Optimizing the Outcomes of Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease
Abstract
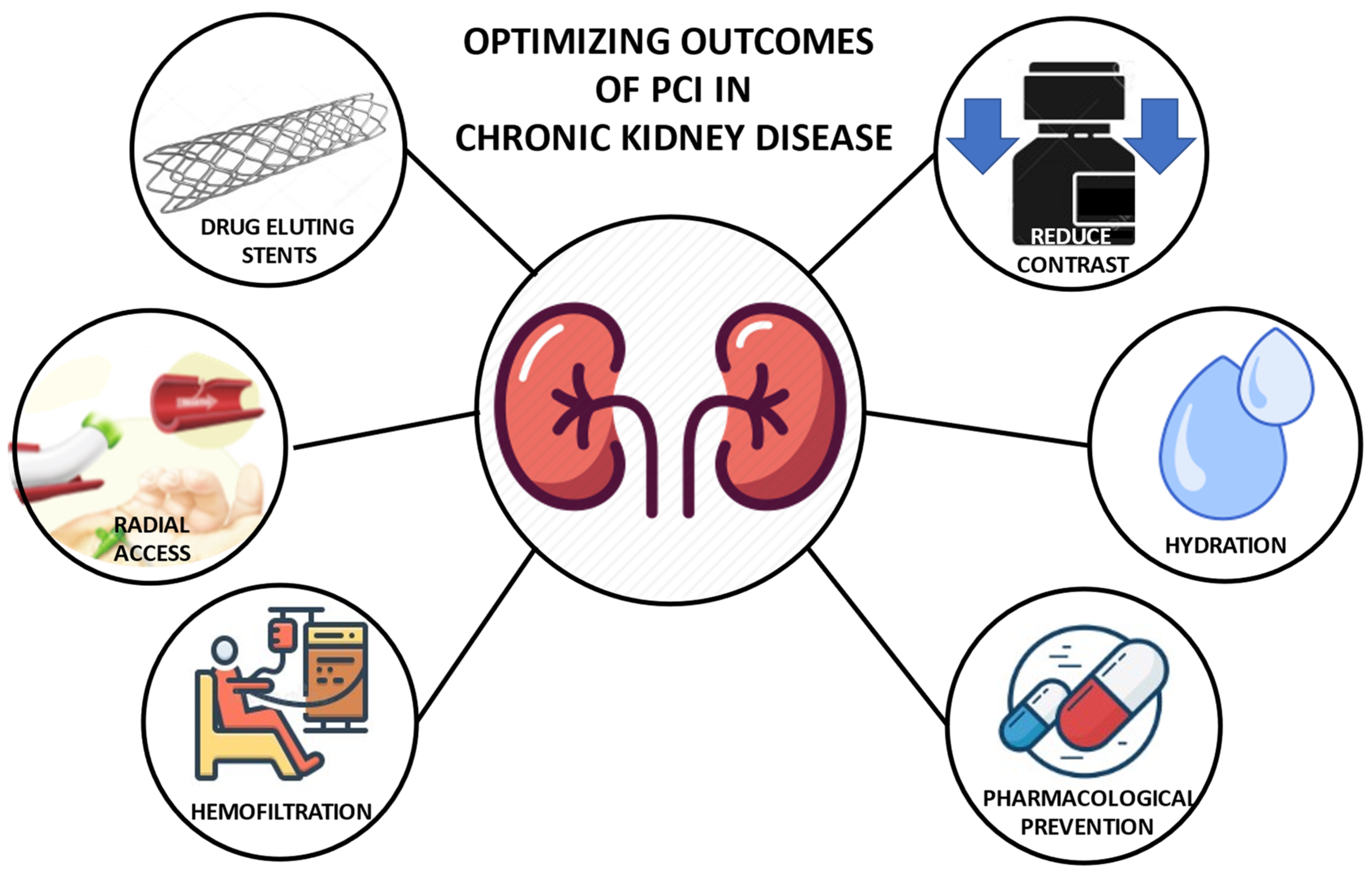
:1. Introduction
2. Contrast-Induced Nephropathy Prophylaxis Strategies
2.1. Fluid Administration
2.2. Pharmacological Prevention
2.3. Renal Replacement Therapies
3. Transradial Artery Access
4. Contrast Dye Reduction for Coronary Angiography and PCI
5. Revascularization Strategy
6. Secondary Prevention Antithrombotic Drug
7. VKA/NOAC for Atrial Fibrillation (AF) in Patients with CKD
8. Optimal Medical Therapy for CKD
8.1. Hypertension Treatment
8.2. Lipid Control
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caracciolo, A.; Mazzone, P.; Laterra, G.; Garcia-Ruiz, V.; Polimeni, A.; Galasso, S.; Saporito, F.; Carerj, S.; D’Ascenzo, F.; Marquis-Gravel, G.; et al. Antithrombotic Therapy for Percutaneous Cardiovascular Interventions: From Coronary Artery Disease to Structural Heart Interventions. J. Clin. Med. 2019, 8, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spertus, J.A.; Jones, P.G.; Maron, D.J.; O’Brien, S.M.; Reynolds, H.; Rosenberg, Y.; Stone, G.W.; Harrell, F.E.; Boden, W.E.; Weintraub, W.S.; et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N. Engl. J. Med. 2020, 382, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, F.; Hoorntje, J.C.; De Boer, M.-J.; Reiffers, S.; Miedema, K.; Ottervanger, J.P.; Hof, A.W.V.T. Long-Term Benefit of Primary Angioplasty as Compared with Thrombolytic Therapy for Acute Myocardial Infarction. N. Engl. J. Med. 2008, 341, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practiceDeveloped by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, G.; Tighiouart, H.; Ibrahim, H.; MacLeod, B.; Salem, D.N.; Griffith, J.L.; Coresh, J.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 2003, 41, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Bangalore, S.; Maron, D.J.; O’Brien, S.M.; Fleg, J.L.; Kretov, E.I.; Briguori, C.; Kaul, U.; Reynolds, H.; Mazurek, T.; Sidhu, M.S.; et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N. Engl. J. Med. 2020, 382, 1608–1618. [Google Scholar] [CrossRef]
- Crimi, G.; Leonardi, S.; Costa, F.; Ariotti, S.; Tebaldi, M.; Biscaglia, S.; Valgimigli, M. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. insights from the all-comer PRODIGY trial. Catheter. Cardiovasc. Interv. 2015, 86, E19–E27. [Google Scholar] [CrossRef]
- McCullough, P.A.; Wolyn, R.; Rocher, L.L.; Levin, R.N.; O’Neill, W.W. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am. J. Med. 1997, 103, 368–375. [Google Scholar] [CrossRef]
- Gruberg, L.; Mintz, G.S.; Mehran, R.; Dangas, G.; Lansky, A.J.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; Leon, M.B. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J. Am. Coll. Cardiol. 2000, 36, 1542–1548. [Google Scholar] [CrossRef] [Green Version]
- Rudnick, M.R.; Goldfarb, S.; Wexler, L.; Ludbrook, P.A.; Murphy, M.J.; Halpern, E.F.; Hill, J.A.; Winniford, M.; Cohen, M.B.; VanFossen, D.B.; et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: A randomized trial. Kidney Int. 1995, 47, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, H.S.; Morcos, S.K. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) Guidelines. Br. J. Radiol. 2003, 76, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Aspelin, P.; Aubry, P.; Fransson, S.-G.; Strasser, R.; Willenbrock, R.; Berg, K.J. Nephrotoxic Effects in High-Risk Patients Undergoing Angiography. N. Engl. J. Med. 2003, 348, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Koreny, M.; Karth, G.D.; Geppert, A.; Neunteufl, T.; Priglinger, U.; Heinz, G.; Siostrzonek, P. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am. J. Med. 2002, 112, 115–119. [Google Scholar] [CrossRef]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.-H.; Youn, T.-J.; Koo, B.-K.; Park, J.-S.; Kang, H.-J.; Cho, Y.-S.; Chung, W.-Y.; Joo, G.-W.; Chae, I.-H.; Choi, D.-J.; et al. Renal Toxicity Evaluation and Comparison Between Visipaque (Iodixanol) and Hexabrix (Ioxaglate) in Patients with Renal Insufficiency Undergoing Coronary Angiography: The RECOVER Study: A Randomized Controlled Trial. J. Am. Coll. Cardiol. 2006, 48, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Laskey, W.K.; Jenkins, C.; Selzer, F.; Marroquin, O.C.; Wilensky, R.L.; Glaser, R.; Holmes, D.R., Jr.; Cohen, H.A. Volume-to-creatinine clearance ratio: A pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Marenzi, G.; Assanelli, E.; Campodonico, J.; Lauri, G.; Marana, I.; De Metrio, M.; Moltrasio, M.; Grazi, M.; Rubino, M.; Veglia, F.; et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann. Intern. Med. 2009, 150, 170–177. [Google Scholar] [CrossRef]
