Corneal Confocal Microscopy Features and Tear Molecular Profile in Study Participants with Discordance between Ocular Surface Disease Clinical Signs and Discomfort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort, Clinical Examination, and Study Groups
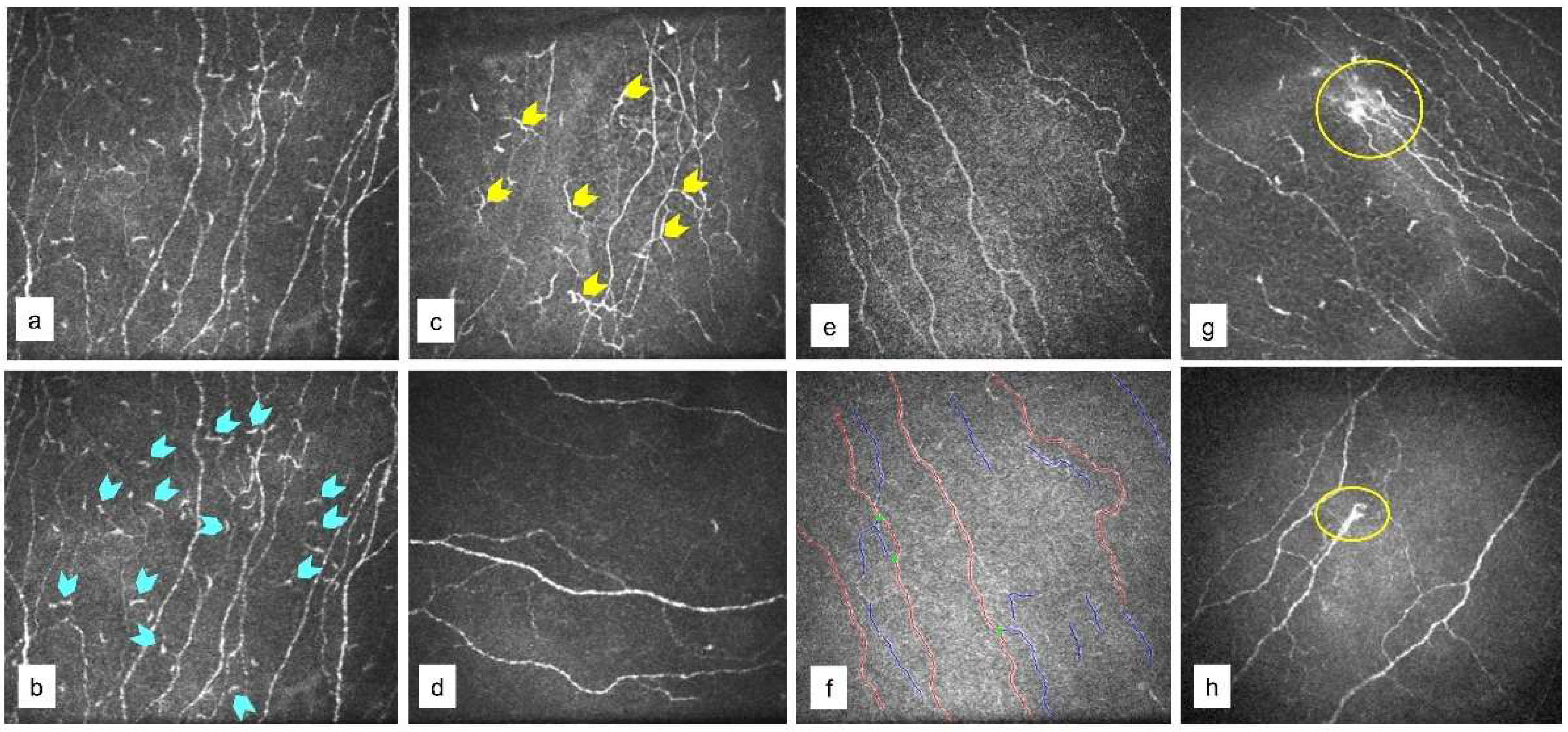
2.2. In Vivo Confocal Microscopy (IVCM) Imaging
2.3. Tear Fluid Collection
2.4. Soluble Factors Level Measurement
2.5. Statistical Analyses
3. Results
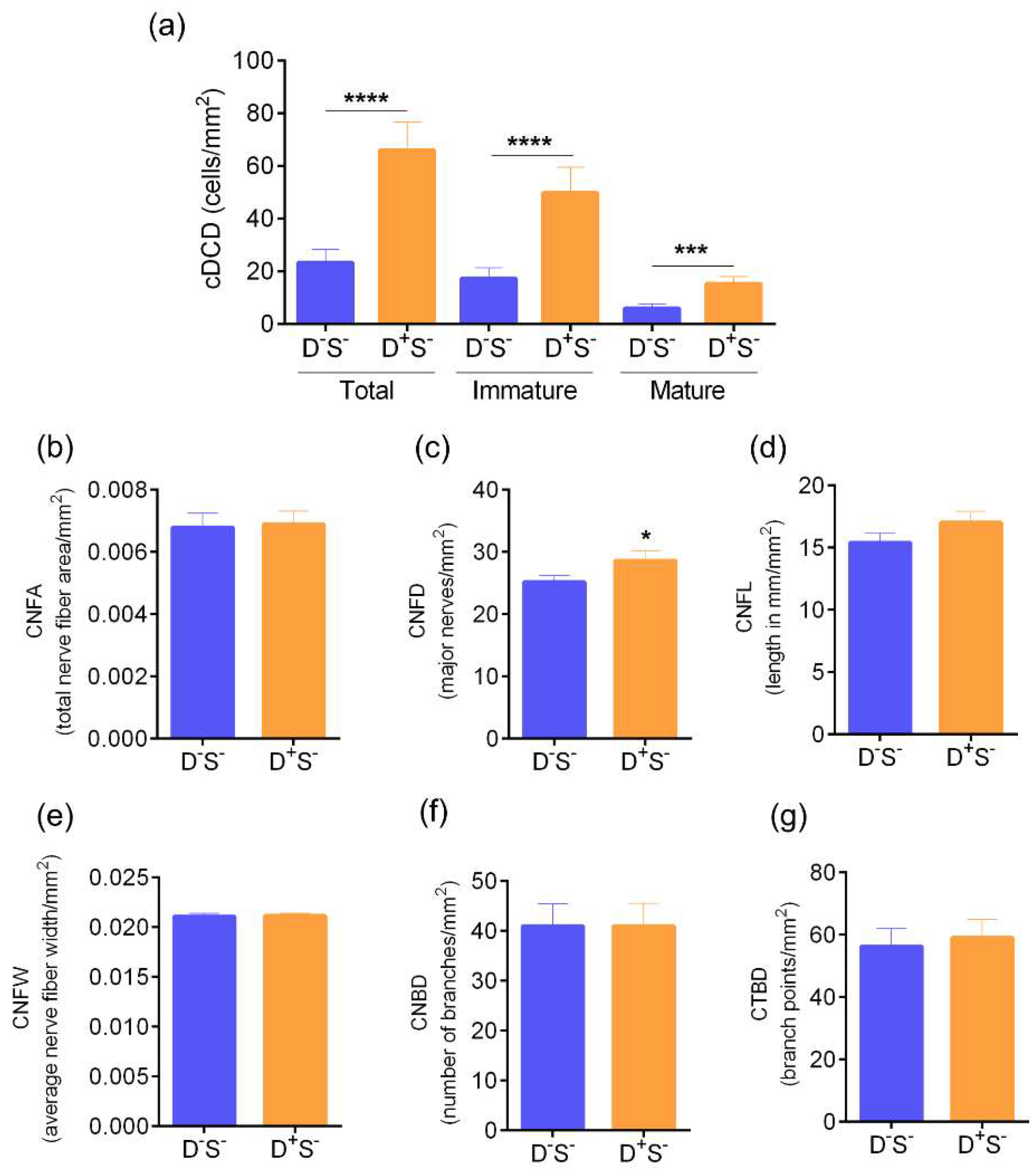
3.1. Ocular Surface Clinical Parameters, IVCM Features, and Tear Soluble Factors in Different Groups
3.2. Ocular Surface Clinical Parameters, IVCM Features, and Tear Soluble Factors in Subjects with High Ocular Surface Discomfort but no Clinical Signs (D++S-)
3.3. Ocular Surface Clinical Parameters, IVCM Features and Tear Soluble Factors in Subjects with DED (Evaporative or Aqueous Deficient)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehra, D.; Cohen, N.K.; Galor, A. Ocular Surface Pain: A Narrative Review. Ophthalmol. Ther. 2020, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dana, R.; Meunier, J.; Markowitz, J.T.; Joseph, C.; Siffel, C. Patient-Reported Burden of Dry Eye Disease in the United States: Results of an Online Cross-Sectional Survey. Am. J. Ophthalmol. 2020, 216, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Dermer, H.; Lent-Schochet, D.; Theotoka, D.; Paba, C.; Cheema, A.A.; Kim, R.S.; Galor, A. A Review of Management Strategies for Nociceptive and Neuropathic Ocular Surface Pain. Drugs 2020, 80, 547–571. [Google Scholar] [CrossRef] [PubMed]
- Morone, N.E.; Weiner, D.K. Pain as the fifth vital sign: Exposing the vital need for pain education. Clin. Ther. 2013, 35, 1728–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.; Treede, R.-D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Galor, A. Painful Dry Eye Symptoms: A Nerve Problem or a Tear Problem? Ophthalmology 2019, 126, 648–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, D.S. Diagnosis and Treatment of Ocular Pain: The Ophthalmologist’s Perspective. Curr. Ophthalmol. Rep. 2017, 5, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galor, A.; Feuer, W.J.; Lee, D.J.; Florez, H.; Venincasa, V.; Perez, V.L. Ocular surface parameters in older male veterans. Investig. Opthalmol. Vis. Sci. 2013, 54, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Vehof, J.; Kozareva, D.; Hysi, P.G.; Harris, J.; Nessa, A.; Williams, F.K.; Bennett, D.L.H.; McMahon, S.B.; Fahy, S.J.; Direk, K.; et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013, 131, 1304–1308. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, P.; Baran, I.; Jacobs, D.S. Corneal pain without stain: Is it real? Ocul. Surf. 2009, 7, 28–40. [Google Scholar] [CrossRef]
