Effects of Type 2 Diabetes Mellitus on Osteoclast Differentiation, Activity, and Cortical Bone Formation in POSTmenopausal MRONJ Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Specimen Harvesting
2.2. Histochemistry
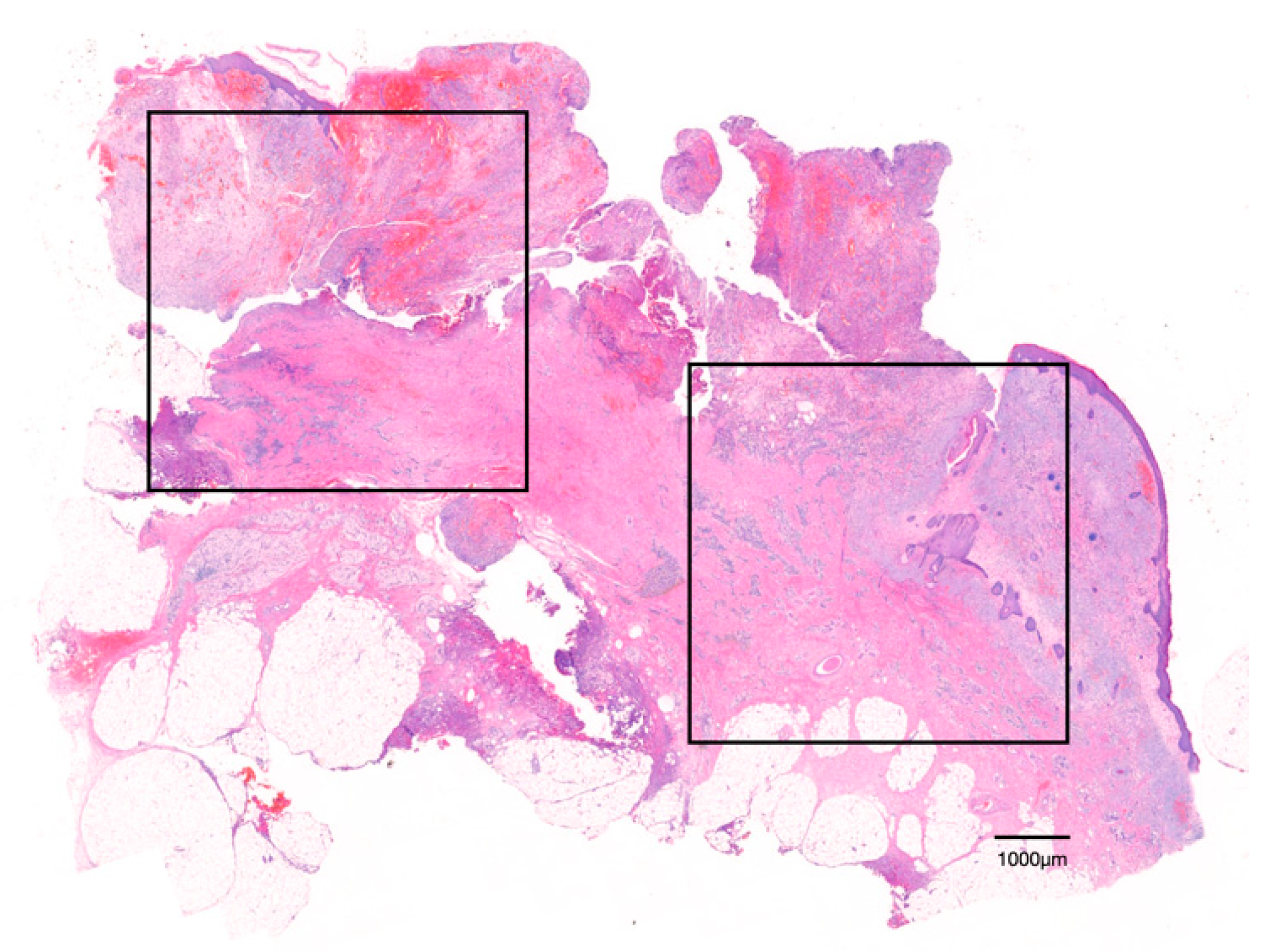
2.2.1. Hematoxylin and Eosin (H&E) Staining
2.2.2. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.3. Immunohistochemistry
2.4. Cortical Bone Formation Measurement
2.4.1. Cortical Bone Thickness Analysis
2.4.2. Cortical Bone Ratio Analysis
2.5. Statistical Analysis
3. Results
3.1. Histomorphometric Analysis and Histological Findings
3.1.1. Hematoxylin and Eosin (H&E) Staining
3.1.2. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
3.2. Immunohistomorphometric Analysis (DC-STAMP Expression)
3.3. Cortical Bone Formation Measurement (Cortical Bone Thickness and Ratio)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, A.V. Diabetes Mellitus: Does it Affect Bone? Calcif. Tissue Res. 2003, 73, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Inzerillo, A.M.; Epstein, S. Osteoporosis and Diabetes Mellitus. Rev. Endocr. Metab. Disord. 2004, 5, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.A.; Joakimsen, R.M.; Berntsen, G.K.; Fønnebø, V.; Schirmer, H. Diabetes mellitus and the risk of non-vertebral fractures: The Tromsø study. Osteoporos. Int. 2005, 17, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Lix, L.M.; Prior, H.J.; Derksen, S.; Metge, C.; O’Neil, J. Biphasic fracture risk in diabetes: A population-based study. Bone 2007, 40, 1595–1601. [Google Scholar] [CrossRef]
- Nicodemus, K.K.; Folsom, A.R. Iowa Women’s Health Study. Type 1 and Type 2 Diabetes and Incident Hip Fractures in Postmenopausal Women. Diabetes Care 2001, 24, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-H.; Hsu, H.-Y.; Tsai, M.-C.; Hsu, L.-Y.; Chien, K.-L.; Yeh, T.-L. Association between type 2 diabetes and osteoporosis risk: A representative cohort study in Taiwan. PLoS ONE 2021, 16, e0254451. [Google Scholar] [CrossRef]
- Janghorbani, M.; van Dam, R.; Willett, W.C.; Hu, F.B. Systematic Review of Type 1 and Type 2 Diabetes Mellitus and Risk of Fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2006, 18, 427–444. [Google Scholar] [CrossRef]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K. Risk of Fracture in Women with Type 2 Diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef] [Green Version]
- Melton, L.J.; Leibson, C.L.; Achenbach, S.J.; Therneau, T.M.; Khosla, S. Fracture Risk in Type 2 Diabetes: Update of a Population-Based Study. J. Bone Miner. Res. 2008, 23, 1334–1342. [Google Scholar] [CrossRef]
