The Impact of an Elevated Uric Acid Level on the Prevalence of Coronary Artery Disease in Pancreas Transplant Candidates with Type 1 Diabetes: A Cross Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements and Definitions of Variables
2.3. Statistical Analysis
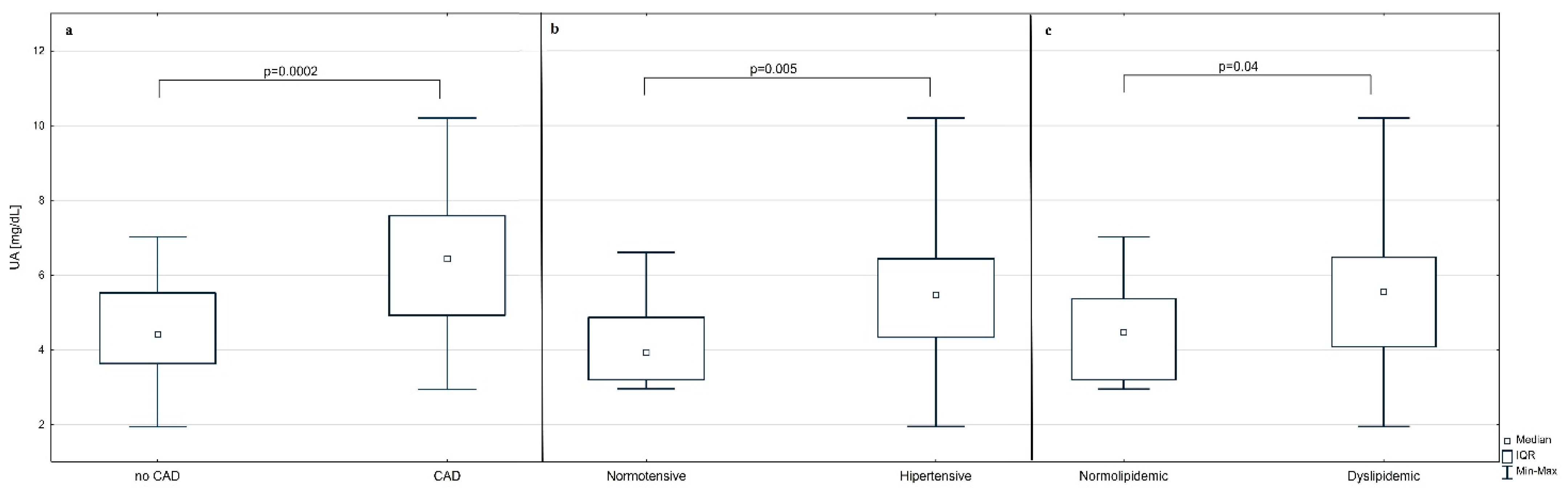
3. Results
3.1. Participant Characteristics
3.2. Uric Acid Concentration and Its Association with CAD
3.3. Relationship between Elevated/Normal UA Levels and CAD
3.4. Relationship between CAD and Selected Clinical and Laboratory Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scalea, J.R.; Pettinato, L.; Fiscella, B.; Bartosic, A.; Piedmonte, A.; Paran, J.; Todi, N.; Siskind, E.J.; Bartlett, S.T. Successful pancreas transplantation alone is associated with excellent self-identified health score and glucose control: A retrospective study from a high-volume center in the United States. Clin. Transplant. 2018, 32, e13177. [Google Scholar] [CrossRef] [PubMed]
- Hau, H.M.; Jahn, N.; Brunotte, M.; Lederer, A.A.; Sucher, E.; Rasche, F.M.; Seehofer, D.; Sucher, R. Short and long-term metabolic outcomes in patients with type 1 and type 2 diabetes receiving a simultaneous pancreas kidney allograft. BMC Endocr. Disord. 2020, 20, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmeijer, K.; Hoogeveen, E.K.; van den Boog, P.J.M.; Konijn, C.; Mallat, M.J.K.; Baranski, A.G.; Dekkers, O.M.; de Fijter, J.W.; Bemelman, F.J.; Nurmohamed, A.; et al. Superior Long-term Survival for Simultaneous Pancreas-Kidney Transplantation as Renal Replacement Therapy: 30-Year Follow-up of a Nationwide Cohort. Diabetes Care 2020, 43, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2016, 13, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Amara, D.; Braun, H.J.; Shui, A.M.; Sorrentino, T.; Ramirez, J.L.; Lin, J.; Liu, I.H.; Mello, A.; Stock, P.G.; Hiramoto, J.S. Long-term Lower Extremity and Cardiovascular Complications after Simultaneous Pancreas-Kidney Transplant. Clin. Transplant. 2021, 35, e14195. [Google Scholar] [CrossRef]
- Mangus, R.S.; Powelson, J.; Kinsella, S.B.; Farar, D.T.; Creal, C.A.; Fridell, J.A. Pretransplant coronary artery disease associated with worse clinical outcomes in pancreas transplantation. Clin. Transplant. 2013, 27, E442–E447. [Google Scholar] [CrossRef]
- St Michel, D.; Donnelly, T.; Jackson, T.; Taylor, B.; Barth, R.N.; Bromberg, J.S.; Scalea, J.R. Assessing Pancreas Transplant Candidate Cardiac Disease: Preoperative Protocol Development at a Rapidly Growing Transplant Program. Methods Protoc. 2019, 2, 82. [Google Scholar] [CrossRef] [Green Version]
- Buksinska-Lisik, M.; Kwasiborski, P.J.; Ryczek, R.; Lisik, W.; Mamcarz, A. Vitamin D Deficiency as a Predictor of a High Prevalence of Coronary Artery Disease in Pancreas Transplant Candidates with Type 1 Diabetes. Front. Endocrinol. 2021, 12, 714728. [Google Scholar] [CrossRef]
