New Advanced Imaging Parameters and Biomarkers—A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy
Abstract
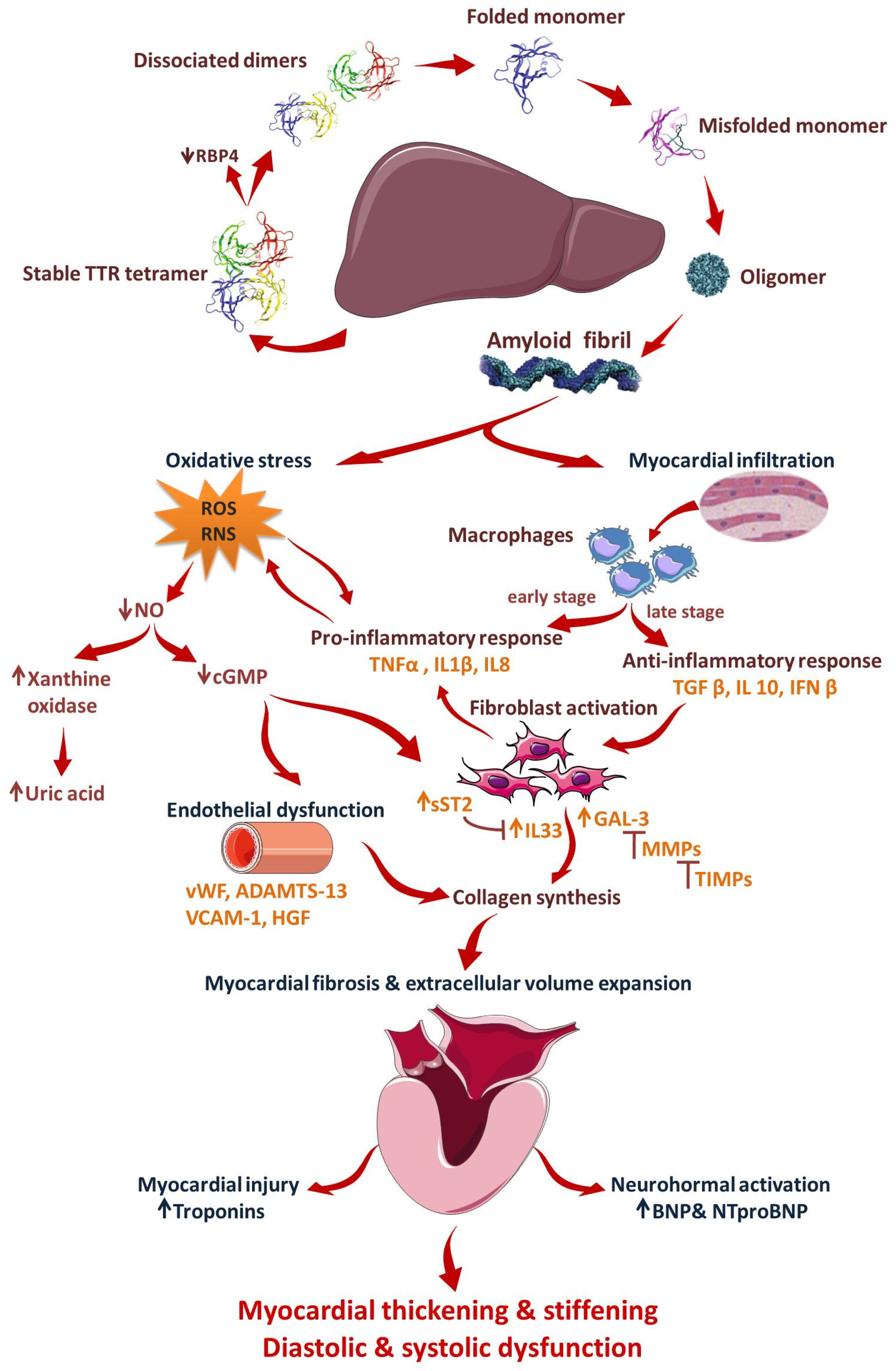
:1. Introduction
2. Clinical Red Flags in ATTR-CM
3. Electrocardiography in ATTR-CM
4. Echocardiography in ATTR Cardiac Amyloidosis
- Tissue Doppler Imaging (TDI). The longitudinal myocardial velocities recorded at the level of the lateral and septal mitral annulus in CA are significantly lower than in patients with true hypertrophy, despite normal LVEF in both categories [59]. TDI in CA demonstrates that longitudinal ventricular contraction is impaired well before deterioration of the LVEF and that longitudinal dysfunction precedes the onset of HF [64]. A decrease in RV free wall systolic velocity has been also recorded [62,65]. However, the prognostic value of the TDI in CA is not clearly established.
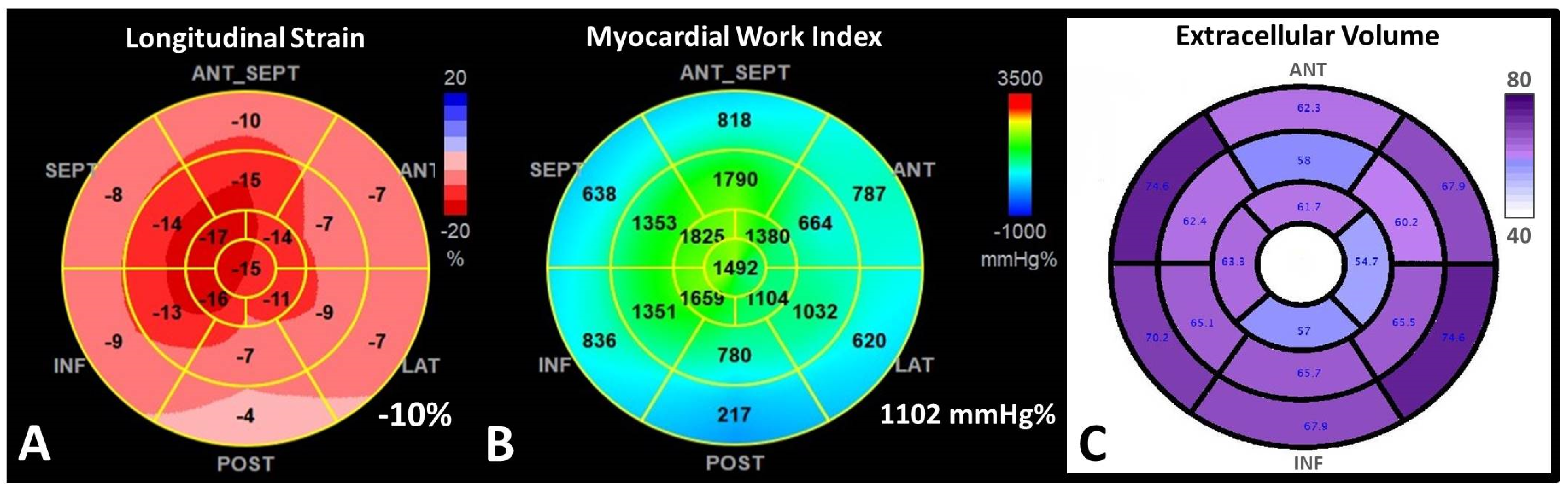
- 2D Speckle Tracking Echocardiography (STE). STE is considered nowadays a more feasible and robust technique than TDI strain. In CA with preserved LVEF, strain and strain rate determined through STE are reduced compared to healthy people, unlike TDI velocities, which remain almost normal in the early stages of the disease [66]. The relative apical-sparing pattern of the global longitudinal strain (LS) is considered highly suggestive of CA [56,63] (Figure 3).