- Solomon, R.J.; Natarajan, M.K.; Doucet, S.; Sharma, S.K.; Staniloae, C.S.; Katholi, R.; Gelormini, J.L.; Labinaz, M.; Moreyra, A.E. Cardiac Angiography in Renally Impaired Patients (CARE) Study. Circulation 2007, 115, 3189–3196. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Maekawa, Y.; Miyata, H.; Inoue, S.; Ishikawa, S.; Sueyoshi, K.; Noma, S.; Kawamura, A.; Kohsaka, S.; Fukuda, K. Impact of periprocedural bleeding on incidence of contrast-induced acute kidney injury in patients treated with percutaneous coronary intervention. J. Am. Coll. Cardiol. 2013, 62, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Solomon, R.; Deray, G. How to prevent contrast-induced nephropathy and manage risk patients: Practical recommendations. Kidney Int. 2006, 69, S51–S53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capodanno, D.; Ministeri, M.; Dipasqua, F.; Dalessandro, V.; Cumbo, S.; Gargiulo, G.; Tamburino, C. Risk prediction of contrast-induced nephropathy by ACEF score in patients undergoing coronary catheterization. J. Cardiovasc. Med. 2016, 17, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.S.; Seth, M.; Kooiman, J.; Share, D. A Novel Tool for Reliable and Accurate Prediction of Renal Complications in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2013, 61, 2242–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.R.; DeVries, J.T.; Piper, W.D.; Robb, J.F.; Hearne, M.J.; Lee, P.M.V.; Kellet, M.A.; Watkins, M.W.; Ryan, T.J.; Silver, M.T.; et al. Serious renal dysfunction after percutaneous coronary interventions can be predicted. Am. Heart J. 2008, 155, 260–266. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Adam, A.; Becker, C.R.; Davidson, C.; Lameire, N.; Stacul, F.; Tumlin, J. Risk Prediction of Contrast-Induced Nephropathy. Am. J. Cardiol. 2006, 98, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ronco, F.; Azzalini, L.; Briguori, C.; Cosmai, L.; D’Amico, M.; Di Luca, M.; Esposito, G.; Granatelli, A.; Maddestra, N.; De Marco, F.; et al. Documento di consenso SICI-GISE/SIN: Danno renale acuto da mezzo di contrasto in cardiologia interventistica. G Ital. Cardiol. 2019, 20, 29–43. [Google Scholar]
- Does Hydration Prevent Radiocontrast-Induced Acute Renal Failure? Nephrol. Dial. Transplant. 1999, 14, 1064–1066. Available online: https://academic.oup.com/ndt/article/14/5/1064/1816200 (accessed on 28 February 2022). [CrossRef] [Green Version]
- Members, T.F.; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.-P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.W.; Head, S.J.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [CrossRef]
- Bader, B.; Berger, E.; Heede, M.; Silberbaur, I.; Duda, S.; Risler, T.; Erley, C. What is the best hydration regimen to prevent contrast media-induced nephrotoxicity? Clin. Nephrol. 2004, 62, 1–7. [Google Scholar] [CrossRef]
- Trivedi, H.S.; Moore, H.; Nasr, S.; Aggarwal, K.; Agrawal, A.; Goel, P.; Hewett, J. A Randomized Prospective Trial to Assess the Role of Saline Hydration on the Development of Contrast Nephrotoxicity. Nephron Clin. Pract. 2003, 93, c29–c34. [Google Scholar] [CrossRef]
- Hiremath, S.; Akbari, A.; Shabana, W.; Fergusson, D.; Knoll, G.A. Prevention of Contrast-Induced Acute Kidney Injury: Is Simple Oral Hydration Similar To Intravenous? A Systematic Review of the Evidence. PLoS ONE 2013, 8, e60009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, C.; Buerkle, G.; Buettner, H.J.; Petersen, J.; Perruchoud, A.P.; Eriksson, U.; Marsch, S.; Roskamm, H. Prevention of Contrast Media–Associated Nephropathy: Randomized Comparison of 2 Hydration Regimens in 1620 Patients Undergoing Coronary Angioplasty. Arch. Intern. Med. 2002, 162, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Chen, S.; Chen, J.; Tan, N.; Zhou, Y.; Liu, Y.; Ye, P.; Ran, P.; Duan, C.; et al. Excessively High Hydration Volume May Not Be Associated With Decreased Risk of Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention in Patients With Renal Insufficiency. J. Am. Heart Assoc. 2016, 5, e003171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, R.; Werner, C.; Mann, D.; D’Elia, J.; Silva, P. Effects of Saline, Mannitol, and Furosemide on Acute Decreases in Renal Function Induced by Radiocontrast Agents. N. Engl. J. Med. 1994, 331, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.A.; McCullough, P.A.; Tobin, K.J.; Speck, J.P.; Westveer, D.C.; Guido-Allen, D.A.; Timmis, G.C.; O’Neill, W.W. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: Results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J. Am. Coll. Cardiol. 1999, 33, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II). Circulation 2011, 124, 1260–1269. Available online: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.111.030759?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 1 March 2022). [CrossRef] [PubMed]
- Prevention of Contrast Nephropathy by Furosemide With Matched Hydration: The MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) Trial. JACC Cardiovasc. Interv. 2012, 5, 92–97. Available online: https://www.sciencedirect.com/science/article/pii/S1936879811007874?via%3Dihub (accessed on 1 March 2022).