- Sundberg, F.; Natman, J.; Franzen, S.; Akesson, K.; Sarnblad, S. A decade of improved glycemic control in young children with type 1 diabetes: A population-based cohort study. Pediatr. Diabetes 2021, 22, 742–748. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Sattar, N.; Franzen, S.; McGuire, D.K.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; Rosengren, A.; et al. Relative Prognostic Importance and Optimal Levels of Risk Factors for Mortality and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation 2019, 139, 1900–1912. [Google Scholar] [CrossRef]
- Colom, C.; Rull, A.; Sanchez-Quesada, J.L.; Perez, A. Cardiovascular Disease in Type 1 Diabetes Mellitus: Epidemiology and Management of Cardiovascular Risk. J. Clin. Med. 2021, 10, 1798. [Google Scholar] [CrossRef] [PubMed]
- Shahin, L.; Patel, K.M.; Heydari, M.K.; Kesselman, M.M. Hyperuricemia and Cardiovascular Risk. Cureus 2021, 13, e14855. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Prattichizzo, F. Variability of risk factors and diabetes complications. Cardiovasc. Diabetol. 2021, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Domienik-Karłowicz, J.; Tykarski, A.; Widecka, K.; Filipiak, K.J.; Jaguszewski, M.J.; Narkiewicz, K.; Mancia, G. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol. J. 2021, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.U.A.; Browne, L.D.; Li, X.; Adeeb, F.; Perez-Ruiz, F.; Fraser, A.D.; Stack, A.G. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: A cohort study. PLoS ONE 2018, 13, e0198197. [Google Scholar] [CrossRef] [Green Version]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Gertler, M.M.; Garn, S.M.; Levine, S.A. Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann. Intern. Med. 1951, 34, 1421–1431. [Google Scholar] [CrossRef]
- Li, J.; Muraki, I.; Imano, H.; Cui, R.; Yamagishi, K.; Umesawa, M.; Hayama-Terada, M.; Ohira, T.; Kiyama, M.; Okada, T.; et al. Serum uric acid and risk of stroke and its types: The Circulatory Risk in Communities Study (CIRCS). Hypertens. Res. 2020, 43, 313–321. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, H.; Chen, X.; Fu, K.; Li, J.; Chen, H.; Teng, W.; Tian, L. Serum uric acid and risk of stroke: A dose-response meta-analysis. J. Clin. Biochem. Nutr. 2021, 68, 221–227. [Google Scholar] [CrossRef]
- Takayama, S.; Kawamoto, R.; Kusunoki, T.; Abe, M.; Onji, M. Uric acid is an independent risk factor for carotid atherosclerosis in a Japanese elderly population without metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Hara, S.; Roncal-Jimenez, C.A.; Bjornstad, P.; Nakagawa, T.; Andres-Hernando, A.; Sato, Y.; Jensen, T.; et al. Uric Acid Is a Strong Risk Marker for Developing Hypertension from Prehypertension: A 5-Year Japanese Cohort Study. Hypertension 2018, 71, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; D’Addato, S.; Borghi, C. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension: Data from the Brisighella Heart Study. J. Hypertens. 