Hemostatic Efficacy of Absorbable Gelatin Sponges for Surgical Nail Matrixectomy after Phenolization—A Blinded Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
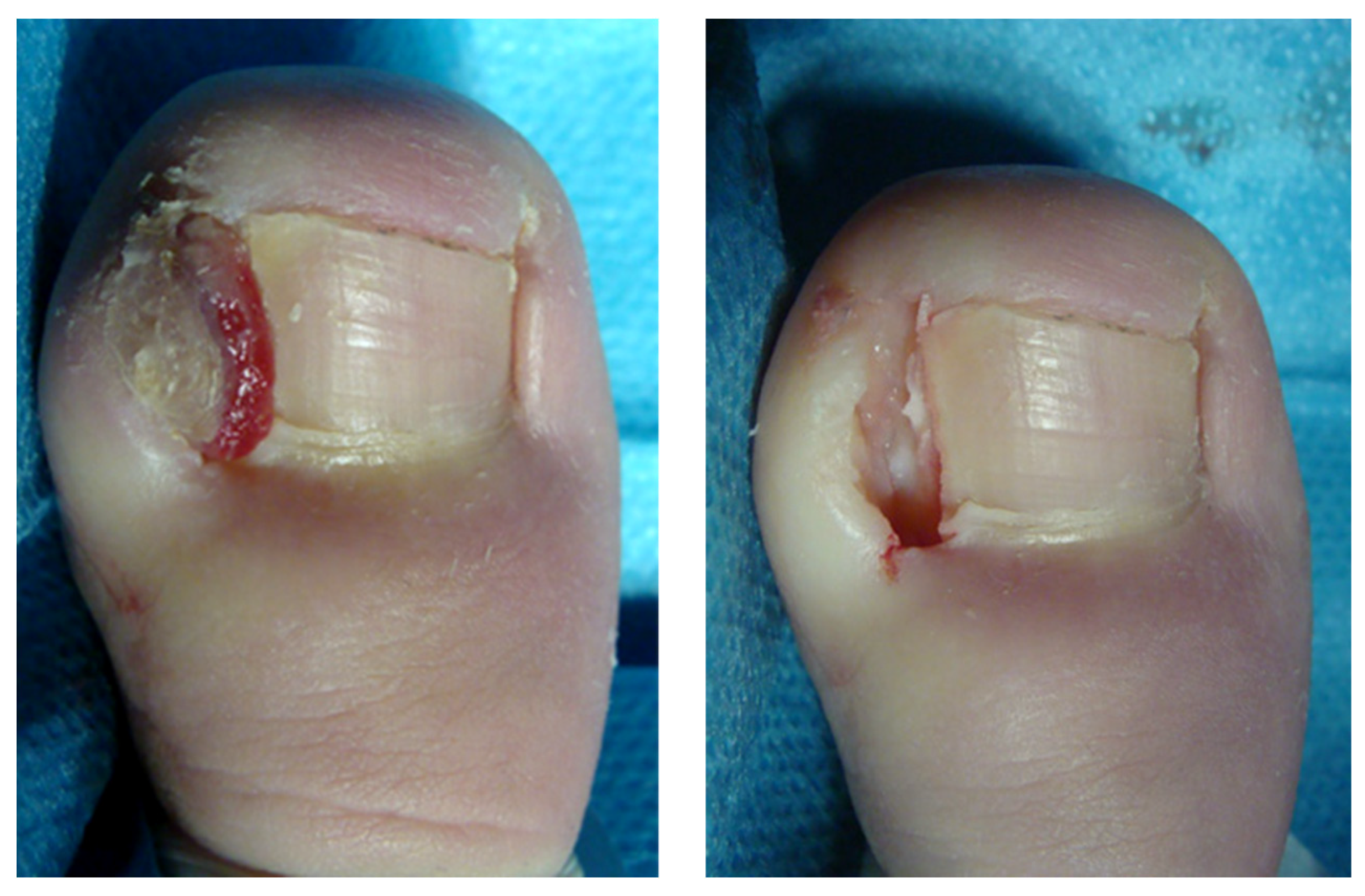
2.2. Surgical Procedure
2.3. Outcome Measurement
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
3.1. Descriptive Dates
3.2. Outcome Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinay, K.; Narayan Ravivarma, V.; Thakur, V.; Choudhary, R.; Narang, T.; Dogra, S.; Varthya, S.B. Efficacy and safety of phenol-based partial matricectomy in treatment of onychocryptosis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Fulton, G.F.; O’Donohoe, M.K.; Reynolds, J.V.; Keane, F.B.; Tanner, W.A. Wedge resection alone or combined with segmental phenolization for the treatment of ingrowing toenail. Br. J. Surg. 1994, 81, 1074–1075. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.M.; Tanner, W.A. Approach to ingrowing toenails: The wedge resection/segmental phenolization combination treatment. Br. J. Surg. 1988, 75, 181–183. [Google Scholar] [CrossRef]
- Shaikh, F.M.; Jafri, M.; Giri, S.K.; Keane, R. Efficacy of wedge resection with phenolization in the treatment of ingrowing toenails. J. Am. Podiatr. Med. Assoc. 2008, 98, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Rounding, C.; Bloomfield, S. Surgical treatments for ingrowing toenails. Cochrane Database Syst. Rev. 2005, 2, CD001541. [Google Scholar]
- Eekhof, J.A.; Van Wijk, B.; Knuistingh Neven, A.; van der Wouden, J.C. Interventions for ingrowing toenails. Cochrane Database Syst. Rev. 2012, 18, CD001541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Jiménez, J.; Córdoba-Fernández, A.; Munuera, P.V. Effect of curettage after segmental phenolization in the treatment of onychocryptosis: A randomized double-blind clinical trial. Dermatol. Surg. 2012, 38, 454–461. [Google Scholar] [CrossRef]
- Morkane, A.J.; Robertson, R.W.; Inglis, G.S. Segmental phenolization of ingrowing toenails: A randomized controlled study. Br. J. Surg. 1984, 71, 526–527. [Google Scholar] [CrossRef]
- Arista, G.F.; Merino, J.E. Onicocriptosis: Estudio comparativo del periodo posoperatorio de una matricectomía parcial lateral con el de una matricectomía parcial lateral con fenolización. Dermatol. Rev. Mex. 2006, 50, 87–93. [Google Scholar]
- Córdoba-Fernandez, A.; Rayo-Rosado, R.; Juarez-Jiménez, J.M. Platelet gel for the surgical treatment of onychocryptosis. J. Am. Podiatr. Med. Assoc. 2008, 98, 296–301. [Google Scholar] [CrossRef]