- Prevention of Contrast-Induced Acute Kidney Injury by Furosemide With Matched Hydration in Patients Undergoing Interventional Procedures: A Systematic Review and Meta-Analysis of Randomized Trials. JACC Cardiovasc. Interv. 2017, 10, 355–363. Available online: https://www.sciencedirect.com/science/article/pii/S1936879816319823?via%3Dihub (accessed on 1 March 2022). [CrossRef]
- Briguori, C.; Marenzi, G. Contrast-induced nephropathy: Pharmacological prophylaxis. Kidney Int. 2006, 69, S30–S38. [Google Scholar] [CrossRef] [Green Version]
- Tepel, M.; van der Giet, M.; Schwarzfeld, C.; Laufer, U.; Liermann, D.; Zidek, W. Prevention of Radiographic-Contrast-Agent–Induced Reductions in Renal Function by Acetylcysteine. N. Engl. J. Med. 2000, 343, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Acetylcysteine Protects against Acute Renal Damage in Patients with Abnormal Renal Function Undergoing a Coronary Procedure. J. Am. Coll. Cardiol. 2002, 40, 1383–1388. Available online: https://www.sciencedirect.com/science/article/pii/S0735109702023082?via%3Dihub (accessed on 1 March 2022). [CrossRef] [Green Version]
- Acetylcysteine for Prevention of Acute Deterioration of Renal Function Following Elective Coronary Angiography and Intervention: A Randomized Controlled Trial. Clinical Pharmacy and Pharmacology. JAMA 2003, 289, 553–558. Available online: https://jamanetwork.com/journals/jama/fullarticle/195894 (accessed on 1 March 2022).
- Briguori, C.; Manganelli, F.; Scarpato, P.; Elia, P.P.; Golia, B.; Riviezzo, G.; Lepore, S.; Librera, M.; Villari, B.; Colombo, A.; et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J. Am. Coll. Cardiol. 2002, 40, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Kshirsagar, A.V.; Poole, C.; Mottl, A.; Shoham, D.; Franceschini, N.; Tudor, G.; Agrawal, M.; Denu-Ciocca, C.; Ohman, E.M.; Finn, W.F. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: A meta-analysis of prospective controlled trials. J. Am. Soc. Nephrol. 2004, 15, 761–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isenbarger, D.W.; Kent, S.M.; O’Malley, P.G. Meta-analysis of randomized clinical trials on the usefulness of acetylcysteine for prevention of contrast nephropathy. Am. J. Cardiol. 2003, 92, 1454–1458. [Google Scholar] [CrossRef]
- Alonso, A.; Lau, J.; Jaber, B.L.; Weintraub, A.; Sarnak, M.J. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: A meta-analysis of randomized, controlled trials. Am. J. Kidney Dis. 2004, 43, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pannu, N.; Manns, B.; Lee, H.; Tonelli, M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004, 65, 1366–1374. [Google Scholar] [CrossRef] [Green Version]
- Guru, V.; Fremes, S.E. The role of N-acetylcysteine in preventing radiographic contrast-induced nephropathy. Clin. Nephrol. 2004, 62, 77–83. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Ghali, W.A. Acetylcysteine for prevention of contrast-induced nephropathy after intravascular angiography: A systematic review and meta-analysis. BMC Med. 2004, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Misra, D.; Leibowitz, K.; Gowda, R.M.; Shapiro, M.; Khan, I.A. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: A meta-analysis. Clin. Cardiol. 2004, 27, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Nallamothu, B.K.; Shojania, K.G.; Saint, S.; Hofer, T.; Humes, H.D.; Moscucci, M.; Bates, E.R. Is acetylcysteine effective in preventing contrast-related nephropathy? A meta-analysis. Am. J. Med. 2004, 117, 938–947. [Google Scholar] [CrossRef]
- Duong, M.H.; MacKenzie, T.A.; Malenka, D.J. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: Comprehensive meta-analysis. Catheter. Cardiovasc. Interv. 2005, 64, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Dwamena, B.; Cronin, P.; Bernstein, S.J.; Carlos, R.C. Meta-analysis: Effectiveness of Drugs for Preventing Contrast-Induced Nephropathy. Ann. Intern. Med. 2008, 148, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.S.R.; Wragg, A.; Kumar, S.; De Palma, R.; Baker, L.R.I.; Knight, C.J. A rapid protocol for the prevention of contrast-induced renal dysfunction: The RAPPID study. J. Am. Coll. Cardiol. 2003, 41, 2114–2118. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, H.; Daram, S.; Szabo, A.; Bartorelli, A.L.; Marenzi, G. High-dose N-acetylcysteine for the Prevention of Contrast-induced Nephropathy. Am. J. Med. 2009, 122, 874.e9–874.e15. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Assanelli, E.; Marana, I.; Lauri, G.; Campodonico, J.; Grazi, M.; de Metrio, M.; Galli, S.; Fabbiocchi, F.; Montorsi, P.; et al. N-Acetylcysteine and Contrast-Induced Nephropathy in Primary Angioplasty. N. Engl. J. Med. 2006, 354, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Arstall, M.A.; Yang, J.; Stafford, I.; Betts, W.H.; Horowitz, J.D. N-Acetylcysteine in Combination With Nitroglycerin and Streptokinase for the Treatment of Evolving Acute Myocardial Infarction. Circulation 1995, 92, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Šochman, J.; Kolc, J.; Vrána, M.; Fabián, J. Cardioprotective effects of N-acetylcysteine: The reduction in the extent of infarction and occurrence of reperfusion arrhythmias in the dog. Int. J. Cardiol. 1990, 28, 191–196. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Massucco, P.; Mattiello, L.; Cavalot, F.; Trovati, M. N-Acetyl-L-Cysteine Exerts Direct Anti-Aggregating Effect on Human Platelets—Anfossi. Eur. J. Clin. Investig. 2001, 314, 452–461. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2362.2001.00815.x (accessed on 3 March 2022). [CrossRef]
- Recio-Mayoral, A.; Chaparro, M.; Prado, B.; Cózar, R.; Méndez, I.; Banerjee, D.; Kaski, J.C.; Cubero, J.; Cruz, J.M. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: The RENO Study. J. Am. Coll. Cardiol. 2007, 49, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Stacul, F.; Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR); van der Molen, A.J.; Reimer, P.; Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.-F.; et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur. Radiol. 2011, 21, 2527–2541. [Google Scholar] [CrossRef]
- Huber, W.; Huber, T.; Baum, S.; Franzen, M.; Schmidt, C.; Stadlbauer, T.; Beitz, A.; Schmid, R.M.; Schmid, S. Sodium Bicarbonate Prevents Contrast-Induced Nephropathy in Addition to Theophylline: A Randomized Controlled Trial. Medicine 2016, 95, e3720. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, J.; Sijpkens, Y.W.J.; Van Buren, M.; Groeneveld, J.H.M.; Ramai, S.R.S.; Van Der Molen, A.J.; Aarts, N.J.M.; Van Rooden, C.J.; Cannegieter, S.C.; Putter, H.; et al. Randomised trial of no hydration vs. sodium bicarbonate hydration in patients with chronic kidney disease undergoing acute computed tomography-pulmonary angiography. J. Thromb. Haemost. 2014, 12, 1658–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodium Bicarbonate for the Prevention of Contrast Induced-Acute Kidney Injury: A Systematic Review and Meta-Analysis. Am. Soc. Nephrol. 2009, 10, 1584–1592. Available online: https://cjasn.asnjournals.org/content/4/10/1584 (accessed on 3 March 2022).
- Weisbord, S.D.; Gallagher, M.; Jneid, H.; Garcia, S.; Cass, A.; Thwin, S.-S.; Conner, T.A.; Chertow, G.M.; Bhatt, D.L.; Shunk, K.; et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N. Engl. J. Med. 2018, 378, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Margaritis, M.; Lee, R.; Channon, K.; Antoniades, C. Statins as anti-inflammatory agents in atherogenesis: Molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 2012, 18, 1519–1530. [Google Scholar] [CrossRef] [Green Version]
- Leoncini, M.; Toso, A.; Maioli, M.; Tropeano, F.; Villani, S.; Bellandi, F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). J. Am. Coll. Cardiol. 2014, 63, 71–79. [Google Scholar] [CrossRef]
- Giacoppo, D.; Gargiulo, G.; Buccheri, S.; Aruta, P.; Byrne, R.A.; Cassese, S.; Dangas, G.; Kastrati, A.; Mehran, R.; Tamburino, C.; et al. Preventive Strategies for Contrast-Induced Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Procedures: Evidence From a Hierarchical Bayesian Network Meta-Analysis of 124 Trials and 28,240 Patients. Circ. Cardiovasc. Interv. 2017, 10, e004383. [Google Scholar] [CrossRef]
- Lai, K.C.; Lam, S.K.; Chu, K.M.; Wong, B.C.Y.; Hui, W.M.; Hu, W.H.C.; Lau, G.K.K.; Wong, W.M.; Yuen, M.F.; Chan, A.O.O.; et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N. Engl. J. Med. 2002, 346, 2033–2038. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.H.; LePendu, P.; Bauer-Mehren, A.; Ghebremariam, Y.T.; Iyer, S.V.; Marcus, J.; Nead, K.T.; Cooke, J.; Leeper, N.J. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS ONE 2015, 10, e0124653. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, M.L.; Braunwald, E.; Antman, E.M.; A Murphy, S.; Bates, E.R.; Rozenman, Y.; Michelson, A.D.; Hautvast, R.W.; Lee, P.N.V.; Close, S.L.; et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: An analysis of two randomised trials. Lancet Lond. Engl. 2009, 374, 989–997. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Cryer, B.L.; Contant, C.F.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; Lapuerta, P.; Goldsmith, M.A.; Laine, L.; et al. Clopidogrel with or without Omeprazole in Coronary Artery Disease. N. Engl. J. Med. 2010, 363, 1909–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS. Eur. Heart J. 2018, 53, 34–78. Available online: https://academic.oup.com/eurheartj/article/39/3/213/4095043?login=false (accessed on 12 April 2022).