- Dede, A.D.; Tournis, S.; Dontas, I.; Trovas, G. Type 2 diabetes mellitus and fracture risk. Metabolism 2014, 63, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Drake, M.T.; Amin, S.; Melton, L.J., 3rd; McCready, L.K.; Khosla, S. In Vivo Assessment of Bone Quality in Postmenopausal Women with Type 2 Diabetes. J. Bone Miner. Res. 2014, 29, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.; DeDe, A.D.; Anagnostis, P.G.; Vryonidou, A.; Morganstein, D.; Goulis, D.G. Type 2 Diabetes and Osteoporosis: A Guide to Optimal Management. J. Clin. Endocrinol. Metab. 2017, 102, 3621–3634. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Pavo, I.; Alam, J.; Disch, D.P.; Schuster, D.; Harris, J.M.; Krege, J.H. Teriparatide in patients with osteoporosis and type 2 diabetes. Bone 2016, 91, 152–158. [Google Scholar] [CrossRef]
- Vagula, M. Osteoporosis: An understated complication of diabetes. US Pharm 2009, 34, 14–16. [Google Scholar]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant Osteoclast Formation and Long-Term Oral Bisphosphonate Therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef]
- Mac-Way, F.; Trombetti, A.; Noël, C.; Lafage-Proust, M.-H. Giant osteoclasts in patients under bisphosphonates. BMC Clin. Pathol. 2014, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Yu, W.; Zhao, H.; Su, J.; Sheng, Q. Skeletal Site-specific Effects of Zoledronate on in vivo Bone Remodeling and in vitro BMSCs Osteogenic Activity. Sci. Rep. 2017, 7, srep36129. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.; Weber, M.; Creutzburg, K.; Möbius, P.; Preidl, R.; Amann, K.; Wehrhan, F. Osteoclast profile of medication-related osteonecrosis of the jaw secondary to bisphosphonate therapy: A comparison with osteoradionecrosis and osteomyelitis. J. Transl. Med. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Ritchlin, C. DC-STAMP: A Key Regulator in Osteoclast Differentiation. J. Cell. Physiol. 2016, 231, 2402–2407. [Google Scholar] [CrossRef] [Green Version]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis—Current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar] [PubMed]
- Zeng, Z.; Zhang, C.; Chen, J. Lentivirus-mediated RNA interference of DC-STAMP expression inhibits the fusion and resorptive activity of human osteoclasts. J. Bone Miner. Metab. 2013, 31, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A.; Jones, S.; Boyde, A.; Foster, D.; Colledge, W.H.; Carlton, M.; Evans, M.; Cox, T. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development 1996, 122, 3151–3162. [Google Scholar] [CrossRef]
- Seeman, E. Bone morphology in response to alendronate as seen by high-resolution computed tomography: Through a glass darkly. J. Bone Miner. Res. 2010, 25, 2553–2557. [Google Scholar] [CrossRef]