2019, 37, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Liu, W.; Zhou, Y.; Liu, Y.; Shi, D.; Zhao, Y.; Liu, X.; Alhelal, J.W.; Ravuru, K.S.S. Hyperuricemia and severity of coronary artery disease: An observational study in adults 35 years of age and younger with acute coronary syndrome. Cardiol. J. 2019, 26, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Jiang, L.; Xu, L.; Tian, J.; Liu, J.; Zhao, X.; Feng, X.; Wang, D.; Zhang, Y.; Sun, K.; et al. Implications of Hyperuricemia in Severe Coronary Artery Disease. Am. J. Cardiol. 2019, 123, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Otaki, Y.; Konta, T.; Ichikawa, K.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Narita, I.; Kondo, M.; et al. Possible burden of hyperuricaemia on mortality in a community-based population: A large-scale cohort study. Sci. Rep. 2021, 11, 8999. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [Green Version]
- Holme, I.; Aastveit, A.H.; Hammar, N.; Jungner, I.; Walldius, G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J. Intern. Med. 2009, 266, 558–570. [Google Scholar] [CrossRef]
- Jin, Y.L.; Zhu, T.; Xu, L.; Zhang, W.S.; Liu, B.; Jiang, C.Q.; Yu, H.; Huang, L.M.; Cheng, K.K.; Thomas, G.N.; et al. Uric acid levels, even in the normal range, are associated with increased cardiovascular risk: The Guangzhou Biobank Cohort Study. Int. J. Cardiol. 2013, 168, 2238–2241. [Google Scholar] [CrossRef]
- Del Pinto, R.; Viazzi, F.; Pontremoli, R.; Ferri, C.; Carubbi, F.; Russo, E. The URRAH study. Panminerva Med. 2021, 63, 416–423. [Google Scholar] [CrossRef]
- Crosta, F.; Occhiuzzi, U.; Passalacqua, G.; Occhiuzzi, E.; Cimini, A.; Grassi, D.; Ferri, C.; Marini, C.; Borghi, C.; Desideri, G. Association Between the Serum Uric Acid Levels and Lacunar Infarcts in the Elderly. J. Mol. Neurosci. 2018, 65, 385–390. [Google Scholar] [CrossRef]
- Pilemann-Lyberg, S.; Hansen, T.W.; Tofte, N.; Winther, S.A.; Theilade, S.; Ahluwalia, T.S.; Rossing, P. Uric Acid Is an Independent Risk Factor for Decline in Kidney Function, Cardiovascular Events, and Mortality in Patients with Type 1 Diabetes. Diabetes Care 2019, 42, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Braffett, B.H.; Basu, A.; Bebu, I.; Dagogo-Jack, S.; Orchard, T.J.; Wallia, A.; Lopes-Virella, M.F.; Garvey, W.T.; Lachin, J.M.; et al. Serum urate and cardiovascular events in the DCCT/EDIC study. Sci. Rep. 2021, 11, 14182. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology, C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality Over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Pereira, L.; Messias, A.; Fonseca, N.; Cotovio, P.; Ferreira, A.; Nolasco, F. The burden of coronary heart disease in simultaneous pancreas-kidney transplantation: Coronary angiography as a diagnostic method for all?—A retrospective study. J. Bras. Nefrol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Kivity, S.; Kopel, E.; Maor, E.; Abu-Bachar, F.; Segev, S.; Sidi, Y.; Olchovsky, D. Association of serum uric acid and cardiovascular disease in healthy adults. Am. J. Cardiol. 2013, 111, 1146–1151. [Google Scholar] [CrossRef]