- Moon, S.S.; Bäck, S.E.; Kurkus, J.; Nilsson-Ehle, P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron 1995, 70, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, T.; Keller, E.; Gondolf, K.; Ffner, T.S.; Dt, H.P.; Schollmeyer, P. Effect of haemodialysis after contrast medium administration in patients with renal insufficiency. Nephrol. Dial. Transplant. 1998, 13, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterner, G.; Frennby, B.; Kurkus, J.; Nyman, U. Does Post-angiographic Hemodialysis Reduce the Risk of Contrast-medium Nephropathy? Scand. J. Urol. Nephrol. 2000, 34, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.; Ferrari, P.; Schönholzer, C.; Marti, H.-P.; Mohaupt, M.; Wiederkehr, M.; Cereghetti, C.; Serra, A.; Huynh-Do, U.; Uehlinger, D.; et al. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am. J. Med. 2001, 111, 692–698. [Google Scholar] [CrossRef]
- Marenzi, G.; Bartorelli, A.L. Recent advances in the prevention of radiocontrast-induced nephropathy. Curr. Opin. Crit. Care 2004, 10, 505–509. [Google Scholar] [CrossRef]
- Marenzi, G.; Lauri, G.; Campodonico, J.; Marana, I.; Assanelli, E.; De Metrio, M.; Grazi, M.; Veglia, F.; Fabbiocchi, F.; Montorsi, P.; et al. Comparison of Two Hemofiltration Protocols for Prevention of Contrast-induced Nephropathy in High-risk Patients. Am. J. Med. 2006, 119, 155–162. [Google Scholar] [CrossRef]
- Scalise, R.F.M.; Salito, A.M.; Polimeni, A.; Garcia-Ruiz, V.; Virga, V.; Frigione, P.; Andò, G.; Tumscitz, C.; Costa, F. Radial Artery Access for Percutaneous Cardiovascular Interventions: Contemporary Insights and Novel Approaches. J. Clin. Med. 2019, 8, 1727. [Google Scholar] [CrossRef] [Green Version]
- Acute kidney Injury after Percutaneous Coronary Intervention: Rationale of the AKI-MATRIX (Acute Kidney Injury-Minimizing Adverse Hemorrhagic Events by TRansradial Access Site and Systemic Implementation of angiox) Sub-Study. Catheter. Cardiovasc. Interv. 2015, 86, 950–987. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ccd.25932 (accessed on 3 March 2022). [CrossRef]
- Steinvil, A.; Garcia-Garcia, H.M.; Rogers, T.; Koifman, E.; Buchanan, K.; Alraies, M.C.; Torguson, R.; Pichard, A.D.; Satler, L.F.; Ben-Dor, I.; et al. Comparison of Propensity Score–Matched Analysis of Acute Kidney Injury After Percutaneous Coronary Intervention With Transradial Versus Transfemoral Approaches. Am. J. Cardiol. 2017, 119, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Andò, G.; Costa, F.; Boretti, I.; Trio, O.; Valgimigli, M. Benefit of radial approach in reducing the incidence of acute kidney injury after percutaneous coronary intervention: A meta-analysis of 22,108 patients. Int. J. Cardiol. 2015, 179, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Andò, G.; Costa, F.; Trio, O.; Oreto, G.; Valgimigli, M. Impact of vascular access on acute kidney injury after percutaneous coronary intervention. Cardiovasc. Revascularization Med. Mol. Interv. 2016, 17, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Gagnor, A.; Calabrò, P.; Frigoli, E.; Leonardi, S.; Mazzarotto, P.; Rubartelli, P.; Briguori, C.; Andò, G.; Repetto, A.; et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015, 385, 2465–2476. [Google Scholar] [CrossRef]
- Vuurmans, T.; Byrne, J.; Fretz, E.; Janssen, C.; Hilton, J.D.; Klinke, W.P.; Djurdjev, O.; Levin, A. Chronic kidney injury in patients after cardiac catheterisation or percutaneous coronary intervention: A comparison of radial and femoral approaches (from the British Columbia Cardiac and Renal Registries). Heart Br. Card Soc. 2010, 96, 1538–1542. [Google Scholar] [CrossRef] [Green Version]
- Scolari, F.; Ravani, P.; Gaggi, R.; Santostefano, M.; Rollino, C.; Stabellini, N.; Colla, L.; Viola, B.F.; Maiorca, P.; Venturelli, C.; et al. The challenge of diagnosing atheroembolic renal disease: Clinical features and prognostic factors. Circulation 2007, 116, 298–304. [Google Scholar] [CrossRef]
- Azzalini, L.; Jolicoeur, E.M. The use of radial access decreases the risk of vascular access-site-related complications at a patient level but is associated with an increased risk at a population level: The radial paradox. EuroIntervention 2014, 10, 531–532. [Google Scholar] [CrossRef]
- Damluji, A.; Cohen, M.G.; Smairat, R.; Steckbeck, R.; Moscucci, M.; Gilchrist, I.C. The incidence of acute kidney injury after cardiac catheterization or PCI: A comparison of radial vs. femoral approach. Int. J. Cardiol. 2014, 173, 595–597. [Google Scholar] [CrossRef]
- Gili, S.; D’Ascenzo, F.; Di Summa, R.; Conrotto, F.; Cerrato, E.; Chieffo, A.; Boccuzzi, G.; Montefusco, A.; Ugo, F.; Omedé, P.; et al. Radial Versus Femoral Access for the Treatment of Left Main Lesion in the Era of Second-Generation Drug-Eluting Stents. Am. J. Cardiol. 2017, 120, 33–39. [Google Scholar] [CrossRef]
- Valgimigli, M.; Frigoli, E.; Leonardi, S.; Vranckx, P.; Rothenbühler, M.; Tebaldi, M.; Varbella, F.; Calabrò, P.; Garducci, S.; Rubartelli, P.; et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): Final 1-year results of a multicentre, randomised controlled trial. Lancet 2018, 392, 835–848. [Google Scholar] [CrossRef]