- 3.
- Myocardial work analysis derived from STE. Myocardial Work (MW) is a novel non-invasive echocardiographic technique for myocardial performance assessment, derived from LV pressure-strain loop analysis. By integration of afterload, MW analysis might have a superior benefit in the evaluation of the prognosis of patients. MW is abnormal in patients with many forms of LVH: hypertrophic cardiomyopathy, hypertensive cardiomyopathy, AS [75]. New evidence demonstrated a potential role for new MW analysis, a novel STE measure of LV systolic function, which may be more sensitive than LS in the diagnosis and prognosis of CA (Figure 3). Both global work index (GWI) and global efficiency (GWE), showed a good correlation with NT-proBNP, eGFR, TpI, and peak oxygen consumption [75,76,77]. These indexes might be better used to assess the efficiency of the treatment than LS or LVEF, because loading conditions are variable over time [75,76]. Furthermore, MW indices seem to predict all-cause mortality in CA better than LVEF.
- 4.
- Left atrial strain analysis by STE. Structural and functional assessment of the LA is an important tool, due to the fact that LA dimensions and functions are independent predictors of survival in HF, especially in HFpEF. Recently, LA functional assessment has become more accurate using STE, from which myocardial strain (S) and strain rate (SR) can be evaluated during different phases of the cardiac cycle [78]. LA function has been assessed comparatively in patients with different types of CA (AL, ATTRm, and ATTRwt) and matched healthy volunteers. Nocioka et al. showed that all LA functions were severely decreased in CA, expressed by conventional LA volumes and functions, and also by S and SR parameters. Among the different CA subtypes, LA reservoir strain (LAr) and LA active emptying fraction were worse in ATTRwt than AL and ATTRm [79]. LA reservoir and pump function are significantly impaired in both ATTR-CM and hypertrophic cardiomyopathy (HCM) patients compared with controls, irrespective of LA volume and LVEF, more severe in CA, mainly determined by the LA wall infiltration [80].
- 5.
- 3D Echocardiography (3DE). 3DE and 3D speckle tracking echocardiography (3DSTE) are increasingly used for characterizing cardiac structure and function, and they have been also used for a more accurate assessment of CA. Deformation and rotational 3DSTE parameters seem to be able to differentiate CA patients from patients with other forms of LVH. Basal rotational strain determined through 3DSTE is significantly lower than apical rotational strain in CA compared to HCM [81]. However, 3DE is not recommended by the current guideline [9], and more clinical studies are needed in order to implement 3DE as a routine diagnostic tool for CA.
- -
- Diastolic dysfunction higher than grade 2;
- -
- Reduced TDI velocities (<5 cm/s);
- -
- LS < −15%.
- -
- Relative LVWT (IVS + PWT)/LVEDD > 0.6—3 points
- -
- E/E’ ratio > 11—1 point
- -
- TAPSE < 19 mm—2 points
- -
- LS < −13%—1 point
- -
- Systolic longitudinal strain apex to base ratio > 2.9—3 points
5. Cardiac Magnetic Resonance
- Early gadolinium enhancement (EGE) images are usually acquired in the first 3 to 5 min after gadolinium contrast agent (GCA) administration. This agent is able to penetrate vascular structures. In CA there is evidence of microvascular coronary obstruction and severe endothelial dysfunction from histological studies. Therefore, EGE imaging is useful for the detection of microvascular obstruction (MVO) [63].
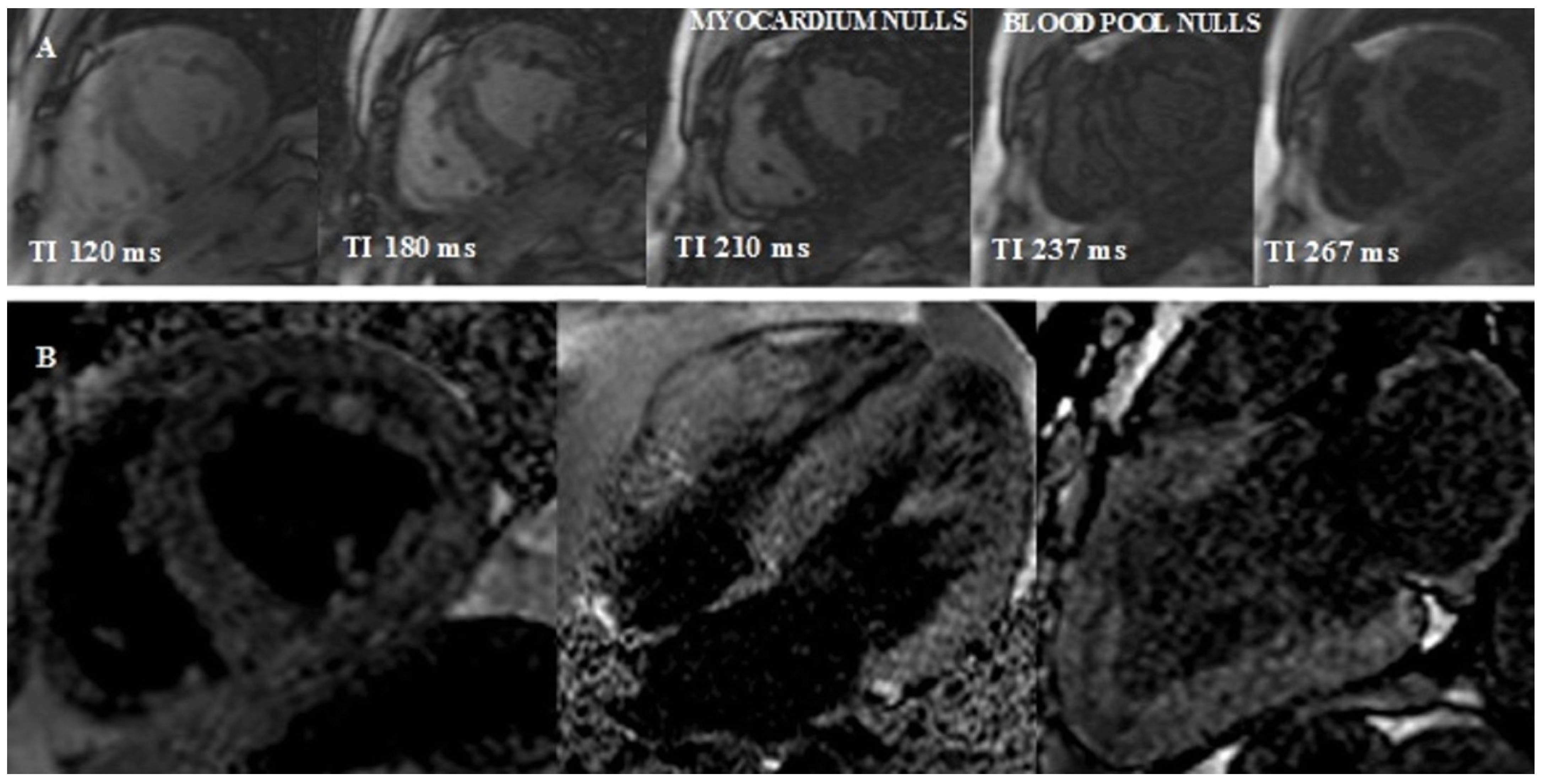
- Look-Locker TI scout. The optimal inversion time to null the normal myocardial signal is determined using a Look–Locker TI scout sequence acquired in the short-axis at the mid-ventricular level ∼5 min after the administration of contrast. The pattern of nulling is classified as normal if the blood pool is nulled before the myocardium. In CA the pattern is reversed, blood pool nulling being coincident with or after myocardial nulling (Figure 4).