- Torres, S.R.; Chen, C.S.; Leroux, B.G.; Lee, P.P.; Hollender, L.G.; Santos, E.C.; Drew, S.P.; Hung, K.-C.; Schubert, M.M. Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Koo, C.-H.; Lee, J.-H. Evaluation of mandibular cortical bone ratio on computed tomography images in patients taking bisphosphonates. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 17. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Serrano, S.; Mariñoso, M.L.; Nacher, M.; Torres, A.; Cuevas, X.; Loreta, J.; Munné, A.; Diez, A. Modulation of osteoblast activity by serum from diabetic and non-diabetic patients on hemodialysis: A three-dimensional culture study. J. Nephrol. 2004, 17, 369–376. [Google Scholar]
- Terada, M.; Inaba, M.; Yano, Y.; Hasuma, T.; Nishizawa, Y.; Morii, H.; Otani, S. Growth-Inhibitory Effect of a High Glucose Concentration on Osteoblast-like Cells. Bone 1998, 22, 17–23. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Stell, C.; Irwin, R.; McCabe, L. Extracellular glucose influences osteoblast differentiation and c-jun expression. J. Cell. Biochem. 2000, 79, 301–310. [Google Scholar] [CrossRef]
- Botolin, S.; McCabe, L.R. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J. Cell. Biochem. 2006, 99, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Wittrant, Y.; Gorin, Y.; Woodruff, K.; Horn, D.; Abboud, H.; Mohan, S.; Abboud-Werner, S. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone 2008, 42, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.-P.; Sheu, S.-Y.; Sun, J.-S.; Chen, M.-H. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE2 synthesis. Phytomedicine 2011, 18, 176–185. [Google Scholar] [CrossRef]
- Singh, D.K.; Winocour, P.; Summerhayes, B.; Viljoen, A.; Sivakumar, G.; Farrington, K. Low serum osteoprotegerin levels in normoalbuminuric type 1 diabetes mellitus. Geol. Rundsch. 2009, 47, 105–110. [Google Scholar] [CrossRef]
- Xu, J.; Yue, F.; Wang, J.; Chen, L.; Qi, W. High glucose inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via downregulation of v-ATPase V0 subunit d2 and dendritic cell-specific transmembrane protein. Mol. Med. Rep. 2014, 11, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Qi, M.; Wang, Y.; Feng, X.; Liu, H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem. Biophys. Res. Commun. 2018, 505, 1195–1202. [Google Scholar] [CrossRef]
- Ishida, N.; Hayashi, K.; Hoshijima, M.; Ogawa, T.; Koga, S.; Miyatake, Y.; Kumegawa, M.; Kimura, T.; Takeya, T. Large Scale Gene Expression Analysis of Osteoclastogenesisin Vitro and Elucidation of NFAT2 as a Key Regulator. J. Biol. Chem. 2002, 277, 41147–41156. [Google Scholar] [CrossRef] [Green Version]
- Yagi, M.; Miyamoto, T.; Toyama, Y.; Suda, T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J. Bone Miner. Metab. 2006, 24, 355–358. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Mensah, K.A.; Schwarz, E.M.; Ju, Y.; Takahata, M.; Feng, C.; McMahon, L.A.; Hicks, D.G.; Panepento, B.; Keng, P.C.; et al. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP). J. Bone Miner. Res. 2011, 27, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.; Kunkel, M.; Weber, A.; Kirkpatrick, C.J. Osteonecrosis of the jaws in patients treated with bisphosphonates-histomorphologic analysis in comparison with infected osteoradionecrosis. J. Oral Pathol. Med. 2006, 35, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Gordon, S.; Benford, H.L.; Coxon, F.P.; Luckman, S.P.; Monkkonen, J.; Frith, J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000, 88, 2961–2978. [Google Scholar] [CrossRef]