- Galor, A.; Levitt, R.C.; Felix, E.R.; Martin, E.R.; Sarantopoulos, C.D. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye 2015, 29, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Moein, H.-R.; Lee, C.; Rodriguez, A.; Felix, E.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Nichols, J.J.; Mph, M.; Mitchell, G.L. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004, 23, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S.; Felix, E.; Levitt, R.C.; Feuer, W.J.; Sarantopoulos, C.D.; Galor, A. Epidemiology of discordance between symptoms and signs of dry eye. Br. J. Ophthalmol. 2018, 102, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Merayo-Lloves, J.; Gallar, J. What Causes Eye Pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Ogawa, Y.; Mukai, S.; Saijo-Ban, Y.; Kamoi, M.; Uchino, M.; Yamane, M.; Ozawa, N.; Fukui, M.; Mori, T.; et al. In Vivo Confocal Microscopy Evaluation of Ocular Surface with Graft-Versus-Host Disease-Related Dry Eye Disease. Sci. Rep. 2017, 7, 10720. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Darabad, R.R.; Cruzat, A.; Hajrasouliha, A.R.; Witkin, D.; Wong, N.; Dana, R.; Hamrah, P. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Investig. Opthalmol. Vis. Sci. 2015, 56, 7179–7185. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Moscardelli, F.; Versura, P.; Campos, E.C. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Tervo, T.M.; Moilanen, J.A.O.; Rosenberg, M.E.; Tuominen, I.S.J.; Valle, T.; Vesaluoma, M.H. In vivo confocal microscopy for studying corneal diseases and conditions associated with corneal nerve damage. Adv. Exp. Med. Biol. 2002, 506, 657–665. [Google Scholar] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Satitpitakul, V.; Kheirkhah, A.; Crnej, A.; Hamrah, P.; Dana, R. Determinants of Ocular Pain Severity in Patients with Dry Eye Disease. Am. J. Ophthalmol. 2017, 179, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal clinically important difference for the ocular surface disease index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Mantopoulos, D.; Cruzat, A.; Hamrah, P. In vivo imaging of corneal inflammation: New tools for clinical practice and research. Semin. Ophthalmol. 