- 3.
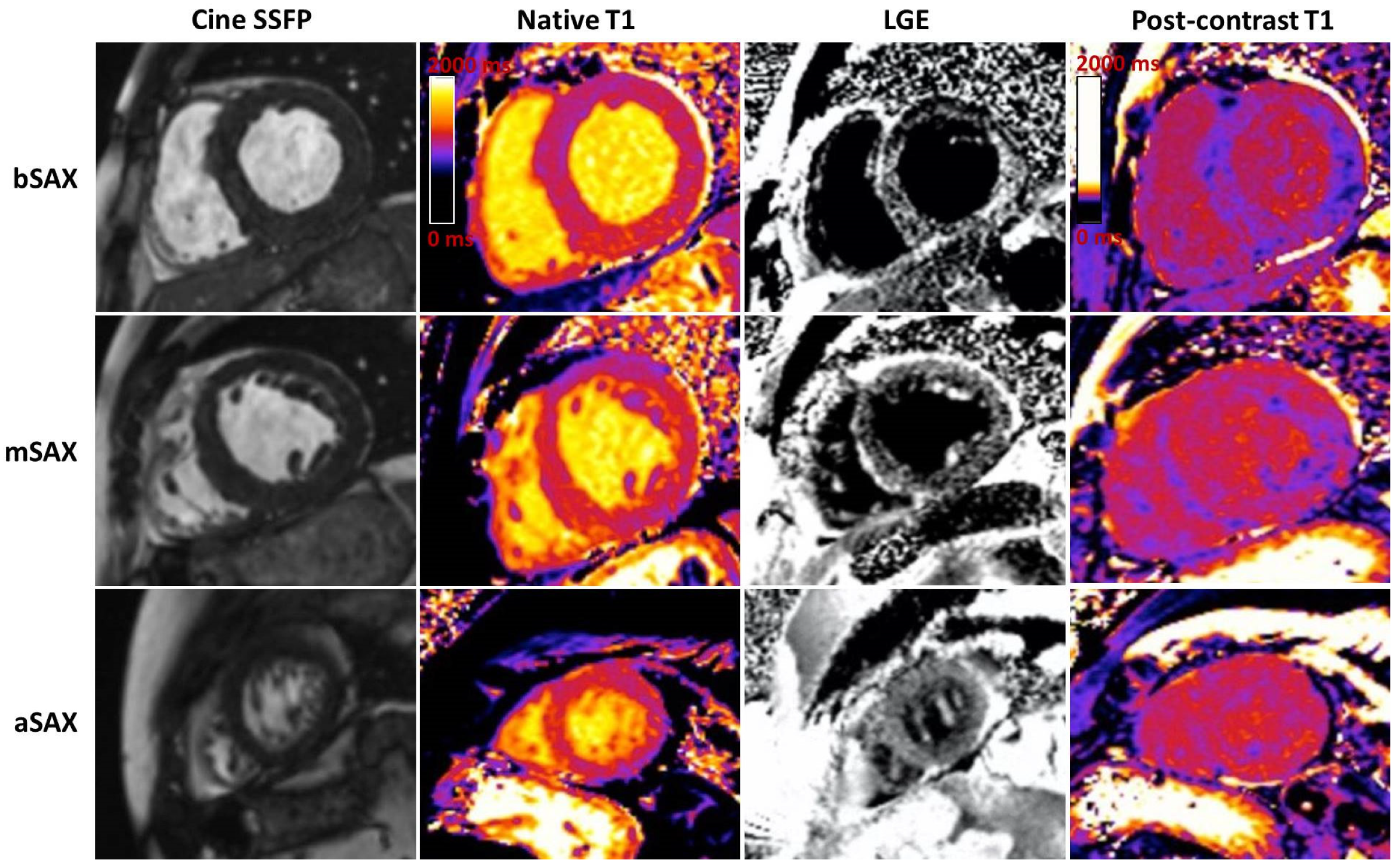
- Late gadolinium enhancement (LGE) can be seen in three possible patterns: no LGE, sub-endocardial -, and transmural enhancement [83]. Sub-endocardial LGE appears to be more prevalent in AL-CA, whereas transmural LGE is more prevalent in ATTR-CM. In addition, RV and LA LGE were found to be more prevalent in patients with ATTR [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83]. Transmural LGE carries higher mortality rates compared with subendocardial LGE [83,84,85]. The mechanism of LGE in CA is due to infiltration of the amyloid protein and fibrosis caused by ischemia due to capillary obstruction by amyloid deposits [63].
- 4.
- T1 mapping. The use of GCA is relatively contraindicated in severe renal failure. Native T1 mapping, before the administration of GCA, can overcome this limitation, as it measures direct quantitative signals from the myocardium [86,87,88]. Post administration of GCA the myocardial extracellular volume (ECV) can be calculated (Figure 3). Native myocardial T1 showed a high diagnostic accuracy to discriminate CA (AUC = 0.93). T1 mapping measures myocardial amyloid load and myocyte response to infiltration, allowing monitoring and eventual change of therapy, even when cardiac function is normal.
- 5.
- T2 imaging is a well-established non-contrast technique that quantifies myocardial edema.
- LVH, restrictive LV pattern (preserved LVEF, non-dilated ventricles, enlarged atria);
- Reduced LV indexed stroke volume;
- Atrial septal hypertrophy;
- Mild pericardial effusions;
- Abnormal nulling time for the myocardium;
- LGE from sub-endocardial to transmural myocardium;
- RV involvement with hypertrophy and in advanced stages with RV LE;
- Atrial LGE is a strong clue, and is associated with atrial contractile dysfunction;
- Significantly increased native T1 time and ECV compared to other causes of LVH (ECV > 40%) [9].