- Yu, J.; Han, J.; Mao, J.; Guo, L.; Gao, W. Association between serum uric acid level and the severity of coronary artery disease in patients with obstructive coronary artery disease. Chin. Med. J. 2014, 127, 1039–1045. [Google Scholar]
- Li, L.; Zhao, M.; Wang, C.; Zhang, S.; Yun, C.; Chen, S.; Cui, L.; Wu, S.; Xue, H. Early onset of hyperuricemia is associated with increased cardiovascular disease and mortality risk. Clin. Res. Cardiol. 2021, 110, 1096–1105. [Google Scholar] [CrossRef]
- Rodrigues, T.C.; Maahs, D.M.; Johnson, R.J.; Jalal, D.I.; Kinney, G.L.; Rivard, C.; Rewers, M.; Snell-Bergeon, J.K. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care 2010, 33, 2471–2473. [Google Scholar] [CrossRef] [Green Version]
- Bjornstad, P.; Maahs, D.M.; Rivard, C.J.; Pyle, L.; Rewers, M.; Johnson, R.J.; Snell-Bergeon, J.K. Serum uric acid predicts vascular complications in adults with type 1 diabetes: The coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014, 51, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Rathmann, W.; Hauner, H.; Dannehl, K.; Gries, F.A. Association of elevated serum uric acid with coronary heart disease in diabetes mellitus. Diabete Metab. 1993, 19, 159–166. [Google Scholar] [PubMed]
- Chen, W.; Lu, D.; Huang, Z.; Xiao, L. Comment on Pilemann-Lyberg et al. Uric Acid Is an Independent Risk Factor for Decline in Kidney Function, Cardiovascular Events, and Mortality in Patients with Type 1 Diabetes. Diabetes Care 2019;42:1088–1094. Diabetes Care 2019, 42, e187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yang, F.; Xu, T.; Wang, Y.; Zhang, K.; Fu, G.; Zhang, W. Genetically predicted serum uric acid levels and the risk of coronary artery disease in patients with diabetes: A Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Efstathiadou, A.; Gill, D.; McGrane, F.; Quinn, T.; Dawson, J. Genetically Determined Uric Acid and the Risk of Cardiovascular and Neurovascular Diseases: A Mendelian Randomization Study of Outcomes Investigated in Randomized Trials. J. Am. Heart Assoc. 2019, 8, e012738. [Google Scholar] [CrossRef]
- Piani, F.; Melena, I.; Severn, C.; Chung, L.T.; Vinovskis, C.; Cherney, D.; Pyle, L.; Roncal-Jimenez, C.A.; Lanaspa, M.A.; Rewers, A.; et al. Tubular injury in diabetic ketoacidosis: Results from the diabetic kidney alarm study. Pediatr. Diabetes 2021, 22, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Cicero, A.F.G.; Borghi, C. Uric Acid and Hypertension: Prognostic Role and Guide for Treatment. J. Clin. Med. 2021, 10, 448. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.L.; Guo, L.L.; Li, H.; Li, D.; Xu, G. The interaction between serum uric acid and triglycerides level on blood pressure in middle-aged and elderly individuals in China: Result from a large national cohort study. BMC Cardiovasc. Disord. 2020, 20, 174. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Park, C.G.; Park, J.S.; Suh, S.Y.; Choi, C.U.; Kim, J.W.; Kim, S.H.; Lim, H.E.; Rha, S.W.; Seo, H.S.; et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: Invasive study. J. Hum. Hypertens. 2007, 21, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Jin, Y.M.; Hwang, S.; Cho, D.H.; Kang, D.H.; Jo, I. Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: A mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide 2013, 32, 36–42. [Google Scholar] [CrossRef]