- Garrido-Castells, X.; Becerro-de-Bengoa-Vallejo, R.; Calvo-Lobo, C.; Losa-Iglesias, M.E.; Palomo-López, P.; Navarro-Flores, E.; López-López, D. Effectiveness of Leukocyte and Platelet-Rich Fibrin versus Nitrofurazone on Nail Post-Surgery Bleeding and Wound Cicatrization Period Reductions: A Randomized Single Blinded Clinical Trial. J. Clin. Med. 2019, 27, 1552. [Google Scholar] [CrossRef] [Green Version]
- Tompeck, A.J.; Gajdhar, A.U.R.; Dowling, M.; Johnson, S.B.; Barie, P.S.; Winchell, R.J.; King, D.; Scalea, T.M.; Britt, L.D.; Narayan, M. A comprehensive review of topical hemostatic agents: The good, the bad, and the novel. J. Trauma Acute Care Surg. 2020, 88, e1–e21. [Google Scholar] [CrossRef] [PubMed]
- Rullan, P.P.; Vallbona, C.; Rullan, J.M.; Mansbridge, J.N.; Morhenn, V.B. Use of gelatin sponges in Mohs micrographic surgery defects and staged melanoma excisions: A novel approach to secondary wound healing. J. Drugs Dermatol. 2011, 10, 68–73. [Google Scholar] [PubMed]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, H.; Huang, X.; Xiong, W.; Zhao, H.; Chua, S.; Li, Z. Using tranexamic acidsoaked absorbable gelatin sponge following complex posterior lumbar spine surgery: A randomized control trial. Clin. Neurol. Neurosurg. 2016, 147, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Hwa, C.; Kovich, O.I.; Stein, J.A. Achieving hemostasis after nail biopsy using absorbable gelatin sponge saturated in aluminum chloride. Dermatol. Surg. 2011, 37, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Hajosch, R.; Suckfuell, M.; Oesser, S.; Ahlers, M.; Flechsenhar, K.; Schlosshauer, B. A novel gelatin sponge for accelerated hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 372–379. [Google Scholar] [CrossRef]
- Goncalves, S.; Chiossone-Kerdel, J.A.; Bianco, A.S.; Ercolino, J.M.; Hernandez-Rojas, J. Effect of absorbable gelatin sponge in the middle ear: In vitro and in vivo animal model. Acta Otolaryngol. 2015, 135, 14–25. [Google Scholar] [CrossRef]
- Hildenbrand, T. A new gelatine-based hemostat for sinonasal surgery: A clinical survey. In Vivo 2013, 27, 523–526. [Google Scholar]
- Sielaff, M.; Hermanns, M.; Uhlig, R.; Abou-Ghazalé, T.; Steinmüller, T. Early experience with a novel gelatine-based sponge for local haemostasis in thyroid surgery. In Vivo 2014, 28, 255–258. [Google Scholar]
- Kline, A. Onychocryptosis: A simple classification system. Foot Ankle J. 2008, 1, 6–13. [Google Scholar]
- Winograd, A.M. A modification in the technic of operation for ingrown toenail. 1929. J. Am. Podiatrc. Med. Assoc. 2007, 97, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, R.; Bilotti, M.A. Surgical nail procedures. Clin. Podiatr. Med. Surg. 1989, 6, 431–451. [Google Scholar] [PubMed]