6. Nuclear Imaging Role in ATTR-CM
7. ATTR-CM in Aortic Stenosis Has a Particular Need for Screening and Treatment
- ▪
- LVH (interventricular septum >18 mm) with infiltrative features (Myocardial granular sparkling), increased thickness of atrioventricular valves, interatrial septum >2 mm, and RV free wall ≥5 mm with RV dysfunction: tricuspid S’ < 9 cm/s and TAPSE <14 mm)
- ▪
- Small LV cavity with reduced stroke volume (SVi < 30 mL/min/m2)
- ▪
- Bi-atrial enlargement and small A wave on mitral inflow Doppler;
- ▪
- Restrictive diastolic pattern with signs of high LVFP in advanced disease;
- ▪
- Pericardial effusion
- ▪
- Early impaired longitudinal strain (LS < −12%, mitral S’ ≤ 6 cm/s);
- ▪
- Apical sparing with normal LVEF (this hallmark appearance on the “bull’s eye” plot on STE is not a typical finding in severe AS patients, probably secondary to diffuse LV remodeling in these settings)
- ▪
- History of carpal tunnel syndrome (3 points)
- ▪
- Right bundle branch block (2 points)
- ▪
- Sokolow/Lyon index < 1.9 mV (1 point)
- ▪
- High sensitivity troponin level >20 ng/mL (1 point)
- ▪
- E/A ratio > 1.4 (1 point)
- ▪
- Age ≥ 85 years (1 point)
8. Conventional and New Added Biomarkers in ATTR-CM
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, M.; Syed, B.; Yvonne, S.E.; Prem, S. Molecular Imaging of Cardiac Amyloidosis. J. Nucl. Med. 2020, 61, 965–970. [Google Scholar]
- Nitsche, C.; Scully, P.R.; Patel, K.P.; Kammerlander, A.A.; Koschutnik, M.; Dona, C.; Wollenweber, T.; Ahmed, N.; Thornton, G.D.; Kelion, A.D.; et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management. A Scientific Statement Fron the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Berk, J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012, 126, 1286–1300. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Lopez, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef] [Green Version]
- Fine, N.M.; Davis, M.K.; Anderson, K.; Delgado, D.H.; Giraldeau, G.; Kitchlu, A.; Massie, R.; Narayan, J.; Swiggum, E.; Venner, C.P.; et al. Canadian Cardiovascular Society/Canadian Heart Failure Society joint position statement on the evaluation and management of patients with cardiac amyloidosis. Can. J. Cardiol. 2020, 36, 322–334. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Wen, I.; Bhat, A.; Chen, H.H.L.; Gan, G.C.H.; Pathan, F.; Tan, T.C. The role of multimodality imaging in the diagnosis of cardiac amylodosis. A focused update. Front. Cardiovasc. Med. 2020, 7, 590057. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2021, 23, 512–526. [Google Scholar] [CrossRef]
- Pun, S.C.; Landau, H.J.; Riedel, E.R.; Jordan, J.; Yu, A.F.; Hassoun, H.; Chen, C.L.; Steingart, R.M.; Liu, J.E. Prognostic and added value of two-dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J. Am. Soc. Echocardiogr. 2018, 31, 64–70. [Google Scholar] [CrossRef]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, M.; Koch, C.M.; Chan, G.G.; Torres-Arancivia, C.; LaValley, M.P.; Jacobson, D.R.; Berk, J.L.; Connors, L.H.; Ruberg, F.L. Identification of transthyretin cardiac amyloidosis using serum retinol-binding protein 4 and a clinical prediction model. JAMA Cardiol. 2017, 2, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaño, A.; Drachman, B.M.; Judge, D.; Maurer, M.S. Natural history and therapy of TTR-cardiac amyloidosis: Emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail. Rev. 2015, 20, 163–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.S.; Hawkins, P.N. Cardiac amyloidosis: Where are we today? J. Intern. Med. 2015, 278, 126–144. [Google Scholar] [CrossRef]
- Castano, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling transthyretin cardiac amyloidoisis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 2879–2887. [Google Scholar] [CrossRef] [Green Version]
- Scully, P.R.; Patel, K.P.; Treibel, T.A.; Thornton, G.D.; Hughes, R.K.; Chadalavada, S.; Katsoulis, M.; Hartman, N.; Fontana, M.; Pugliese, F.; et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 2759–2767. [Google Scholar] [CrossRef] [Green Version]
- Treibel, T.A.; Fontana, M.; Gilbertson, J.A.; Castelletti, S.; White, S.K.; Scully, P.R.; Roberts, N.; Hutt, D.F.; Rowczenio, D.M.; Whelan, C.J.; et al. Occult Transthyretin Cardiac Amyloid in Severe Calcific Aortic Stenosis: Prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ. Cardiovasc. Imaging 2016, 9, e005066. [Google Scholar] [CrossRef] [Green Version]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef]
- Li, W.; Uppal, D.; Wang, Y.C.; Xu, X.; Kokkinidis, D.G.; Travin, M.I.; Tauras, J.M. Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021. Diagnostics 2021, 11, 996. [Google Scholar] [CrossRef]
- Witteles, R.M.; Bokhari, S.; Damy, T.; Elliott, P.M.; Falk, R.H.; Fine, N.M.; Gospodinova, M.; Obici, L.; Rapezzi, C.; Pavia-Garcia, P. Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice. JACC Heart Fail. 2019, 7, 709–716. [Google Scholar] [CrossRef]
- Pibarot, P.; Lancellotti, P.; Narula, J. Concomitant Cardiac Amyloidosis in Severe Aortic Stenosis. The Trojan Horse? J. Am. Coll. Cardiol. 2021, 77, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Ando, Y.; Beirão, J.M.; Coelho, T.; Gertz, M.A.; Gillmore, J.D.; Hawkins, P.N.; Lousada, I.; Suhr, O.B.; Merlini, G. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J. Neurol. 2020, 268, 2109–2122. [Google Scholar] [CrossRef] [Green Version]
- Benson, M.D.; Dasgupta, M.R. Amyloid cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Immunoglobulin light chain amyloidosis: 2018 update on diagnosis, prognosis, and treatment. Am. J. Hematol. 2018, 93, 1169–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, M.A.; Dispenzieri, A. Systemic amyloidosis recognition, prognosis, and therapy A systematic review. JAMA 2020, 324, 79–89. [Google Scholar] [CrossRef]
- Merlini, G.; Dispenzieri, A.; Sanchorawal, V.; Schonland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Primers 2018, 4, 38. [Google Scholar] [CrossRef]
- Agha, A.M.; Parwani, P.; Guha, A.; Durand, J.B.; Iliescu, C.A.; Hassan, S.; Palaskas, N.L.; Gladish, G.; Kim, P.Y.; Lopez-Mattei, J. Role of cardiovascular imaging for the diagnosis and prognosis of cardiac amyloidosis. Open Heart 2018, 5, e000881. [Google Scholar] [CrossRef]
- Ng, B.; Connors, L.H.; Davidoff, R.; Skinner, M.; Falk, R.H. Senile systemic amyloidosis presenting with heart failure. Arch. Intern. Med. 2005, 165, 1425–1429. [Google Scholar] [CrossRef] [Green Version]
- Perfetto, F.; Bergesio, F.; Emdin, M.; Capelli, F. Troponins in cardiac amyloidosis: Multipurpose markers. Nat. Rev. Cardiol. 2014, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Neben-Wittich, M.A.; Wittich, C.M.; Mueller, P.S.; Larson, D.R.; Gertz, M.A.; Edwards, W.D. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am. J. Med. 2005, 118, 1287. [Google Scholar] [CrossRef]
- Dorbala, S.; Vangala, D.; Bruyere, J.; Quarta, C.; Kruger, J.; Padera, R.; Foster, C.; Hanley, M.; Di Carli, M.F.; Falk, R. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail. 2014, 2, 358–367. [Google Scholar] [CrossRef]