- Andò, G.; Cortese, B.; Russo, F.; Rothenbühler, M.; Frigoli, E.; Gargiulo, G.; Briguori, C.; Vranckx, P.; Leonardi, S.; Guiducci, V.; et al. Acute Kidney Injury After Radial or Femoral Access for Invasive Acute Coronary Syndrome Management: AKI-MATRIX. J. Am. Coll. Cardiol. 2017, 69, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Rothenbühler, M.; Valgimigli, M.; Odutayo, A.; Frigoli, E.; Leonardi, S.; Vranckx, P.; Turturo, M.; Moretti, L.; Amico, F.; Uguccioni, L.; et al. Association of acute kidney injury and bleeding events with mortality after radial or femoral access in patients with acute coronary syndrome undergoing invasive management: Secondary analysis of a randomized clinical trial. Eur. Heart J. 2019, 40, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, W.; Yu, M.; Yang, P. Comparison of acute kidney injury with radial vs. femoral access for patients undergoing coronary catheterization: An updated meta-analysis of 46,816 patients. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Akindavyi, G.; Fu, Q.; Li, Z.L.; Wang, H.M.; Wen, L.H. Research Progress on the Relationship between Coronary Artery Calcification and Chronic Renal Failure. Chin. Med. J. Engl. 2018, 131, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, N.-H.; Shin, I.S.; Noh, D.H.; Kim, Y.C.; Kim, S.H.; Choi, J.H.; Park, E.M.; Lee, S.J.; Yun, K.H.; et al. Alteration of Ventricular Repolarization by Intracoronary Infusion of Normal Saline in Patients With Variant Angina. Korean Circ. J. 2009, 39, 223–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacha, J.; Gierlotka, M.; Feusette, P.; Dudek, D. Ultra-low contrast coronary angiography and zero-contrast percutaneous coronary intervention for prevention of contrast-induced nephropathy: Step-by-step approach and review. Adv. Interv. Cardiol. 2019, 15, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.R.; Mehta, H.S.; Price, M.J.; Russo, R.J.; Stinis, C.T.; Moses, J.W.; Mehran, R.; Leon, M.B.; Kandzari, D.E.; Teirstein, P.S. A novel technique for ultra-low contrast administration during angiography or intervention. Catheter. Cardiovasc. Interv. 2010, 75, 1076–1083. [Google Scholar] [CrossRef]
- Imaging—And Physiology-Guided Percutaneous Coronary Intervention without Contrast Administration in Advanced Renal Failure: A Feasibility, Safety, and Outcome Study. Eur. Heart J. 2016, 37, 3090–3095. Available online: https://academic.oup.com/eurheartj/article/37/40/3090/2420804?login=false (accessed on 3 March 2022). [CrossRef]
- Karimi Galougahi, K.; Mintz, G.S.; Karmpaliotis, D.; Ali, Z.A. Zero-contrast percutaneous coronary intervention on calcified lesions facilitated by rotational atherectomy. Catheter. Cardiovasc. Interv. 2017, 90, E85–E89. [Google Scholar] [CrossRef]
- Hruska, K.; Mathew, S.; Lund, R.; Fang, Y.; Sugatani, T. Cardiovascular risk factors in chronic kidney disease: Does phosphate qualify? Kidney Int. Suppl. 2011, 79, S9–S13. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary artery calcification: Pathogenesis and prognostic implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chonchol, M.; Whittle, J.; Desbien, A.; Orner, M.B.; Petersen, L.A.; Kressin, N.R. Chronic kidney disease is associated with angiographic coronary artery disease. Am. J. Nephrol. 2008, 28, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Coskun, U.; Kilickesmez, K.O.; Abaci, O.; Kocas, C.; Bostan, C.; Yildiz, A.; Baskurt, M.; Arat, A.; Ersanli, M.K.; Gurmen, T. The relationship between chronic kidney disease and SYNTAX score. Angiology 2011, 62, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Ma, J.Z.; Collins, A.J. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N. Engl. J. Med. 1998, 339, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anavekar, N.S.; McMurray, J.J.V.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.-L.; White, H.D.; Nordlander, R.; Maggioni, A.P.; Dickstein, K.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Lundman, P.; Jacobson, S.H.; Schön, S.; Lindbäck, J.; Stenestrand, U.; Wallentin, L.; Jernberg, T. Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: Data from the Swedeheart register. J. Intern. Med. 2010, 268, 40–49. [Google Scholar] [CrossRef]
- Ezekowitz, J.; McAlister, F.A.; Humphries, K.H.; Norris, C.; Tonelli, M.; Ghali, W.A.; Knudtson, M.L. The association among renal insufficiency, pharmacotherapy, and outcomes in 6427 patients with heart failure and coronary artery disease. J. Am. Coll. Cardiol. 2004, 44, 1587–1592. [Google Scholar] [CrossRef] [Green Version]
- Konstantinidis, I.; Patel, S.; Camargo, M.; Patel, A.; Poojary, P.; Coca, S.G.; Nadkarni, G.N. Repres.sentation and reporting of kidney disease in cerebrovascular disease: A systematic review of randomized controlled trials. PLoS ONE 2017, 12, e0176145. [Google Scholar] [CrossRef]
- Baber, U.; Stone, G.W.; Weisz, G.; Moreno, P.; Dangas, G.; Maehara, A.; Mintz, G.S.; Cristea, E.; Fahy, M.; Xu, K.; et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc. Imaging 2012, 5, S53–S61. [Google Scholar] [CrossRef] [Green Version]
- Park, D.-W.; Kim, Y.-H.; Yun, S.-C.; Ahn, J.-M.; Lee, J.-Y.; Kim, W.-J.; Kang, S.-J.; Lee, S.-W.; Lee, C.W.; Park, S.-W. Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur. Heart J. 2013, 34, 1662–1669. [Google Scholar] [CrossRef] [Green Version]
- Zeitouni, M.; Silvain, J.; Guedeney, P.; Kerneis, M.; Yan, Y.; Overtchouk, P.; Barthelemy, O.; Hauguel-Moreau, M.; Choussat, R.; Helft, G.; et al. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur. Heart J. 2018, 39, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Patialiakas, A.; Thury, A.; McFadden, E.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I.; Tondi, S.; Roffi, M.; et al. Zotarolimus-Eluting Versus Bare-Metal Stents in Uncertain Drug-Eluting Stent Candidates. J. Am. Coll. Cardiol. 2015, 65, 805–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariotti, S.; Adamo, M.; Costa, F.; Patialiakas, A.; Briguori, C.; Thury, A.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I.; et al. Is Bare-Metal Stent Implantation Still Justifiable in High Bleeding Risk Patients Undergoing Percutaneous Coronary Intervention? A Pre-Specified Analysis from the ZEUS Trial. JACC Cardiovasc. Interv. 2016, 9, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Crimi, G.; Leonardi, S.; Costa, F.; Adamo, M.; Ariotti, S.; Valgimigli, M. Role of stent type and of duration of dual antiplatelet therapy in patients with chronic kidney disease undergoing percutaneous coronary interventions. Is bare metal stent implantation still a justifiable choice? A post-hoc analysis of the all comer PRODIGY trial. Int. J. Cardiol. 2016, 212, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Xu, J.; Hannan, E.L. Revascularization in Patients With Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J. Am. Coll. Cardiol. 2015, 66, 1209–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Giustino, G.; Costa, F. Characterization of the Individual Patient Risk After Percutaneous Coronary Intervention: At the Crossroads of Bleeding and Thrombosis. JACC Cardiovasc. Interv. 2019, 12, 831–834. [Google Scholar] [CrossRef]