2010, 25, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; McGhee, C.N. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: A review. Br. J. Ophthalmol. 2008, 93, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagali, N.S.; Badian, R.A.; Liu, X.; Feldreich, T.R.; Arnlov, J.; Utheim, T.P.; Dahlin, L.B.; Rolandsson, O. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci. Rep. 2018, 8, 14248. [Google Scholar] [CrossRef] [Green Version]
- Shetty, R.; Deshmukh, R.; Shroff, R.; Dedhiya, C.; Jayadev, C. Subbasal Nerve Plexus Changes in Chronic Migraine. Cornea 2018, 37, 72–75. [Google Scholar] [CrossRef]
- Shetty, R.; Sethu, S.; Deshmukh, R.; Deshpande, K.; Ghosh, A.; Agrawal, A.; Shroff, R. Corneal Dendritic Cell Density Is Associated with Subbasal Nerve Plexus Features, Ocular Surface Disease Index, and Serum Vitamin D in Evaporative Dry Eye Disease. BioMed Res. Int. 2016, 2016, 4369750. [Google Scholar] [CrossRef] [Green Version]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef]
- Goyal, S.; Hamrah, P. Understanding Neuropathic Corneal Pain—Gaps and Current Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.R.; Al-Aqaba, M.A.; Almaazmi, A.; Messina, M.; Nubile, M.; Mastropasqua, L.; Dua, H.S.; Said, D.G. Clinical and in vivo confocal microscopic features of neuropathic corneal pain. Br. J. Ophthalmol. 2020, 104, 768–775. [Google Scholar] [CrossRef]
- Khamar, P.; Nair, A.P.; Shetty, R.; Vaidya, T.; Subramani, M.; Ponnalagu, M.; Dhamodaran, K.; D’Souza, S.; Ghosh, A.; Pahuja, N.; et al. Dysregulated Tear Fluid Nociception-Associated Factors, Corneal Dendritic Cell Density, and Vitamin D Levels in Evaporative Dry Eye. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2532–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hucho, T.; Levine, J.D. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron 2007, 55, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Dana, M.R.; Hamrah, P. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv. Exp. Med. Biol. 2002, 506, 729–738. [Google Scholar]
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- McKay, T.B.; Seyed-Razavi, Y.; Ghezzi, C.E.; Dieckmann, G.; Nieland, T.J.F.; Cairns, D.M.; Pollard, R.E.; Hamrah, P.; Kaplan, D.L. Corneal pain and experimental model development. Prog. Retin. Eye Res. 2019, 71, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kheirkhah, A.; Cavalcanti, B.M.; Cruzat, A.; Colon, C.; Brown, E.; Borsook, D.; Pruss, H.; Hamrah, P. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul. Surf. 2015, 13, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, T.; Takahashi, H.; Dana, R. Novel Insights Into the Immunoregulatory Function and Localization of Dendritic Cells. Cornea 2016, 35, S49–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastjerdi, M.H.; Dana, R. Corneal nerve alterations in dry eye-associated ocular surface disease. Int. Ophthalmol. Clin. 2009, 49, 11–20. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P.; Yu, C.; Liu, Y.; Hu, S.; Di, G. In vivo Confocal Microscopic Evaluation of Corneal Dendritic Cell Density and Subbasal Nerve Parameters in Dry Eye Patients: A Systematic Review and Meta-analysis. Front. Med. 2021, 8, 578233. [Google Scholar] [CrossRef] [PubMed]
- Moein, H.R.; Akhlaq, A.; Dieckmann, G.; Abbouda, A.; Pondelis, N.; Salem, Z.; Muller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; et al. Visualization of microneuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain? Ocul. Surf. 2020, 18, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [Green Version]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.F.; Moalem-Taylor, G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 2011, 12, 370–383. [Google Scholar] [CrossRef]
- Hulse, R.P.; Beazley-Long, N.; Ved, N.; Bestall, S.M.; Riaz, H.; Singhal, P.; Ballmer Hofer, K.; Harper, S.J.; Bates, D.O.; Donaldson, L.F. Vascular endothelial growth factor-A165b prevents diabetic neuropathic pain and sensory neuronal degeneration. Clin. Sci. 2015, 129, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Hulse, R.P. Role of VEGF-A in chronic pain. Oncotarget 2017, 8, 10775–10776. [Google Scholar] [CrossRef]










| D-S- | D+S- | p Value | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | SEM | Mean | SD | SEM | ||
| IL-1⍺ | 44 | 45 | 8 | 38 | 41 | 15 | 0.601 |
| IL-1β | 5.9 | 11.5 | 2.2 | 7.3 | 11.9 | 4.2 | 0.522 |
| IL-6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.000 |
| IL-8 | 272 | 281 | 53 | 247 | 178 | 63 | 0.950 |
| IL-10 | 0.2 | 0.3 | 0.1 | 0.3 | 0.5 | 0.2 | 0.830 |
| IL-17A | 1.2 | 2.3 | 0.4 | 64.3 | 176.5 | 62.4 | 0.250 |
| IL-17F | 74 | 236 | 45 | 76 | 195 | 69 | 0.929 |
| TNF-⍺ | 0.5 | 0.9 | 0.2 | 0.7 | 1.1 | 0.4 | 0.865 |
| IFN-γ | 2.7 | 6.1 | 1.1 | 117.3 | 324.7 | 114.8 | 0.504 |
| RANTES | 102 | 131 | 25 | 48 | 58 | 21 | 0.196 |
| MCP-1 | 300 | 290 | 55 | 320 | 202 | 72 | 0.672 |
| VEGF-A | 1275 | 822 | 155 | 794 | 829 | 293 | 0.075 |