- Ihne, S.; Morbach, C.; Obici, L.; Palladini, G.; Störk, S. Amyloidosis in Heart Failure. Curr. Heart Fail. Rep. 2019, 16, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef] [PubMed]
- Dubrey, S.W.; Hawkins, P.N.; Falk, R.H. Amyloid diseases of the heart: Assessment, diagnosis, and referral. Heart 2011, 97, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Sekijima, Y.; Yazaki, M.; Tojo, K.; Yoshinaga, T.; Doden, T.; Koyama, J.; Yanagisawa, S.; Ikeda, S.I. Carpal tunnel syndrome: A common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016, 23, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Westermark, P.; Westermark, G.T.; Suhr, O.B.; Berg, S. Transthyretin derived amyloidosis: Probably a common cause of lumbar spinal stenosis. Ups. J. Med. Sci. 2014, 119, 223–228. [Google Scholar] [CrossRef]
- Rimbas, R.C.; Balinesteanu, A.; Chitroceanu, A.M.; Vinereanu, D. Cardiac amyloidosis an underdiagnosed cause of heart failure with preserved ejection fraction—updated diagnosis and treatment options. Rom. J. Cardiol. 2021, 31, 284–302. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef] [Green Version]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current Status of Endomyocardial Biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Damy, T.; Costes, B.; Hagege, A.A.; Donal, E.; Eicher, J.C.; Slama, M.; Guellich, A.; Rappeneau, S.; Gueffet, J.P.; Logeart, D.; et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular thickness. Eur. Heart J. 2016, 37, 1834–1926. [Google Scholar] [CrossRef] [Green Version]
- Cyrille, N.B.; Goldsmith, J.; Alvarez, J.; Maurer, M.S. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am. J. Cardiol. 2014, 114, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Maurer, M.S.; Rapezzi, C.; Planté-Bordeneuve, V.; Karayal, O.N.; Mundayat, R.; Suhr, O.B.; Kristen, A.V. Clinical, ECG and echocardiographic clues to the diagnosis of TTR-related cardiomyopathy. Open Heart 2016, 3, e000289. [Google Scholar] [CrossRef] [Green Version]
- Jercan, A.; Ene, A.; Jurcut, R.; Draghici, M.; Badelita, S.; Dragomir, M.; Dobrea, C.; Popescu, M.; Jardan, D.; Stoica, E.; et al. Clinical characteristics in patients with hereditary amyloidosis with Glu54Gln transthyretin identified in the Romanian population. Orphanet J. Rare Dis. 2020, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Dungu, J.N.; Sattianayagam, P.T.; Whelan, C.J.; Gibbs, S.D.; Pinney, J.H.; Banypersad, S.M.; Rowczenio, D.; Gilbertson, J.A.; Lachmann, H.J.; Wechalekar, A.; et al. The electrocardiographic features associated with cardiac amyloidosis of variant transthyretin isoleucine 122 type in afro-caribbean patients. Am. Heart J. 2012, 164, 72–79. [Google Scholar] [CrossRef]
- Murtagh, B.; Hammill, S.C.; Gertz, M.A.; Kyle, R.A.; Tajik, A.J.; Grogan, M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am. J. Cardiol. 2005, 95, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Mussinelli, R.; Salinaro, F.; Alogna, A.; Boldrini, M.; Raimondi, A.; Musca, F.; Palladini, G.; Merlini, G.; Perlini, S. Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann. Noninvasive Electrocardiol. 2013, 18, 271–280. [Google Scholar] [CrossRef]
- Rahman, J.E.; Helou, E.F.; Gelzer-Bell, R.; Thompson, R.E.; Kuo, C.; Rodriguez, E.R.; Hare, J.M.; Baughman, K.L.; Kasper, E.K. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J. Am. Coll. Cardiol. 2004, 43, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Granstam, S.O.; Rosengren, S.; Vedin, O.; Kero, T.; Sorensen, J.; Carlson, K.; Flachsckampf, F.A.; Wikstrom, G. Evaluation of patients with cardiac amyloidosis using echocardiography, ECG and right heart catheterization. Amyloid 2013, 20, 27–33. [Google Scholar] [CrossRef]
- Boldrini, M.; Salinaro, F.; Mussinelli, R.; Raimondi, A.; Alogna, A.; Musca, F.; Palladini, G.; Merlini, G.; Perlini, S. Prevalence and prognostic value of conduction disturbances at the time of diagnosis of cardiac al amyloidosis. Ann. Noninvasive Electrocardiol. 2013, 18, 327–335. [Google Scholar] [CrossRef]
- Perlini, S.; Salinaro, F.; Cappelli, F.; Perfetto, F.; Bergesio, F.; Alogna, A.; Mussinelli, R.; Boldrini, M.; Raimondi, A.; Musca, F.; et al. Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int. J. Cardiol. 2013, 167, 2156–2161. [Google Scholar] [CrossRef]
- Namdar, M.; Steffel, J.; Jetzer, S.; Schmied, C.; Hurlimann, D.; Camici, G.G.; Bayrak, F.; Ricciardi, D.; Rao, J.Y.; de Asmundis, C.; et al. Value of electrocardiogram in the differentiation of hypertensive heart disease, hypertrophic cardiomyopathy, aortic stenosis, amyloidosis, and Fabry disease. Am. J. Cardiol. 2012, 109, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Kristen, A.V.; Prez, J.B.; Schonland, S.O.; Hegenbart, U.; Schnabel, P.A.; Kristen, J.H.; Goldcshmidt, H.; Katus, H.A.; Dengler, T.J. Non-invasive predictors of survival in cardiac amyloidosis. Eur. J. Heart Fail. 2007, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, G.Y.; Choi, J.O.; Kim, K.; Kim, S.J.; Jeon, E.S. Associations of electrocardiographic parameters with left vetricular longitudinal strain and prognosis in cardiac light chain amyloidosis. Sci. Rep. 2019, 9, 7746. [Google Scholar] [CrossRef] [PubMed]
- Slivnick, J.A.; Wallner, A.L.; Vallakati, A.; Truong, V.T.; Mazur, W.; Elamin, M.B.; Tong, M.S.; Raman, S.V.; Zareba, K.M. Indexed left ventricular mass to QRS voltage ratio is associated with heart failure hospitalizations in patients with cardiac amyloidosis. Int. J. Cardiovasc. Imaging 2021, 37, 1043–1051. [Google Scholar] [CrossRef]
- Boldrini, M.; Cappelli, F.; Chacko, L.; Restrepo-Cordoba, M.A.; Lopez-Sainz, A.; Giannoni, A.; Aimo, A.; Baggiano, A.; Martinez-Naharro, A.; Whelan, C.; et al. Multiparametric Echocardiography Scores for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yokochi, T. Transthyretin cardiac amyloidosis: An update on diagnosis and treatment. ESC Heart Fail 2019, 6, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Chacko, L.; Martone, R.; Cappelli, F.; Fontana, M. Cardiac Amyloidosis: Updates in Imaging. Curr. Cardiol. Rep. 2019, 21, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurmann, R.; Mankad, S.V.; Mankad, R. Echocardiography in Sarcoidosis. Curr. Cardiol. Rep. 2018, 20, 118. [Google Scholar] [CrossRef]
- Cacciapuoti, F. The role of echocardiography in the non-invasive diagnosis of cardiac amyloidosis. J. Echocardiogr. 2015, 13, 84–89. [Google Scholar] [CrossRef]
- Di Nunzio, D.; Recupero, A.; de Gregorio, C.; Zito, C.; Carerj, S.; Di Bella, G. Echocardiographic Findings in Cardiac Amyloidosis: Inside Two-Dimensional, Doppler, and Strain Imaging. Curr. Cardiol. Rep. 2019, 21, 7. [Google Scholar] [CrossRef]
- Dubrey, S.W.; Cha, K.; Skinner, M.; LaValley, M.; Falk, R.H. Familial and primary (AL) cardiac amyloidosis: Echocardiographically similar diseases with distinctly different clinical outcomes. Heart 1997, 78, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Sascău, R.; Anghel, L.; Clement, A.; Bostan, M.; Radu, R.; Stătescu, C. The Importance of Multimodality Imaging in the Diagnosis and Management of Patients with Infiltrative Cardiomyopathies: An Update. Diagnostics 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Jurcut, R.; Onciul, S.; Adam, R.; Stan, C.; Coriu, D.; Rapezzi, C.; Popescu, B.A. Multimodality imaging in cardiac amyloidosis: A primer for cardiologists. Eur. Heart J. Cardiovasc Imaging 2020, 21, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H.; Quarta, C.C. Echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2015, 20, 125–131. [Google Scholar] [CrossRef]