- Matsuo, T.; Koide, M.; Kario, K.; Suzuki, S.; Matsuo, M. Extrinsic Coagulation Factors and Tissue Factor Pathway Inhibitor in End-Stage Chronic Renal Failure. Pathophysiol. Haemost. Thromb. 1997, 27, 163–167. [Google Scholar] [CrossRef]
- Landray, M.J.; Wheeler, D.C.; Lip, G.Y.; Newman, D.J.; Blann, A.D.; McGlynn, F.J.; Ball, S.; Townend, J.; Baigent, C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: The chronic renal impairment in Birmingham (CRIB) study. Am. J. Kidney Dis. 2004, 43, 244–253. [Google Scholar] [CrossRef]
- Eknoyan, G., III; Brown, I.C.H. Biochemical Abnormallities of Platelets in Renal Failure. Am. J. Nephrol. 1981, 1, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Boccardo, P.; Galbusera, M.; Monteagudo, J.; De Marco, L.; Remuzzi, G.; Ruggeri, Z.M. Reversible Activation Defect of the Platelet Glycoprotein IIb-IIIa Complex in Patients With Uremia. Am. J. Kidney Dis. 1993, 22, 668–676. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, W.; Park, C.S.; Kang, W.Y.; Hwang, S.H.; Kim, W. A Comparison of Clopidogrel Responsiveness in Patients With Versus Without Chronic Renal Failure. Am. J. Cardiol. 2009, 104, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; James, S.K.; Lakic, T.G.; Budaj, A.J.; Cornel, J.H.; Katus, H.A.; Keltai, M.; Kontny, F.; Lewis, B.S.; Storey, R.F.; et al. Impact of Diabetes Mellitus and Chronic Kidney Disease on Cardiovascular Outcomes and Platelet P2Y12 Receptor Antagonist Effects in Patients With Acute Coronary Syndromes: Insights From the PLATO Trial. J. Am. Heart Assoc. 2019, 8, e011139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baber, U.; Mehran, R.; Kirtane, A.J.; Gurbel, P.A.; Christodoulidis, G.; Maehara, A.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Metzger, D.C.; et al. Prevalence and Impact of High Platelet Reactivity in Chronic Kidney Disease. Circ. Cardiovasc. Interv. 2015, 8, e001683. [Google Scholar] [CrossRef] [Green Version]
- Rollini, F.; Cho, J.R.; DeGroat, C.; Bhatti, M.; Alobaidi, Z.; Ferrante, E.; Jakubowski, J.A.; Sugidachi, A.; Zenni, M.M.; Bass, T.A.; et al. Impact of chronic kidney disease on platelet P2Y12 receptor signalling in patients with type 2 diabetes mellitus. Thromb. Haemost. 2017, 117, 201–203. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.-H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; ten Berg, J.; et al. Consensus and Update on the Definition of On-Treatment Platelet Reactivity to Adenosine Diphosphate Associated With Ischemia and Bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef]
- Morel, O.; El Ghannudi, S.; Jesel, L.; Radulescu, B.; Meyer, N.; Wiesel, M.-L.; Caillard, S.; Campia, U.; Moulin, B.; Gachet, C.; et al. Cardiovascular Mortality in Chronic Kidney Disease Patients Undergoing Percutaneous Coronary Intervention Is Mainly Related to Impaired P2Y12 Inhibition by Clopidogrel. J. Am. Coll. Cardiol. 2011, 57, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Best, P.J.; Steinhubl, S.R.; Berger, P.B.; Dasgupta, A.; Brennan, D.M.; Szczech, L.A.; Califf, R.M.; Topol, E. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: Results from the Clopidogrel for the Reduction of Events During Observation (CREDO) Trial. Am. Heart J. 2008, 155, 687–693. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Li, Y.; Tang, Y.; Li, C.; Li, J.; Han, Y. Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br. J. Clin. Pharmacol. 2018, 84, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Small, D.S.; Wrishko, R.E.; Ii, C.S.E.; Ni, L.; Winters, K.J.; Farid, N.A.; Li, Y.G.; Brandt, J.T.; Salazar, D.E.; Borel, A.G.; et al. Prasugrel pharmacokinetics and pharmacodynamics in subjects with moderate renal impairment and end-stage renal disease. J. Clin. Pharm. Ther. 2009, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Ariyoshi, N.; Nakayama, T.; Fujimoto, Y.; Sugimoto, K.; Wakabayashi, S.; Hanaoka, H.; Kobayashi, Y. Impact of chronic kidney disease on platelet inhibition of clopidogrel and prasugrel in Japanese patients. J. Cardiol. 2017, 69, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, C.P.; A Harrington, R.; James, S.; Ardissino, D.; Becker, R.C.; Emanuelsson, H.; Husted, S.; Katus, H.; Keltai, M.; Khurmi, N.S.; et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): A randomised double-blind study. Lancet 2010, 375, 283–293. [Google Scholar] [CrossRef]
- Edfors, R.; Sahlén, A.; Szummer, K.; Renlund, H.; Evans, M.; Carrero, J.-J.; Spaak, J.; James, S.K.; Lagerqvist, B.; Varenhorst, C.; et al. Outcomes in patients treated with ticagrelor versus clopidogrel after acute myocardial infarction stratified by renal function. Heart 2018, 104, 1575–1582. [Google Scholar] [CrossRef]
- Melloni, C.; Cornel, J.; Hafley, G.; Neely, M.L.; Clemmensen, P.; Zamoryakhin, D.; Prabhakaran, D.; White, H.D.; Fox, K.; Ohman, E.M.; et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: Insights from the TRILOGY ACS Trial. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 443–454. [Google Scholar] [CrossRef]