- Ye, D.; Wu, S.; Zhang, B.; Hong, C.; Yang, L. Characteristics and clinical potential of a cellularly modified gelatin sponge. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211035061. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Fernández, A.; Rayo-Rosado, R.; Juárez-Jiménez, J.M. The use of autologous platelet gel in toenail surgery: A within-patient clinical trial. J. Foot Ankle Surg. 2010, 49, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Ham, A.C.; Hackeng, C.A.; Yo, T.I. The treatment of ingrowing toenails. A randomised comparison of wedge excision and phenol cauterisation. J. Bone Jt. Surg. Br. 1990, 72, 507–509. [Google Scholar] [CrossRef] [Green Version]
- Herold, N.; Houshian, S.; Riegels-Nielsen, P. A prospective comparison of wedge matrix resection with nail matrix phenolization for the treatment of ingrown toenail. J. Foot Ankle Surg. 2001, 40, 390–395. [Google Scholar] [CrossRef]
- Grieg, J.D.; Anderson, J.H.; Ireland, A.J.; Anderson, J.R. The surgical treatment of ingrowing toenails. J. Bone Jt. Surg. Br. Vol. 1991, 73, 131–133. [Google Scholar] [CrossRef]




| Characteristics | Group A (Control) | Group B (CGS) | Group C (HPGS) | Total |
|---|---|---|---|---|
| Average age ± SD (years) | 27.4 ± 18.1 | 30.3 ± 18.3 | 28.2 ± 18.7 | 28.7 ± 18.2 |
| Halluces of male | 6 (30%) | 11 (44%) | 10 (34.5%) | 27 |
| Halluces of female | 14 (70%) | 14 (56%) | 19 (65.5%) | 47 |
| Nail morphology: | ||||
| Normal | 2 (10%) | 4 (16%) | 2 (6.9%) | 8 (10.8%) |
| Abnormal | 17 (90%) | 21 (84%) | 27 (93.1%) | 66 (89.1%) |
| Laterality: | ||||
| Right | 5 (25%) | 14 (56%) | 20 (69%) | 39 (52.7%) |
| Left | 15 (75%) | 11 (44%) | 9 (31%) | 35 (47.2%) |
| ONC Stage: | ||||
| Stage 1 | 2 (10%) | 2 (8%) | 2 (6.9%) | 6 (8.1%) |
| Stage 2 | 1 (5%) | 6 (24%) | 1 (3.4%) | 8 (10.8%) |
| Stage 3 | 17 (85%) | 17(68%) | 26 (89.7%) | 60 (81.0%) |
| Outcome Measurements | Control (n = 20) Mean ± SD (95% CI) Median (IR) | Group CGS (n = 25) Mean ± SD (95% CI) Median (IR) | Group HPGS (n = 29) Mean ± SD (95% CI) Median (IR) |
|---|---|---|---|
| Post-surgical blood loss (g) | 3.5 ± 0.8 | 1.9 ± 0.8 | 1.9 ± 0.8 |
| 2.8 (2.3–4.0) | 2.1 (1.5–2.6) | 1.8 (1.2–2.2) | |
| Digital circumference, pre-operative and at 48 h (cm) | 8.3 ± 0.7 | 8.4 ± 0.7 | 8.3 ± 0.7 |
| 8.8 (7.9–9.0) | 8.1 (8.0–9.1) | 8.1 (7.8–8.9) | |
| 8.8 ± 0.6 | 8.8 ± 0.7 | 8.8 ± 0.6 | |
| 8.9 (8.3–9.2) | 8.8 (8.4–9.4) | 8.7 (8.3–9.4) | |
| Pain day 1 | 4.2 ± 1.9 | 5.0 ± 2.3 | 4.2 ± 2.5 |
| 4.1 (3.1–5.8) | 5.0 (3.0–7.1) | 4 (2–6) | |
| 2.4 ± 2.1 | 3.6 ± 2.7 | 2.3 ± 2.4 | |
| Pain day 2 | 2.0 (0.3–4.0) | 4 (1–6) | 0 (0–1.5) |