- Mazzali, M.; Kanellis, J.; Han, L.; Feng, L.; Xia, Y.Y.; Chen, Q.; Kang, D.H.; Gordon, K.L.; Watanabe, S.; Nakagawa, T.; et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Ren. Physiol. 2002, 282, F991–F997. [Google Scholar] [CrossRef] [Green Version]
- Inserra, F.; Forcada, P.; Castellaro, A.; Castellaro, C. Chronic Kidney Disease and Arterial Stiffness: A Two-Way Path. Front. Med. 2021, 8, 765924. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Llaurado, G.; Cano, A.; Albert, L.; Ballesta, S.; Mazarico, I.; Luchtenberg, M.F.; Gonzalez-Sastre, M.; Megia, A.; Simo, R.; Vendrell, J.; et al. Arterial stiffness is highly correlated with the scores obtained from the Steno Type 1 Risk Engine in subjects with T1DM. PLoS ONE 2019, 14, e0220206. [Google Scholar] [CrossRef] [Green Version]
- Vistisen, D.; Andersen, G.S.; Hansen, C.S.; Hulman, A.; Henriksen, J.E.; Bech-Nielsen, H.; Jorgensen, M.E. Prediction of First Cardiovascular Disease Event in Type 1 Diabetes Mellitus: The Steno Type 1 Risk Engine. Circulation 2016, 133, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lin, L.H.; Gao, M.; Tang, R.N.; Ma, K.L.; Tu, Y.; Liu, H.; Liu, B.C. Association Between the Serum Uric Acid Level and the Severity of Coronary Artery Disease in a Retrospective Study of China Nondialysis CKD Patients. Metab. Syndr. Relat. Disord. 2020, 18, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Latif, W.; Karaboyas, A.; Tong, L.; Winchester, J.F.; Arrington, C.J.; Pisoni, R.L.; Marshall, M.R.; Kleophas, W.; Levin, N.W.; Sen, A.; et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin. J. Am. Soc. Nephrol. 2011, 6, 2470–2477. [Google Scholar] [CrossRef]
- Kim, C.S.; Jin, D.C.; Yun, Y.C.; Bae, E.H.; Ma, S.K.; Kim, S.W. Relationship between serum uric acid and mortality among hemodialysis patients: Retrospective analysis of Korean end-stage renal disease registry data. Kidney Res. Clin. Pract. 2017, 36, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Al-Attas, O.S.; Wani, K.; Sabico, S.; Alokail, M.S. Serum Uric Acid to Creatinine Ratio and Risk of Metabolic Syndrome in Saudi Type 2 Diabetic Patients. Sci. Rep. 2017, 7, 12104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoszkiewicz, K.; Czerwinski, J. Pobieranie i przeszczepianie narządów w Polsce w 2020 r. In Poltransplant Biuletyn Informacyjny; Poltransplant: Warsaw, Poland, 2021; Volume 1, pp. 17–19. [Google Scholar]
| Variable | Total (N = 63) | Elevated UA Level (N = 26) | Normal UA Level (N = 37) | p-Value |
|---|---|---|---|---|
| Age (years) | 41 (35–46.5) | 40.5 (33–48.75) | 41 (36–46) | 0.8 |
| Sex (Female) | 33 (52.4%) | 13 (50%) | 20 (54%) | 0.95 |
| Duration of T1D (years) | 26 (21.5–31) | 26.5 (24.25–33) | 25 (21–29) | 0.4 |