- Perry, R.; Selvanayagam, J.B. Echocardiography in Infiltrative Cardiomyopathy. Heart Lung Circ. 2019, 28, 1365–1413. [Google Scholar] [CrossRef] [Green Version]
- Bellavia, D.; Abraham, T.P.; Pellikka, P.A.; Al-Zahrani, G.B.; Dispenzieri, A.; Oh, J.K.; Bailey, K.R.; Wood, C.M.; Novo, S.; Miyazaki, C.; et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J. Am. Soc. Echocardiogr. 2007, 20, 1194–1202. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Van Cleemput, J.; Delforge, M.; Bogaert, J.; Kuznetsova, T.; Voigt, J.U. Echo parameters for differential diagnosis in cardiac amyloidosis: A head-to-head comparison of deformation and nondeformation parameters. Circ. Cardiovasc. Imaging 2017, 10, e005588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popovic, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef]
- Liu, D.; Hu, K.; Niemann, M.; Herrmann, S.; Cikes, M.; Stork, S.; Gaudron, P.D.; Knop, S.; Ertl, G.; Bijnens, B.; et al. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ. Cardiovasc. Imaging 2013, 6, 1066–1072. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.; Kim, H.K.; Choi, J.O.; Chang, S.A.; Oh, J.K.; Jeon, J.S. Visual assessment of relative apical sparing pattern is more useful than quantitative assessment for diagnosing cardiac amyloidosis in borderline or mildly increased LV thickness. Circ. J. 2015, 79, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Arvidsson, S.; Henein, M.Y.; Wikström, G.; Suhr, O.B.; Lindqvist, P. Right ventricular involvement in transthyretin amyloidosis. Amyloid 2018, 25, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Fine, N.M.; White, J.A.; Jimenez-Zepeda, V.; Howlett, J.G. Determinants and prognostic significance of serial right heart function changes in patients with cardiac amyloidosis. Can. J. Cardiol. 2020, 36, 432–440. [Google Scholar] [CrossRef]
- Chacko, L.; Martone, R.; Bandera, F.; Lane, T.; Martinez-Naharro, A.; Boldrini, M.; Rezk, T.; Whelan, C.; Quarta, C.; Rowczenio, D.; et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur. Heart J. 2020, 41, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.S.; Zumbo, G.; Barcella, W.; Steeden, J.A.; Muthurangu, V.; Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Kotecha, T.; et al. Cardiac sructural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc. Imaging 2019, 12, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Roger-Rollé, A.; Cariou, E.; Rguez, K.; Fournier, P.; Lavie-Badie, Y.; Blanchard, V.; Roncalli, J.; Galinier, M.; Carrié, D.; Lairez, O. Can myocardial work indices contribute to the exploration of patients with cardiac amyloidosis? Open Heart 2020, 7, e001346. [Google Scholar] [CrossRef]
- Stricagnoli, M.; Cameli, M.; Incampo, E.; Lunghetti, S.; Mondillo, S. Speckle tracking echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2019, 24, 701–707. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Eiskjær, H.; Mikkelsen, F.; Granstam, S.O.; Flachskampf, F.A.; Sørensen, J.; Poulsen, S.H. Left ventricular pressure-strain-derived myocardial work at rest and during exercise in patients with cardiac amyloidosis. J. Am. Soc. Echocardiogr. 2020, 33, 573–582. [Google Scholar] [CrossRef]
- Henein, M.; Suhr, O.; Arvidsson, S.; Pilebro, B.; Westermark, P.; Hornsten, R.; Lindqvist, P. Reduced left atrial myocardial deformation irrespective of cavity size: A potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid 2018, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nochioka, K.; Quarta, C.C.; Claggett, B.; Roca, G.Q.; Rapezzi, C.; Falk, R.H.; Solomon, S.D. Left atrial structure and function in cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, C.; Dattilo, G.; Casale, M.; Terrizzi, A.; Donato, R.; Di Bella, G. Left Atrial Morphology, Size and Function in Patients With Transthyretin Cardiac Amyloidosis and Primary Hypertrophic Cardiomyopathy-Comparative Strain Imaging Study. Circ. J. 2016, 80, 1830–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccouche, H.; Maunz, M.; Beck, T.; Gaa, E.; Banzhaf, M.; Knayer, U.; Fogarassy, P.; Beyer, M. Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography 2012, 29, 668–677. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Falk, R.H.; Quarta, C.C.; Dorbala, S. How to image cardiac amyloidosis. Circ. Cardiovasc. Imaging 2014, 7, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Response to letters regarding article, ‘prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis’. Circulation 2016, 133, e450–e451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, M.; Nava, S.; Quattrocchi, G.; Milazzo, A.; Giannattasio, C.; Roghi, A.; Pedrotti, P. Role of cardiovascular magnetic resonance in suspected cardiac amyloidosis: Late gadolinium enhancement pattern as mortality predictor. Neth. Heart J 2018, 26, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Raina, S.; Lensin, S.Y.; Nairooz, R.S.; Pothineni, N.V.; Hakeem, A.; Bhatti, S.; Pandey, T. Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc. Imaging 2016, 9, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Chung, R.; Hawkins, P.N.; Moon, J.C. Cardiovascular magnetic resonance for amyloidosis. Heart Fail. Rev. 2015, 20, 133–144. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Baggiano, A.; Boldrini, M.; Martinez-Naharro, A.; Kotecha, T.; Petrie, A.; Rezk, T.; Gritti, M.; Quarta, C.; Knight, D.S.; Wechalekar, A.D.; et al. Noncontrast Magnetic Resonance for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; Flett, A.S.; Banypersad, S.M.; White, S.K.; Maestrini, V.; Quarta, G.; Lachmann, R.H.; Murphy, E.; Mehta, A.; Hughes, D.A.; et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012, 98, 1436–1441. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordin, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Maestrini, V.; et al. Multiparametric Mapping to Understand Pathophysiology in Cardiac Amyloidosis. Heart 2017, 103, A1–A2. [Google Scholar] [CrossRef] [Green Version]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Vidal-Perez, R.; Vázquez-García, R.; Barge-Caballero, G.; Bouzas-Mosquera, A.; Soler-Fernandez, R.; Larrañaga-Moreira, J.M.; Crespo-Leiro, M.G.; Vazquez-Rodriguez, J.M. Diagnostic and prognostic value of cardiac imaging in amyloidosis. World J. Cardiol. 2020, 12, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Hutt, D.F.; Fontana, M.; Burniston, M.; Quigley, A.; Petrie, A.; Ross, J.C.; Page, J.; Martinez-Naharro, A.; Wechalekar, A.D.; Lachmann, H.J.; et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur. Heart J Cardiovasc. Imaging 2017, 18, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Stats, M.A.; Stone, J.R. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: Implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc. Pathol. 2016, 25, 413–417. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Treglia, G.; Glaudemans, A.W.J.M.; Bertagna, F.; Hazenberg, B.P.C.; Erba, P.A.; Giubbini, R.; Ceriani, L.; Prior, J.O.; Giovanella, L.; Slart, R.H.J.A. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: A bivariate meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1945–1955. [Google Scholar] [CrossRef]