| ICAM-1 | 2793 | 2710 | 512 | 1449 | 759 | 268 | 0.305 |
| Analytes (pg/mL) | Controls | EDED | ADED | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SEM | Mean | SD | SEM | Mean | SD | SEM | Ctrls vs. EDED | Ctrls vs. ADED | EDED vs. ADED | |
| IL-1⍺ | 44 | 45 | 8 | 33 | 42 | 6 | 29 | 13 | 5 | 0.095 | 0.789 | 0.474 |
| IL-1β | 6 | 12 | 2 | 4 | 6 | 1 | 7 | 8 | 3 | 0.254 | 0.208 | 0.421 |
| IL-6 | 0 | 0 | 0 | 6 | 22 | 3 | 42 | 68 | 28 | 0.022 | <0.0001 | 0.499 |
| IL-8 | 272 | 281 | 53 | 287 | 199 | 30 | 632 | 806 | 329 | 0.395 | 0.092 | 0.209 |
| IL-10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.217 | 0.997 | 0.507 |
| IL-17A | 1 | 2 | 0 | 9 | 17 | 2 | 3 | 2 | 1 | 0.003 | <0.0001 | 0.583 |
| IL-17F | 74 | 236 | 45 | 62 | 195 | 29 | 0 | 0 | 0 | 0.713 | 0.440 | 0.583 |
| TNF-⍺ | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0.480 | 0.441 | 0.447 |
| IFN-γ | 3 | 6 | 1 | 9 | 21 | 3 | 0 | 0 | 0 | 0.427 | 0.228 | 0.113 |
| RANTES | 102 | 131 | 25 | 188 | 483 | 72 | 381 | 242 | 99 | 0.812 | 0.002 | 0.013 |
| MCP-1 | 300 | 290 | 55 | 166 | 232 | 35 | 261 | 340 | 139 | 0.008 | 0.388 | 0.704 |
| VEGF-A | 1275 | 822 | 155 | 971 | 1303 | 194 | 446 | 326 | 133 | 0.009 | 0.008 | 0.663 |
| ICAM-1 | 2793 | 2710 | 512 | 2526 | 4162 | 620 | 2610 | 1238 | 505 | 0.220 | 0.713 | 0.254 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Souza, S.; Shetty, R.; Nair, A.P.; Agrawal, R.; Dickman, M.M.; Khamar, P.; Nuijts, R.M.M.A.; Ghosh, A.; Sethu, S. Corneal Confocal Microscopy Features and Tear Molecular Profile in Study Participants with Discordance between Ocular Surface Disease Clinical Signs and Discomfort. J. Clin. Med. 2022, 11, 2407. https://doi.org/10.3390/jcm11092407
D’Souza S, Shetty R, Nair AP, Agrawal R, Dickman MM, Khamar P, Nuijts RMMA, Ghosh A, Sethu S. Corneal Confocal Microscopy Features and Tear Molecular Profile in Study Participants with Discordance between Ocular Surface Disease Clinical Signs and Discomfort. Journal of Clinical Medicine. 2022; 11(9):2407. https://doi.org/10.3390/jcm11092407
Chicago/Turabian StyleD’Souza, Sharon, Rohit Shetty, Archana Padmanabhan Nair, Ruchika Agrawal, Mor M. Dickman, Pooja Khamar, Rudy M. M. A. Nuijts, Arkasubhra Ghosh, and Swaminathan Sethu. 2022. "Corneal Confocal Microscopy Features and Tear Molecular Profile in Study Participants with Discordance between Ocular Surface Disease Clinical Signs and Discomfort" Journal of Clinical Medicine 11, no. 9: 2407. https://doi.org/10.3390/jcm11092407