| Hemodialyzed patients | 39 (61.9%) | 18 (69.2%) | 21 (56.8%) | 0.5 |
| Duration of HD (months) | 24 (15–34.5); N = 39 | 24 (17.25–34); N = 18 | 26 (14–33); N = 21 | 0.98 |
| BMI (kg/m2) | 22.77 (20.82–24.49) | 21.3 (20.53–26.32) | 22.94 (21.36–24.24) | 0.6 |
| Hypertension | 49 (77.8%) | 24 (92.3%) | 25 (67.6%) | 0.03 |
| Dyslipidemia | 44 (69.8%) | 23 (88.5%) | 21 (56.8%) | 0.01 |
| Statin intake | 31 (49.2%) | 17 (65.4%) | 14 (37.8%) | 0.06 |
| Smoking habit | 19 (30.2%) | 8 (30.8%) | 11 (29.7%) | 1.0 |
| CAD | 23 (36.5%) | 15 (57.7%) | 8 (21.6%) | 0.008 |
| SBP (mmHg) | 132 (125–148.5) | 137.5 (129–150.25) | 131 (124–147) | 0.2 |
| DBP (mmHg) | 77 (72–83.5) | 77 (71.25–83.75) | 77 (73–83) | 0.9 |
| PP (mmHg) | 58 (50–66) | 60 (54–67.25) | 53 (47–64) | 0.06 |
| HbA1c (%) | 7.61 (7.11–8.84) | 8.31 (7.25–9.52) | 7.47 (6.95–8.22) | 0.1 |
| eGFR (mL/min/1.73 m2) | 88.1 (26.63–109.1); N = 24 | 19.75 (14.55–31.68); N = 8 | 91.65 (86.03–114.57); N = 16 | 0.005 |
| TC (mmol/L) | 4.7 (3.65–5.6) | 4.95 (3.73–5.65) | 4.3 (3.6–5.1) | 0.3 |
| LDL-C (mmol/L) | 2.5 (1.95–2.95) | 2.65 (1.9–2.98) | 2.4 (2–2.8) | 0.6 |
| HDL-C (mmol/L) | 1.4 (1.2–1.6) | 1.35 (1.2–1.6) | 1.4 (1.2–1.6) | 0.9 |
| TG (mmol/L) | 1.5 (1.1–1.9) | 1.85 (1.25–2.08) | 1.3 (1–1.8) | 0.006 |
| UA (mg/dL) | 4.92 (3.94–6.34) | N/A | N/A | N/A |
| Variable | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Age (years) | 0.129 * | 0.314 |
| Duration of T1D (years) | 0.079 * | 0.540 |
| Duration of HD (months); N = 39 | −0.037 ** | 0.825 |
| BMI (kg/m2) | 0.022 ** | 0.862 |
| SBP (mmHg) | 0.271 * | 0.032 |
| DBP (mmHg) | 0.081 * | 0.528 |
| PP (mmHg) | 0.327 ** | 0.009 |
| eGFR (mL/min/1.73 m2); N = 24 | −0.534 * | 0.007 |
| TC (mmol/L) | 0.147 * | 0.249 |
| LDL-C (mmol/L) | 0.067 * | 0.601 |
| HDL-C (mmol/L) | −0.005 ** | 0.970 |
| TG (mmol/L) | 0.354 ** | 0.004 |
| HbA1c (%) | 0.166 ** | 0.195 |
| Variable | Total (N = 63) | CAD (N = 23) | No CAD (N = 40) | p-Value |
|---|---|---|---|---|
| Age (years) | 41 (35–46.5) | 43 (37–51) | 40 (34.5–46) | 0.2 |
| Sex (Female) | 33 (52.4%) | 13 (56.5%) | 20 (50%) | 0.8 |
| Duration of T1D (years) | 26 (21.5–31) | 27 (24–34) | 25.5 (20.5–29) | 0.3 |
| Hemodialyzed patients | 39 (61.9%) | 18 (78.3%) | 21 (52.5%) | 0.06 |
| Duration of HD (months) | 24 (15–34.5); N = 39 | 27.5 (18–36); N = 18 | 22 (10–27); N = 21 | 0.06 |
| BMI (kg/m2) | 22.77 (20.82–24.49) | 21.4 (20.2–25.1) | 22.8 (20.9–24.4) | 0.8 |
| Hypertension | 49 (77.8%) | 21 (91.3%) | 28 (70%) | 0.06 |
| Dyslipidemia | 44 (69.8%) | 19 (82.6%) | 25 (62.5%) | 0.2 |
| Statin intake | 31 (49.2%) | 15 (65.2%) | 16 (40%) | 0.07 |
| Smoking habit | 19 (30.2%) | 9 (39.1%) | 10 (25%) | 0.3 |
| CAD | 23 (36.5%) | N/A | N/A | N/A |
| SBP (mmHg) | 132 (125–148.5) | 145 (127–158) | 131 (124.5–138) | 0.02 |
| DBP (mmHg) | 77 (72–83.5) | 78 (71–88) | 76.5 (72.5–83.5) | 0.5 |
| PP (mmHg) | 58 (50–66) | 63 (59–74) | 53 (48–59.5) | 0.002 |
| HbA1c (%) | 7.61 (7.11–8.84) | 7.82 (7.21–9.3) | 7.46 (6.895–8.64) | 0.1 |