- Pinney, J.H.; Whelan, C.J.; Petrie, A.; Dungu, J.; Banypersad, S.M.; Sattianayagam, P.; Wechalekar, A.; Gibbs, S.D.J.; Venner, C.P.; Wassef, N.; et al. Senile systemic amyloidosis: Clinical features at presentation and outcome. J. Am. Heart Assoc. 2013, 2, e000098. [Google Scholar] [CrossRef] [Green Version]
- Asif, T.; Gomez, J.; Singh, V.; Doukky, R.; Nedeltcheva, A.; Malhotra, S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: Perils and pitfalls. J. Nucl. Cardiol. 2021, 28, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hutt, D.F.; Quigley, A.M.; Page, J.; Hall, M.L.; Burniston, M.; Gopaul, D.; Lane, T.; Whelan, C.J.; Lachmann, H.J.; Gillmore, J.D.; et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Bukhari, S.; Ahmad, S.; Nieves, R.; Eisele, Y.S.; Follansbee, W.; Brownell, A.; Wong, T.C.; Schelbert, E.; Soman, P. Efficient 1-Hour Technetium-99 m Pyrophosphate Imaging Protocol for the Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Cardiovasc. Imaging 2020, 13, e010249. [Google Scholar] [CrossRef]
- Grigoratos, C.; Aimo, A.; Rapezzi, C.; Genovesi, D.; Barison, A.; Aquaro, G.D.; Vergaro, G.; Pucci, A.; Passino, C.; Marzullo, P.; et al. Diphosphonate single-photon emission computed tomography in cardiac transthyretin amyloidosis. Int. J. Cardiol. 2020, 307, 187–192. [Google Scholar] [CrossRef]
- Sperry, B.W.; Vranian, M.N.; Tower-Rader, A.; Hachamovitch, R.; Hanna, M.; Brunken, R.; Phelan, D.; Cerqueira, M.D.; Jaber, W.A. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc. Imaging 2018, 11, 234–242. [Google Scholar] [CrossRef]
- Löfbacka, V.; Axelsson, J.; Pilebro, B.; Suhr, O.B.; Lindqvist, P.; Sundström, T. Cardiac transthyretin amyloidosis 99mTc-DPD SPECT correlates with strain echocardiography and biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1822–1832. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ha, S. Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and metaanalysis. J. Nucl. Cardiol. 2020, 27, 123–132. [Google Scholar] [CrossRef]
- Cohen, A.D.; Rabinovici, G.D.; Mathis, C.A.; Jagust, W.J.; Klunk, W.E.; Ikonomovic, M.D. Using Pittsburgh Compound B for in vivo PET imaging of fibrillar amyloid-beta. Adv. Pharmacol. 2012, 64, 27–81. [Google Scholar]
- Takasone, K.; Katoh, N.; Takahashi, Y.; Abe, R.; Ezawa, N.; Yoshinaga, T.; Yanagisawa, S.; Yazaki, M.; Oguchi, K.; Koyama, J.; et al. Non-invasive detection and differentiation of cardiac amyloidosis using 99mTc-pyrophosphate scintigraphy and 11C-Pittsburgh compound B PET imaging. Amyloid 2020, 27, 266–274. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, G.Y.; Kim, S.J.; Kim, K.H.; Jeon, E.-S.; Lee, K.-H.; Kim, B.-T.; Choi, J.Y. Imaging Findings and Literature Review of 18F-FDG PET/CT in Primary Systemic AL Amyloidosis. Nucl. Med. Mol. Imaging 2015, 49, 182–190. [Google Scholar] [CrossRef]
- Oda, S.; Kidoh, M.; Nagayama, Y.; Takashio, S.; Usuku, H.; Ueda, M.; Yamashita, T.; Ando, Y.; Tsujita, K.; Yamashita, Y. Trends in Diagnostic Imaging of Cardiac Amyloidosis: Emerging Knowledge and Concepts. Radiographics 2020, 40, 961–981. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; Bandula, S.; Fontana, M.; White, S.K.; Gilbertson, J.A.; Herrey, A.S.; Gillmore, J.D.; Punwani, S.; Hawkins, P.N.; Taylor, S.A.; et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J. Cardiovasc. Comput. Tomogr. 2015, 9, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ternacle, J.; Krapf, L.; Mohty, D.; Magne, J.; Nguyen, A.; Galat, A.; Gallet, R.; Teiger, E.; Côté, N.; Clavel, M.A.; et al. Aortic Stenosis and Cardiac Amyloidosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2638–2651. [Google Scholar] [CrossRef]
- Oda, S.; Takashio, S.; Nagamatsu, S.; Yamashita, T.; Uchimura, R.; Kidoh, M.; Utsunomiya, D.; Nakaura, T.; Tsujita, K.; Yamashita, Y. Myocardial extracellular volume quantification using CT for the identification of occult cardiac amyloidosis in patients with severe aortic stenosis referred for transcatheter aortic valve replacement. Amyloid 2019, 26, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Basset, M.; Russo, F.; Foli, A.; Palladini, G.; Merlini, G. The lung in amyloidosis. Eur. Respir. Rev. 2017, 26, 170046. [Google Scholar] [CrossRef] [Green Version]
- Penalver, J.; Abrosino, M.; Jeon, H.D.; Agrawal, A.; Kanjanahattakij, N.; Pitteloud, M.; Stempel, J.; Amanullah, A. Transthyretin Cardiac Amyloidosis and Aortic Stenosis: Connection and Therapeutic Implications. Curr. Cardiol. Rev. 2020, 16, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.; Burkhoff, D.; Maurer, M.S. Untangling the physiology of transthyretin cardiac amyloidosis by leveraging echocardiographically derived pressure–volume indices. Eur. Heart J. 2020, 41, 1448–1450. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Solomon, S.D.; Adams, D.; Kristen, A.; Grogan, M.; Gonzalez-Duarte, A.; Maurer, M.S.; Merlini, G.; Damy, T.; Slama, M.S.; Brannagan, T.H., 3rd; et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation 2019, 139, 431–443. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Plante Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef]
- Pregenzer-Wenzler, A.; Abraham, J.; Barrell, K.; Kovacsovics, T.; Nativi-Nicolau, J. Utility of biomarkers in cardiac amyloidosis. JACC Heart Fail. 2020, 8, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.S.; Arvanitis, M.; Koch, C.M.; Berk, J.L.; Ruberg, F.L.; Prokaeva, T.; Connors, L.H. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ. Heart Fail. 2018, 11, e004000. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, F.; Martone, R.; Gabriele, M.; Taborchi, G.; Morini, S.; Vignini, E.; Allinovi, M.; Di Gioia, M.; Bartolini, S.; Di Mario, C.; et al. Biomarkers and prediction of prognosis in transthyretin-related cardiac amyloidosis; direct comparison of two staging systems. Can. J. Cardiol. 2020, 36, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Scott, C.G.; Kyle, R.A.; Zeldenrust, S.R.; Gertz, M.A.; Lin, G.; Klarich, K.W.; Miller, W.L.; Maleszewski, J.J.; Dispnezieri, A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J. Am. Coll. Cardiol. 2016, 68, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef]