| 0.6 ± 1.1 | 1.0 ± 1.6 | 0.9 ± 2.0 | |
| Pain day 3 | 0 (0–1.0) | 0 (0–1.5) | 0 (0–1) |
| Recovery time (days) | 15.1 ± 4.2 | 15.3 ± 3.6 | 16.0 ± 4.0 |
| 14 (13–15) | 15 (14–20) | 14 (14–17.3) | |
| Number of cures | 3.0 ± 1.1 | 3.4 ± 1.0 | 3.5 ± 1.1 |
| 3 (2–3) | 3 (3–4) | 3 (3–4) |
| Outcome Measurements | Control (n = 20) Mean ± SD (95% CI) | Group CGS (n = 25) Mean ± SD (95% CI) | p-Value |
|---|---|---|---|
| Post-surgical blood loss (g) | 3.5 ± 2.3 | 1.9 ± 0.8 | 0.005 * |
| Circumference, pre-operative and at 48 h (cm) | 8.3 ± 0.7 | 8.4 ± 0.7 | 0.616 ** |
| 8.8 ± 0.6 | 8.8 ± 0.7 | 0.979 ** | |
| Recovery time (days) | 15.1 ± 4.2 | 16.3 ± 3.6 | 0.144 * |
| Number of cures | 3.0 ± 1.1 | 3.4 ± 1.0 | 0.051 * |
| Outcome Measurements | Control (n = 20) Mean ± SD (95% CI) | Group HPGS (n = 29) Mean ± SD (95% CI) | p-Value |
|---|---|---|---|
| Post-surgical blood loss (g) | 3.5 ± 2.3 | 1.9 ± 0.8 | 0.001 * |
| Circumference, pre-operative and at 48 h (cm) | 8.3 ± 0.7 | 8.3 ± 0.7 | 0.860 ** |
| 8.8 ± 0.6 | 8.8 ± 0.6 | 0.970 ** | |
| Recovery time (days) | 15.1 ± 4.2 | 16.0 ± 4.0 | 0.258 * |
| Number of cures | 3.0 ± 1.1 | 3.5 ± 1.1 | 0.094 * |
| Recurrence: No Median (IR) | Recurrence: Yes Median (IR) | p-Value | |
|---|---|---|---|
| Number of nail folds evaluated (%) | 114 (85.07%) | 10 (7.4%) | |
| Follow-up (months) | 28.0 ± 20.3 | 40.8 ± 9.5 | 0.071 * |
| 26 (8–50) | 39 (34–51) | ||
| Satisfaction scale score (0–10) | 9.4 ± 0.9 | 6.8 ± 3.9 | 0.004 * |
| 10 (9–10) | 9 (4.5–9.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdoba-Fernández, A.; Lobo-Martín, A. Hemostatic Efficacy of Absorbable Gelatin Sponges for Surgical Nail Matrixectomy after Phenolization—A Blinded Randomized Controlled Trial. J. Clin. Med. 2022, 11, 2420. https://doi.org/10.3390/jcm11092420
Córdoba-Fernández A, Lobo-Martín A. Hemostatic Efficacy of Absorbable Gelatin Sponges for Surgical Nail Matrixectomy after Phenolization—A Blinded Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(9):2420. https://doi.org/10.3390/jcm11092420
Chicago/Turabian StyleCórdoba-Fernández, Antonio, and Adrián Lobo-Martín. 2022. "Hemostatic Efficacy of Absorbable Gelatin Sponges for Surgical Nail Matrixectomy after Phenolization—A Blinded Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 9: 2420. https://doi.org/10.3390/jcm11092420
APA StyleCórdoba-Fernández, A., & Lobo-Martín, A. (2022). Hemostatic Efficacy of Absorbable Gelatin Sponges for Surgical Nail Matrixectomy after Phenolization—A Blinded Randomized Controlled Trial. Journal of Clinical Medicine, 11(9), 2420. https://doi.org/10.3390/jcm11092420






