Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
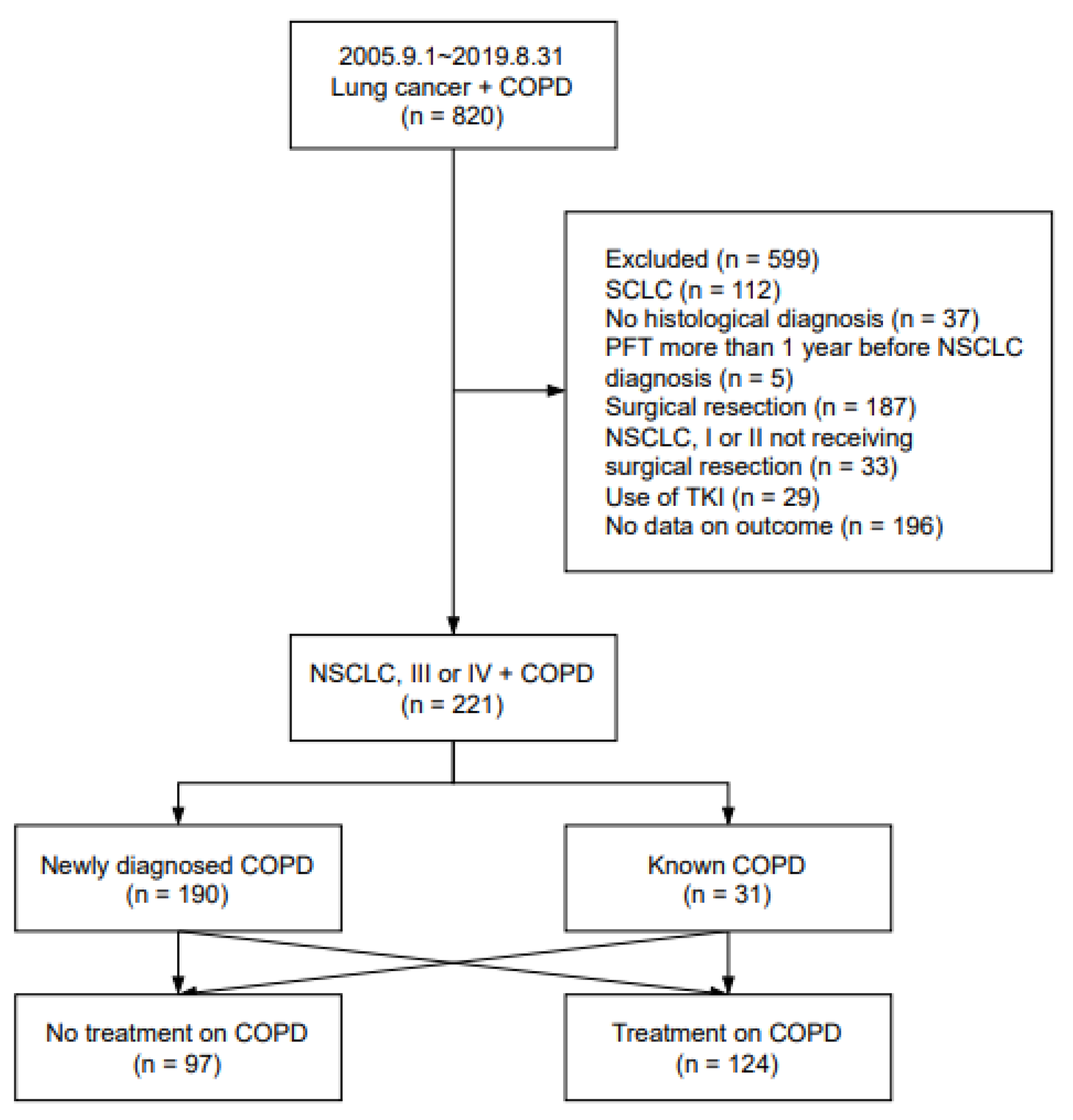
2.1. Study Population
2.2. Data Collection and Assessment
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loganathan, R.S.; Stover, D.E.; Shi, W.; Venkatraman, E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006, 129, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Collar, D.P.; Guerra, M.P.; Rodriguez, P.; Gotera, C.; Mahíllo-Fernández, I.; Peces-Barba, G.; Seijo, L.M. COPD is commonly underdiagnosed in patients with lung cancer: Results from the RECOIL study (retrospective study of COPD infradiagnosis in lung cancer). Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1033. [Google Scholar]
- Win, T.; Jackson, A.; Sharples, L.; Groves, A.; Wells, F.; Ritchie, A.; Laroche, C. Relationship between pulmonary function and lung cancer surgical outcome. Eur. Respir. J. 2005, 25, 594–599. [Google Scholar] [CrossRef]
- Greillier, L.; Thomas, P.; Loundou, A.; Doddoli, C.; Badier, M.; Auquier, P.; Barlési, F. Pulmonary function tests as a predictor of quantitative and qualitative outcomes after thoracic surgery for lung cancer. Clin. Lung Cancer 2007, 8, 554–561. [Google Scholar] [CrossRef]
- Zhai, R.; Yu, X.; Shafer, A.; Wain, J.C.; Christiani, D.C. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014, 145, 346–353. [Google Scholar] [CrossRef]
- Kobayashi, S.; Suzuki, S.; Niikawa, H.; Sugawara, T.; Yanai, M. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology 2009, 14, 675–679. [Google Scholar] [CrossRef]
- Nojiri, T.; Inoue, M.; Yamamoto, K.; Maeda, H.; Takeuchi, Y.; Nakagiri, T.; Shintani, Y.; Minami, M.; Sawabata, N.; Okumura, M. RETRACTED ARTICLE: Inhaled tiotropium to prevent postoperative cardiopulmonary complications in patients with newly diagnosed chronic obstructive pulmonary disease requiring lung cancer surgery. Surg. Today 2014, 44, 285–290. [Google Scholar] [CrossRef]
- Makino, T.; Otsuka, H.; Hata, Y.; Koezuka, S.; Azuma, Y.; Isobe, K.; Sugino, K.; Ebihara, S.; Homma, S.; Iyoda, A. Long-acting muscarinic antagonist and long-acting β2-agonist therapy to optimize chronic obstructive pulmonary disease prior to lung cancer surgery. Mol. Clin. Oncol. 2018, 8, 647–652. [Google Scholar] [CrossRef]
- Bölükbas, S.; Eberlein, M.; Eckhoff, J.; Schirren, J. Short-term effects of inhalative tiotropium/formoterol/budenoside versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: A prospective randomized trial. Eur. J. Cardio-Thorac. Surg. 2011, 39, 995–1000. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, E.M.; Sim, Y.S.; Ryu, Y.J.; Chang, J.H. Forced expiratory volume in one second as a prognostic factor in advanced non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-y.; Choi, Y.J.; Seo, J.H.; Lee, S.Y.; Kim, J.S.; Kang, E.J. Pulmonary function is implicated in the prognosis of metastatic non-small cell lung cancer but not in extended disease small cell lung cancer. J. Thorac. Dis. 2019, 11, 4562. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Yeo, C.D.; Rhee, C.K.; Kang, H.S.; Park, C.K.; Kim, J.S.; Kim, J.W.; Kim, S.J.; Yoon, H.K.; Lee, S.H. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (COPD) and non-COPD never-smoking stage I-IV non-small cell lung cancer patients. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; de Oca, M.M.; Mendez, R.A.; Plata, V.P.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Krishnan, J.A.; Lechtzin, N.; Diette, G.B. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch. Intern. Med. 2003, 163, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Lowe, D.; Hosker, H.; Anstey, K.; Pearson, M.; Roberts, C.M. UK National COPD Audit 2003: Impact of hospital resources and organisation of care on patient outcome following admission for acute COPD exacerbation. Thorax 2006, 61, 837–842. [Google Scholar] [CrossRef]
- Chen, D.; Restrepo, M.I.; Fine, M.J.; Pugh, M.J.V.; Anzueto, A.; Metersky, M.L.; Nakashima, B.; Good, C.; Mortensen, E.M. Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am. J. Respir. Crit. Care Med. 2011, 184, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; St. Rose, E.; Ballal, S.; McLaren, J. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, H.C.; Choi, C.-M. Recent Trends of Lung Cancer in Korea. Tuberc. Respir. Dis. 2021, 84, 89. [Google Scholar] [CrossRef]
- Semrau, S.; Klautke, G.; Virchow, J.; Kundt, G.; Fietkau, R. Impact of comorbidity and age on the outcome of patients with inoperable NSCLC treated with concurrent chemoradiotherapy. Respir. Med. 2008, 102, 210–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duong, M.; Islam, S.; Rangarajan, S.; Leong, D.; Kurmi, O.; Teo, K.; Killian, K.; Dagenais, G.; Lear, S.; Wielgosz, A. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): An international, community-based cohort study. Lancet Glob. Health 2019, 7, e613–e623. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Jones, P.W.; Calverley, P.M.; Celli, B.; Anderson, J.A.; Ferguson, G.T.; Yates, J.C.; Willits, L.R.; Vestbo, J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled TORCH study. Respir. Res. 2009, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Papi, A.; Corradi, M.; Pavlišová, I.; Montagna, I.; Francisco, C.; Cohuet, G.; Vezzoli, S.; Scuri, M.; Vestbo, J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): A double-blind, parallel group, randomised controlled trial. Lancet 2016, 388, 963–973. [Google Scholar] [CrossRef]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, S.J.; Lee, J.H.; Ha, E. Inhaled corticosteroids in COPD and the risk of lung cancer. Int. J. Cancer 2018, 143, 2311–2318. [Google Scholar] [CrossRef]