- Collet, J.-P.; Roffi, M.; Byrne, R.A.; Costa, F.; Valgimigli, M.; Bueno, H.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; et al. Case-based implementation of the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease. Eur. Heart J. 2018, 39, e1–e33. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Adamo, M.; Ariotti, S.; Ferrante, G.; Navarese, E.P.; Leonardi, S.; Garcia-Garcia, H.; Vranckx, P.; Valgimigli, M. Left main or proximal left anterior descending coronary artery disease location identifies high-risk patients deriving potentially greater benefit from prolonged dual antiplatelet therapy duration. EuroIntervention 2016, 11, e1222–e1230. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Gargiulo, G.; Santucci, A.; Piccolo, R.; Franzone, A.; Ariotti, S.; Baldo, A.; Esposito, G.; Moschovitis, A.; Windecker, S.; Valgimigli, M. Impact of chronic kidney disease on 2-year clinical outcomes in patients treated with 6-month or 24-month DAPT duration: An analysis from the PRODIGY trial. Catheter. Cardiovasc. Interv. 2017, 90, E73–E84. [Google Scholar] [CrossRef]
- Costa, F.; Van Klaveren, D.; Feres, F.; James, S.; Räber, L.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; Steg, P.G.; et al. Dual Antiplatelet Therapy Duration Based on Ischemic and Bleeding Risks After Coronary Stenting. J. Am. Coll. Cardiol. 2019, 73, 741–754. [Google Scholar] [CrossRef]
- Gargiulo, G.; Costa, F.; Ariotti, S.; Biscaglia, S.; Campo, G.; Esposito, G.; Leonardi, S.; Vranckx, P.; Windecker, S.; Valgimigli, M. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am. Heart J. 2016, 174, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Brand, E.; Mesters, R.; Schäbitz, W.-R.; Fisher, M.; Pavenstädt, H.; Breithardt, G. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andò, G.; Costa, F. Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials. Int. J. Cardiol. 2020, 302, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Valgimigli, M.; Steg, P.G.; Bhatt, D.L.; Hohnloser, S.H.; Ten Berg, J.M.; Miede, C.; Nordaby, M.; Lip, G.Y.; Oldgren, J.; et al. Antithrombotic Therapy according to Baseline Bleeding Risk in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: Applying the PRECISE-DAPT Score in RE-DUAL PCI. Eur. Heart J. 2020. Available online: https://academic.oup.com/ehjcvp/advance-article/doi/10.1093/ehjcvp/pvaa135/6015238 (accessed on 16 April 2022).
- Hijazi, Z.; Alexander, J.H.; Li, Z.; Wojdyla, D.M.; Mehran, R.; Granger, C.B.; Parkhomenko, A.; Bahit, M.C.; Windecker, S.; Aronson, R.; et al. Apixaban or Vitam.min K Antagonists and Aspirin or Placebo according to Kidney Function in Patients with Atrial Fibrillation after Acute Coronary Syndrome or Percutaneous Coronary Intervention: Insights from the AUGUSTUS Trial. Circulation. 2021, 143, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Steg, P.G.; Oldgren, J.; Nickenig, G.; Kiss, R.G.; Ongen, Z.; Estrada, J.L.N.; Ophuis, T.O.; Lip, G.Y.; Nordaby, M.; et al. Renal Function and Outcomes With Dabigatran Dual Antithrombotic Therapy in Atrial Fibrillation Patients After PCI. JACC Cardiovasc. Interv. 2019, 12, 1553–1561. [Google Scholar] [CrossRef]
- Jamrozik, K. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Sim, J.J.; Shi, J.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Jacobsen, S.J. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J. Am. Coll. Cardiol. 2014, 64, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Earley, A.; Haynes, S.M.; Uhlig, K. Systematic review: Blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann. Intern. Med. 2011, 154, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.K.; Rahman, M.; Reboussin, D.M.; Craven, T.E.; Greene, T.; Kimmel, P.L.; Cushman, W.C.; Hawfield, A.T.; Johnson, K.C.; Lewis, C.E.; et al. Effects of Intensive BP Control in CKD. J. Am. Soc. Nephrol. 2017, 28, 2812–2823. [Google Scholar] [CrossRef]
- Bakris, G.L.; A Sarafidis, P.; Weir, M.R.; Dahlöf, B.; Pitt, B.; Jamerson, K.; Velazquez, E.J.; Staikos-Byrne, L.; Kelly, R.Y.; Shi, V.; et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): A prespecified secondary analysis of a randomised controlled trial. Lancet Lond. Engl. 2010, 375, 1173–1181. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, C.; Tonelli, M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; A Hegele, R.; Hovingh, G.K.; A Jacobson, T.; et al. Adverse effects of statin therapy: Perception vs. the evidence—Focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracciolo, A.; Scalise, R.F.M.; Ceresa, F.; Bagnato, G.; Versace, A.G.; Licordari, R.; Perfetti, S.; Lofrumento, F.; Irrera, N.; Santoro, D.; et al. Optimizing the Outcomes of Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease. J. Clin. Med. 2022, 11, 2380. https://doi.org/10.3390/jcm11092380
Caracciolo A, Scalise RFM, Ceresa F, Bagnato G, Versace AG, Licordari R, Perfetti S, Lofrumento F, Irrera N, Santoro D, et al. Optimizing the Outcomes of Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2022; 11(9):2380. https://doi.org/10.3390/jcm11092380
Chicago/Turabian StyleCaracciolo, Alessandro, Renato Francesco Maria Scalise, Fabrizio Ceresa, Gianluca Bagnato, Antonio Giovanni Versace, Roberto Licordari, Silvia Perfetti, Francesca Lofrumento, Natasha Irrera, Domenico Santoro, and et al. 2022. "Optimizing the Outcomes of Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 11, no. 9: 2380. https://doi.org/10.3390/jcm11092380
APA StyleCaracciolo, A., Scalise, R. F. M., Ceresa, F., Bagnato, G., Versace, A. G., Licordari, R., Perfetti, S., Lofrumento, F., Irrera, N., Santoro, D., Patanè, F., Di Bella, G., Costa, F., & Micari, A. (2022). Optimizing the Outcomes of Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 11(9), 2380. https://doi.org/10.3390/jcm11092380