- Takashio, S.; Yamamuro, M.; Izumyia, Y.; Hirakawa, K.; Marume, K.; Yamamoto, M.; Ueda, M.; Yamashita, T.; Ishibashi-Ueda, H.; Yasuda, S.; et al. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail. 2017, 5, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Hendren, N.S.; Roth, L.R.; Grodin, J.L. Disease-specific biomarkers in transthyretin cardiac amyloidosis. Curr. Heart Fail. Rep. 2020, 17, 77–83. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, S.; Rahman, S.; Singh, L.R. The extracellular protein, transthyretin is an oxidative stress biomarker. Front. Physiol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Suhr, O.B.; Coelho, T.; Buades, J.; Pouget, J.; Conceicao, I.; Berk, J.; Schmidt, H.; Waddington-Cruz, M.; Campistol, J.M.; Bettencourt, B.R.; et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: A phase II multi-dose study. Orphanet J. Rare Dis. 2015, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Arvanitis, M.; Simon, S.; Chan, G.; Fine, D.; Beardsley, P.; La Valley, M.P.; Jacobson, M.; Koch, C.; Berk, J.L.; Connors, L.H.; et al. Retino binding protein 4 (RBP4) concentration identifies V122I transthyretin cardiac amyloidosis. Amyloid 2017, 2, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Essick, E.E.; Doros, G.; Tanriverdi, K.; Connors, L.H.; Seldin, D.C.; Sam, F. Circulating matrix metalloproteinases and tissue inhibitors of metalloproteinases in cardiac amyloidosis. J. Am. Heart Assoc. 2013, 2, e005868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiger, K.J.; Friedman, E.A.; Brittain, E.L.; Tomasek, K.A.; Huang, S.; Su, Y.R.; Sawyer, D.B.; Lenihan, D.J. Plasma hepatocyte growth factor is a novel marker of AL cardiac amyloidosis. Amyloid 2016, 23, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.P.; Guimaraes-Costa, A.B.; Bandeira-Melo, C.; Chimelli, L.; Waddington-Cruz, M.; Saraiva, E.M.; Palhano, F.L.; Foguel, D. Inflammatory profiling of patients with familial amyloid polyneuropathy. BMC Neurol. 2019, 19, 146. [Google Scholar] [CrossRef]
- Gertz, M.A.; Dispenzieri, A.; Sher, T. Pathophysiology and treatment of cardiac amyloidosis. Nat. Rev. Cardiol. 2015, 12, 91–102. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Ordonez-Llanos, J. Multiple biomarker strategies for risk stratification in heart failure. Clin. Chim. Acta 2015, 443, 120–125. [Google Scholar] [CrossRef]
- Ticau, S.; Sridharan, G.V.; Tsour, S.; Cantley, W.L.; Chan, A.; Gilbert, J.A.; Erbe, D.; Aldinc, E.; Reilly, M.M.; Adams, D.; et al. Neurofilament light chain as a biomarker of hereditary transthyretin-mediated amyloidosis. Neurology 2021, 96, e412–e422. [Google Scholar] [CrossRef]
- Misumi, Y.; Ando, Y.; Goncalves, N.P.; Saraiva, M.J. Fibroblasts endocytose and degrade tranthyretin aggregates in transthyretin-related amyloidosis. Lab. Investig. 2013, 93, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, G.; Ikeda, T.; Komohara, Y.; Takamatsu, K.; Kakuma, T.; Tasaki, M.; Misumi, Y.; Ueda, M.; Ito, T.; Senju, S.; et al. Involvement of macrophages in the pathogenesis of familial amyloid polyneuropathy and efficacy of human iPS cell-derived macrophages in its treatment. PLoS ONE 2016, 11, e0163944. [Google Scholar] [CrossRef] [Green Version]
- Nakov, R.; Sarafov, S.; Nakov, V.; Gospodinova, M.; Ianiro, G.; Todorov, T.; Todorova, A.; Tournev, I. Fecal calprotectin levels are elevated in transthyretin amyloidosis patients with gastrointestinal manifestations. Medicine 2020, 99, e19509. [Google Scholar] [CrossRef]
- Modesto, K.M.; Dispenzieri, A.; Gertz, M.; Cauduro, S.A.; Khandheria, B.K.; Seward, J.B.; Kyle, R.; Wood, C.M.; Bailey, K.R.; Tajik, A.J.; et al. Vascular abnormalities in primary amyloidosis. Eur. Heart J. 2007, 28, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migrino, R.Q.; Truran, S.; Gutterman, D.D.; Franco, D.A.; Bright, M.; Schlundt, B.; Timmons, M.; Motta, A.; Phillips, S.A.; Hari, P. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, H.; Katsuno, M. Transthyretin amyloidosis: Update on the clinical spectrum, pathogenesis, and disease-modifying therapies. Neurol. Ther. 2020, 9, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and biomarkers of endothelium; when something is rotten in the state. Oxid. Med. Cell. Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef]
- Kastritis, E.; Papassotiriou, I.; Terpos, E.; Roussou, M.; Gavriatopoulou, M.; Komitopoulou, A.; Skevaki, C.; Eleutherakis-Papaiakovou, E.; Pamboucas, C.; Psimenou, E.; et al. Clinical and prognostic significance of serum levels of von Willebrand factor and ADAMSTS-13 antigens in AL amyloidosis. Blood 2016, 128, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Coelho, T.; Merlini, G.; Bulawa, C.E.; Fleming, J.A.; Judge, D.P.; Kelly, J.W.; Maurer, M.S.; Plante´-Bordeneuve, V.; Labaudinière, R.; Mundayat, R.; et al. Mechanism of Action and Clinical Application of Tafamidis in Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2016, 5, 1–25. [Google Scholar] [CrossRef] [Green Version]


| Cardiac Amyloidosis | Systemic Involvement |
|---|---|
| HFpEF with increased LV wall thickness | Carpal tunnel syndrome, particularly if bilateral |
| HFpEF particularly in older men | Lumbar spinal stenosis (mainly ATTRwt) |
| Biventricular HF | Spontaneous biceps tendon rupture |
| HF with intolerance to βblocker, ACEI, or ARB, ARNI | Autonomic dysfunction, |
| Newly diagnosed HCMP in elderly patients | Orthostatic hypotension, |
| Paradoxical low flow, low gradient AS in elderly patients | Peripheral neuropathy |
| Low to normal blood pressure | Deafness |
| Syncope, conduction, or AV blocks needing pacemaker associated with increased LV wall thickness | Recurrent urinary tract infections |
| Mild increase in high sensitivity troponin levels (>20 ng/L) on repeated occasions in the absence of coronary artery disease or renal dysfunction | Sexual dysfunction |
| NT-proBNP, often disproportionately for the degree of HF | Alternating constipation/diarrhea |
| Low/normal voltage on ECG, with LVH on echocardiography (QRS voltage amplitude <0.5 mV in all limb leads or <1 mV in all precordial leads) | Sweating abnormalities |
| Pseudo-infarction pattern with no history of myocardial infarction | Unintentional weight loss |
| Atrioventricular block + LVH + AS (amyloid infiltration of the atrioventricular node) | Pseudo claudication |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimbas, R.C.; Balinisteanu, A.; Magda, S.L.; Visoiu, S.I.; Ciobanu, A.O.; Beganu, E.; Nicula, A.I.; Vinereanu, D. New Advanced Imaging Parameters and Biomarkers—A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy. J. Clin. Med. 2022, 11, 2360. https://doi.org/10.3390/jcm11092360
Rimbas RC, Balinisteanu A, Magda SL, Visoiu SI, Ciobanu AO, Beganu E, Nicula AI, Vinereanu D. New Advanced Imaging Parameters and Biomarkers—A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy. Journal of Clinical Medicine. 2022; 11(9):2360. https://doi.org/10.3390/jcm11092360
Chicago/Turabian StyleRimbas, Roxana Cristina, Anca Balinisteanu, Stefania Lucia Magda, Simona Ionela Visoiu, Andrea Olivia Ciobanu, Elena Beganu, Alina Ioana Nicula, and Dragos Vinereanu. 2022. "New Advanced Imaging Parameters and Biomarkers—A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy" Journal of Clinical Medicine 11, no. 9: 2360. https://doi.org/10.3390/jcm11092360
APA StyleRimbas, R. C., Balinisteanu, A., Magda, S. L., Visoiu, S. I., Ciobanu, A. O., Beganu, E., Nicula, A. I., & Vinereanu, D. (2022). New Advanced Imaging Parameters and Biomarkers—A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy. Journal of Clinical Medicine, 11(9), 2360. https://doi.org/10.3390/jcm11092360