- Shin, J.; Yoon, H.-Y.; Lee, Y.M.; Ha, E.; Lee, J.H. Inhaled corticosteroids in COPD and the risk for coronary heart disease: A nationwide cohort study. Sci. Rep. 2020, 10, 18973. [Google Scholar] [CrossRef]
- Simmons, C.P.; Koinis, F.; Fallon, M.T.; Fearon, K.C.; Bowden, J.; Solheim, T.S.; Gronberg, B.H.; McMillan, D.C.; Gioulbasanis, I.; Laird, B.J. Prognosis in advanced lung cancer—A prospective study examining key clinicopathological factors. Lung Cancer 2015, 88, 304–309. [Google Scholar] [CrossRef]
- Crim, C.; Dransfield, M.T.; Bourbeau, J.; Jones, P.W.; Hanania, N.A.; Mahler, D.A.; Vestbo, J.; Wachtel, A.; Martinez, F.J.; Barnhart, F. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann. Am. Thorac. Soc. 2015, 12, 27–34. [Google Scholar] [CrossRef]
- Yang, M.; Du, Y.; Chen, H.; Jiang, D.; Xu, Z. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2019, 77, 105950. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, Y.H.; Kang, D.R.; Lee, S.J.; Lee, M.K.; Kim, S.-H.; Yong, S.J.; Lee, W.-Y. Risk of pneumonia associated with inhaled corticosteroid in patients with chronic obstructive pulmonary disease: A Korean population-based study. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3397. [Google Scholar] [CrossRef] [PubMed]
- de Molina, R.M.; Mortensen, E.; Restrepo, M.; Copeland, L.; Pugh, M.; Anzueto, A. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur. Respir. J. 2010, 36, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P. Corticosteroid effects on cell signalling. Eur. Respir. J. 2006, 27, 413–426. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, D.S.; Nikcevich, D.A.; Long, G.D.; Vredenburgh, J.J.; Rizzieri, D.; Smith, C.A.; Broadwater, G.; Loftis, J.S.; McDonald, C.; Morris, A.K. Inhaled steroids as prophylaxis for delayed pulmonary toxicity syndrome in breast cancer patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2001, 7, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Zhou, W.; Yang, X.; Li, J.; Cao, J. Risk of pneumonia with different inhaled corticosteroids in COPD patients: A meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Crowley, J.J.; LeBlanc, M.; Livingston, R.B. Survival determinants in extensive-stage non-small-cell lung cancer: The Southwest Oncology Group experience. J. Clin. Oncol. 1991, 9, 1618–1626. [Google Scholar] [CrossRef]
