe Disease in Korean Patients with Type 2 Diabetes. J. Clin. Med. 2022, 11, 2327. https://doi.org/10.3390/jcm11092327
Yoo J, Choi Y, Park SA, Seo JY, Ahn CW, Han J. Glycated Albumin and Glycated Albumin/HbA1c Predict the Progression of Coronavirus Disease 2019 from Mild to Severe Disease in Korean Patients with Type 2 Diabetes. Journal of Clinical Medicine. 2022; 11(9):2327. https://doi.org/10.3390/jcm11092327
Chicago/Turabian StyleYoo, Jeongseon, Youngah Choi, Shin Ae Park, Ji Yeon Seo, Chul Woo Ahn, and Jaehyun Han. 2022. "Glycated Albumin and Glycated Albumin/HbA1c Predict the Progression of Coronavirus Disease 2019 from Mild to Severe Disease in Korean Patients with Type 2 Diabetes" Journal of Clinical Medicine 11, no. 9: 2327. https://doi.org/10.3390/jcm11092327
APA StyleYoo, J., Choi, Y., Park, S. A., Seo, J. Y., Ahn, C. W., & Han, J. (2022). Glycated Albumin and Glycated Albumin/HbA1c Predict the Progression of Coronavirus Disease 2019 from Mild to Severe Disease in Korean Patients with Type 2 Diabetes. Journal of Clinical Medicine, 11(9), 2327. https://doi.org/10.3390/jcm11092327