| eGFR (mL/min/1.73 m2) | 88.1 (26.63–109.1); N = 24 | 28.6 (20.7–40.9); N = 5 | 91 (33.8–118.4); N = 19 | 0.08 |
| TC (mmol/L) | 4.7 (3.65–5.6) | 5 (3.6–5.7) | 4.35 (3.65–5.45) | 0.4 |
| LDL-C (mmol/L) | 2.5 (1.95–2.95) | 2.7 (1.8–3.2) | 2.45 (1.95–2.8) | 0.4 |
| HDL-C (mmol/L) | 1.4 (1.2–1.6) | 1.3 (1.2–1.5) | 1.4 (1.2–1.7) | 0.4 |
| TG (mmol/L) | 1.5 (1.1–1.9) | 1.8 (1.5–2.1) | 1.2 (0.95–1.7) | 0.0004 |
| UA (mg/dL) | 4.92 (3.94–6.34) | 6.43 (4.92–7.58) | 4.41 (3.63–5.52) | 0.0002 |
| UA/Cr | 1.09 (0.64–3.50) | 1.022 (0.76–1.94) | 1.26 (0.62–4.73) | 0.4 |
| Univariate Logistic Regression Analysis | ||||
|---|---|---|---|---|
| Variable | β | OR | 95% CI | p-Value |
| Sex (Male) | −0.262 | 0.769 | 0.274–2.158 | 0.6 |
| Age | 0.047 | 1.049 | 0.979–1.123 | 0.2 |
| Hypertension | 1.504 | 4.500 | 0.908–22.296 | 0.066 |
| BMI (kg/m2) | 0.012 | 1.012 | 0.878–1.166 | 0.9 |
| Statin intake | 1.034 | 2.812 | 0.969–8.167 | 0.057 |
| Smoking | 0.657 | 1.929 | 0.641–5.803 | 0.2 |
| eGFR (mL/min/1.73 m2) * | −0.028 | 0.973 | 0.945–1.001 | 0.063 |
| LDL-C (mmol/L) | 0.312 | 1.367 | 0.712–2.623 | 0.3 |
| TG (mmol/L) | 1.383 | 3.986 | 1.472–10.791 | 0.007 |
| UA (mg/dL) | 0.761 | 2.140 | 1.373–3.337 | 0.0008 |
| UA/Cr | −0.290 | 0.749 | 0.546–1.026 | 0.072 |
| SBP (mmHg) | 0.045 | 1.046 | 1.010–1.084 | 0.01 |
| DBP (mmHg) | 0.032 | 1.032 | 0.973–1.096 | 0.3 |
| PP (mmHg) | 0.087 | 1.091 | 1.030–1.156 | 0.003 |
| HD | 1.181 | 3.257 | 1.012–10.485 | 0.048 |
| Multivariate logistic regression analysis | ||||
| UA (mg/dL) | 0.715 | 2.044 | 1.261–3.311 | 0.004 |
| PP (mmHg) | 0.074 | 1.077 | 1.009–1.15 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buksińska-Lisik, M.; Kwasiborski, P.; Ryczek, R.; Lisik, W.; Mamcarz, A. The Impact of an Elevated Uric Acid Level on the Prevalence of Coronary Artery Disease in Pancreas Transplant Candidates with Type 1 Diabetes: A Cross Sectional Study. J. Clin. Med. 2022, 11, 2421. https://doi.org/10.3390/jcm11092421
Buksińska-Lisik M, Kwasiborski P, Ryczek R, Lisik W, Mamcarz A. The Impact of an Elevated Uric Acid Level on the Prevalence of Coronary Artery Disease in Pancreas Transplant Candidates with Type 1 Diabetes: A Cross Sectional Study. Journal of Clinical Medicine. 2022; 11(9):2421. https://doi.org/10.3390/jcm11092421
Chicago/Turabian StyleBuksińska-Lisik, Małgorzata, Przemysław Kwasiborski, Robert Ryczek, Wojciech Lisik, and Artur Mamcarz. 2022. "The Impact of an Elevated Uric Acid Level on the Prevalence of Coronary Artery Disease in Pancreas Transplant Candidates with Type 1 Diabetes: A Cross Sectional Study" Journal of Clinical Medicine 11, no. 9: 2421. https://doi.org/10.3390/jcm11092421
APA StyleBuksińska-Lisik, M., Kwasiborski, P., Ryczek, R., Lisik, W., & Mamcarz, A. (2022). The Impact of an Elevated Uric Acid Level on the Prevalence of Coronary Artery Disease in Pancreas Transplant Candidates with Type 1 Diabetes: A Cross Sectional Study. Journal of Clinical Medicine, 11(9), 2421. https://doi.org/10.3390/jcm11092421