- Di Maio, M.; Lama, N.; Morabito, A.; Smit, E.F.; Georgoulias, V.; Takeda, K.; Quoix, E.; Hatzidaki, D.; Wachters, F.M.; Gebbia, V. Clinical assessment of patients with advanced non-small-cell lung cancer eligible for second-line chemotherapy: A prognostic score from individual data of nine randomised trials. Eur. J. Cancer 2010, 46, 735–743. [Google Scholar] [CrossRef]
- Zukin, M.; Barrios, C.H.; Rodrigues Pereira, J.; De Albuquerque Ribeiro, R.; de Mendonça Beato, C.A.; do Nascimento, Y.N.; Murad, A.; Franke, F.A.; Precivale, M.; de Lima Araujo, L.H. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non–small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J. Clin. Oncol. 2013, 31, 2849–2853. [Google Scholar] [CrossRef]

| Total n = 221 | Treatment n = 124 | No Treatment n = 97 | p-Value | |
|---|---|---|---|---|
| Sex, men | 200 (90.5) | 114 (91.9) | 86 (88.7) | 0.410 |
| Age, years | 70.7 ± 8.97 | 71.2 ± 7.79 | 70.0 ± 10.3 | 0.330 |
| BMI, kg/m2 | 22.3 ± 3.19 | 22.3 ± 3.29 | 22.4 ± 3.06 | 0.873 |
| Smoking | 0.084 | |||
| Never smoker | 37 (16.7) | 16 (12.9) | 21 (21.6) | |
| Ever smoker | 184 (83.3) | 108 (87.1) | 76 (78.4) | |
| Histology | 0.090 | |||
| SqCC | 117 (52.9) | 70 (56.5) | 47 (48.5) | |
| ADC | 77 (34.8) | 36 (29.0) | 41 (42.3) | |
| P/D carcinoma | 27 (12.2) | 18 (14.5) | 9 (9.3) | |
| COPD diagnosis | <0.001 | |||
| New COPD | 190 (86.0) | 94 (75.8) | 96 (99.0) | |
| Known COPD | 31 (14.0) | 30 (24.2) | 1 (1.0) | |
| Clinical stage | 0.010 | |||
| III | 106 (48.0) | 69 (55.6) | 37 (38.1) | |
| IV | 115 (52.0) | 55 (44.4) | 60 (61.9) | |
| Chemotherapy | 0.451 | |||
| Chemotherapy | 165 (74.7) | 95 (76.6) | 70 (72.2) | |
| No chemotherapy | 56 (25.3) | 29 (23.4) | 27 (27.8) | |
| First-line chemotherapy | 0.098 | |||
| Platinum + gemcitabine | 48 (29.1) | 34 (35.8) | 14 (20.0) | |
| Platinum + pemetrexed | 32 (19.4) | 16 (16.8) | 16 (22.9) | |
| Platinum + taxane | 56 (33.9) | 26 (27.4) | 30 (42.9) | |
| Taxane only | 17 (10.3) | 11 (11.6) | 6 (8.6) | |
| Others | 12 (7.3) | 8 (8.4) | 4 (5.7) | |
| Radiotherapy | 0.452 | |||
| CCRT | 54 (24.4) | 33 (26.6) | 21 (21.6) | |
| Palliative | 12 (5.4) | 5 (4.0) | 7 (7.2) | |
| No radiotherapy | 155 (70.1) | 86 (69.4) | 69 (71.1) | |
| Follow up duration, months | 9.5 (5.3–16.1) | 10.7 (5.8–18.3) | 8.7 (4.4–15.1) | 0.013 |
| Total n = 221 | Treatment n = 124 | No Treatment n = 97 | p-Value | |
|---|---|---|---|---|
| FVC, liters | 2.91 ± 0.81 | 2.81 ± 0.76 | 3.04 ± 0.86 | 0.035 |
| FVC % predicted | 77.8 ± 18.2 | 75.7 ± 17.9 | 80.5 ± 18.3 | 0.053 |
| FEV1, liters | 1.68 ± 0.55 | 1.53 ± 0.48 | 1.87 ± 0.58 | <0.001 |
| FEV1 % predicted | 65.3 ± 18.7 | 60.2 ± 16.8 | 71.8 ± 19.0 | <0.001 |
| FEV1/FVC | 57.9 ± 10.4 | 55.1 ± 11.2 | 61.5 ± 7.90 | <0.001 |
| DLco % predicted | 68.7 ± 27.5 a | 64.7 ± 25.8 b | 74.0 ± 28.8 c | 0.033 |