- Touaitahuata, H.; Blangy, A.; Vives, V. Modulation of osteoclast differentiation and bone resorption by Rho GTPases. Small GTPases 2014, 5, e28119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsdal, M.A.; Martin, T.J.; Bollerslev, J.; Christiansen, C.; Henriksen, K. Are Nonresorbing Osteoclasts Sources of Bone Anabolic Activity? J. Bone Miner. Res. 2007, 22, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone 2003, 33, 960–969. [Google Scholar] [CrossRef]
- Ma, L.; Oei, L.; Jiang, L.; Estrada, K.; Chen, H.; Wang, Z.; Yu, Q.; Zillikens, M.C.; Gao, X.; Rivadeneira, F. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur. J. Epidemiol. 2012, 27, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Zebaze, R.; Seeman, E. Cortical Bone: A Challenging Geography. J. Bone Miner. Res. 2014, 30, 24–29. [Google Scholar] [CrossRef]




| Experimental Group (T2D + MRONJ) | Control Group (MRONJ) | p Value 1 | |
|---|---|---|---|
| Number of patients | 10 | 10 | - |
| Gender | F | F | - |
| Age | 76.9 ± 4.1 | 77.0 ± 6.5 | >0.05 |
| T2D duration, year | 11.7 ± 5.9 | - | - |
| HbA1c, % | 7.3 ± 0.7 | - | - |
| BP duration, year | 5.4 ± 1.8 | 6.7 ± 2.8 | >0.05 |
| Experimental Group (T2D + MRONJ) | Control Group (MRONJ) | p Value | |
|---|---|---|---|
| Diameter of osteoclasts (μm) | 21.2 ± 1.8 | 23.4 ± 2.7 | >0.05 |
| Nuclearity of osteoclasts (nuclei/osteoclast) | 3.7 ± 0.4 | 3.8 ± 0.6 | >0.05 |
| Osteoclasts per ROI | 42.9 ± 13.2 | 45.7 ± 18.4 | >0.05 |
| Experimental Group (T2D + MRONJ) | Control Group (MRONJ) | p Value | |
|---|---|---|---|
| Cortical bone thickness (mm) | 4.89 ± 1.26 | 3.41 ± 0.69 | <0.05 |
| Cortical bone ratio (%) | 46.13 ± 10.42 | 35.38 ± 4.87 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-M.; Lee, J.-H. Effects of Type 2 Diabetes Mellitus on Osteoclast Differentiation, Activity, and Cortical Bone Formation in POSTmenopausal MRONJ Patients. J. Clin. Med. 2022, 11, 2377. https://doi.org/10.3390/jcm11092377
Park S-M, Lee J-H. Effects of Type 2 Diabetes Mellitus on Osteoclast Differentiation, Activity, and Cortical Bone Formation in POSTmenopausal MRONJ Patients. Journal of Clinical Medicine. 2022; 11(9):2377. https://doi.org/10.3390/jcm11092377
Chicago/Turabian StylePark, Sung-Min, and Jae-Hoon Lee. 2022. "Effects of Type 2 Diabetes Mellitus on Osteoclast Differentiation, Activity, and Cortical Bone Formation in POSTmenopausal MRONJ Patients" Journal of Clinical Medicine 11, no. 9: 2377. https://doi.org/10.3390/jcm11092377