| COPD severity by GOLD classification d | <0.001 | |||
| GOLD 1 | 52 (23.5) | 17 (13.7) | 35 (36.1) | |
| GOLD 2 | 121 (54.8) | 72 (58.1) | 49 (50.5) | |
| GOLD 3 | 44 (19.9) | 33 (26.6) | 11 (11.3) | |
| GOLD 4 | 4 (1.8) | 2 (1.6) | 2 (2.1) | |
| Median overall survival by GOLD classification e, f | ||||
| GOLD 1 | 9.7 (7.3–12.1) | 11.7 (6.3–17.0) | 9.0 (6.9–11.1) | 0.414 |
| GOLD 2 | 10.6 (8.4–12.9) | 11.8 (9.8–13.8) | 8.9 (6.6–11.2) | 0.007 |
| GOLD 3 | 7.4 (5.2–9.6) | 7.9 (4.9–10.9) | 6.4 (5.2–9.6) | 0.253 |
| GOLD 4 | 3.9 (0.8–7.0) | 4.0 (NE) | 0.8 (NE) | 0.090 |
| Type of Treatment | n = 124 |
|---|---|
| Inhalers | |
| LAMA | 20 (16.1) |
| LABA | 2 (1.6) |
| LAMA/LABA | 22 (17.7) |
| ICS/LABA | 16 (12.9) |
| ICS/LAMA/LABA | 45 (36.3) |
| No use | 19 (15.3) |
| Theophylline | |
| Use | 69 (55.6) |
| No use | 55 (44.4) |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex, men | 1.62 | 1.02–2.56 | 0.042 | 2.49 | 1.52–4.10 | <0.001 |
| Age, years | 1.00 | 0.98–1.02 | 0.846 | 0.99 | 0.98–1.01 | 0.313 |
| BMI, kg/m2 | 0.95 | 0.91–0.99 | 0.038 | 0.95 | 0.91–0.99 | 0.033 |
| Ever smoker | 1.02 | 0.72–1.46 | 0.896 | |||
| FEV1 < 50% predicted | 1.40 | 1.01–1.95 | 0.044 | 1.26 | 0.90–1.79 | 0.184 |
| Histology | ||||||
| ADC | 1.00 | |||||
| SqCC | 1.05 | 0.79–1.41 | 0.736 | |||
| P/D carcinoma | 1.17 | 0.75–1.81 | 0.497 | |||
| Clinical stage | ||||||
| III | 1.00 | 1.00 | ||||
| IV | 1.62 | 1.24–2.13 | <0.001 | 1.94 | 1.44–2.62 | <0.001 |
| Chemotherapy | 0.59 | 0.44–0.81 | 0.001 | 0.44 | 0.31–0.62 | <0.001 |
| CCRT | 0.98 | 0.66–1.24 | 0.542 | |||
| COPD treatment | 0.69 | 0.52–0.91 | 0.007 | 0.71 | 0.53–0.95 | 0.021 |
| Inhaled therapy | 0.73 | 0.55–0.95 | 0.021 | |||
| ICS | 0.65 | 0.48–0.89 | 0.006 | |||
| Theophylline | 0.70 | 0.52–0.94 | 0.017 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Park, S.; Kim, N.E.; Park, S.Y.; Ryu, Y.J.; Chang, J.H.; Lee, J.H. Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer. J. Clin. Med. 2022, 11, 2391. https://doi.org/10.3390/jcm11092391
Jo H, Park S, Kim NE, Park SY, Ryu YJ, Chang JH, Lee JH. Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2022; 11(9):2391. https://doi.org/10.3390/jcm11092391
Chicago/Turabian StyleJo, Hyunji, Sojung Park, Nam Eun Kim, So Young Park, Yon Ju Ryu, Jung Hyun Chang, and Jin Hwa Lee. 2022. "Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer" Journal of Clinical Medicine 11, no. 9: 2391. https://doi.org/10.3390/jcm11092391
APA StyleJo, H., Park, S., Kim, N. E., Park, S. Y., Ryu, Y. J., Chang, J. H., & Lee, J. H. (2022). Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 11(9), 2391. https://doi.org/10.3390/jcm11092391






