Impella to Treat Acute Myocardial Infarct-Related Cardiogenic Shock
Abstract
:1. Introduction
2. Axial Flow Pump Technology
3. Pathophysiological Rationale for Axial Flow Pump in AMICS
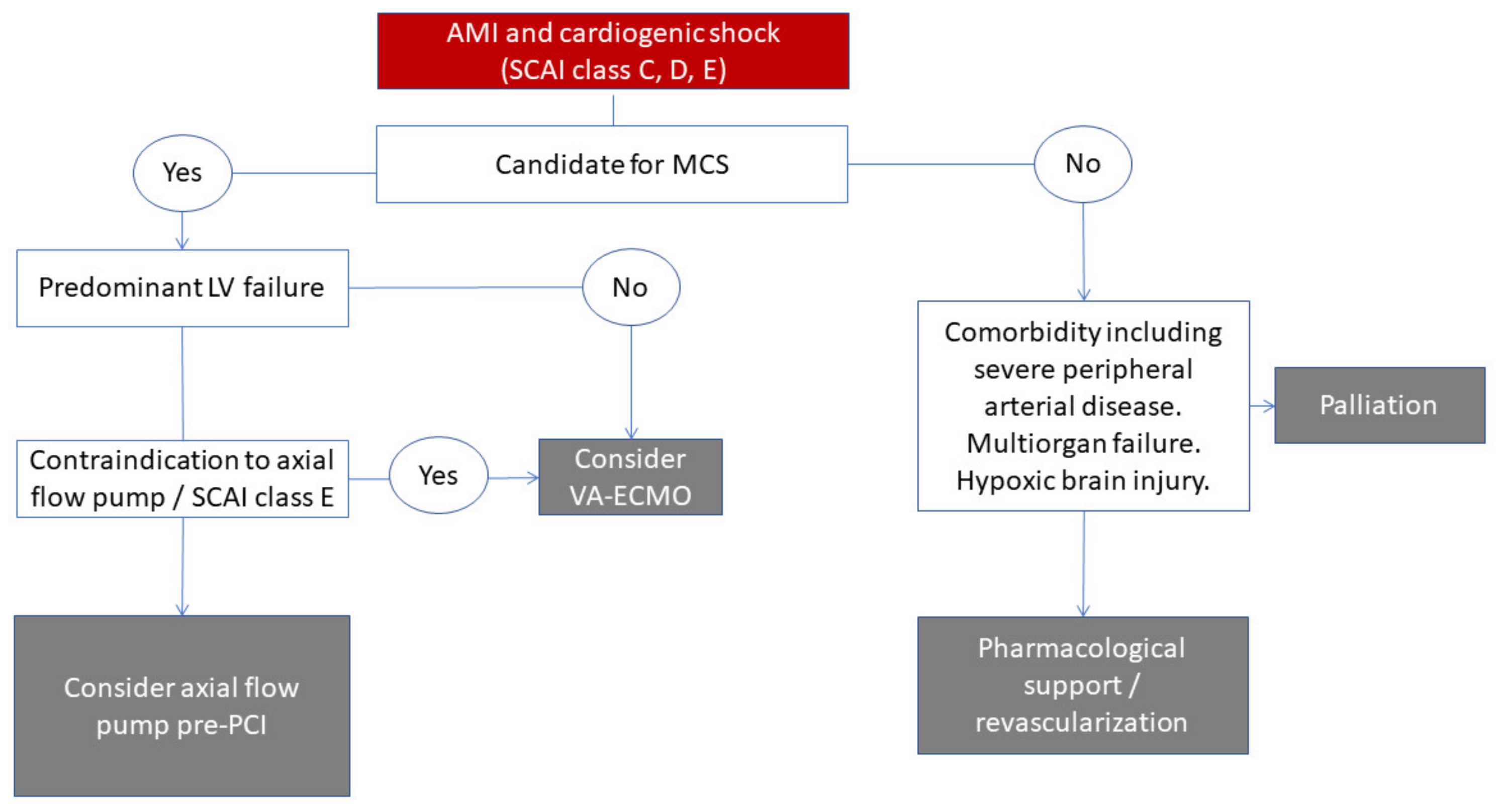
4. Patient Identification
5. Futility Criteria
6. Timing of Therapy
7. Evidence for Routine Use of Impella in AMICS
8. Escalation Strategies
9. Weaning
10. Complications
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helgestad, O.K.; Josiassen, J.; Hassager, C.; Jensen, L.O.; Holmvang, L.; Sørensen, A.; Frydland, M.; Lassen, A.; Udesen, N.L.; Schmidt, H.; et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: A Danish cohort study. Eur. J. Heart Fail. 2019, 21, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Zhang, Y.; Qiao, X.; Reyelt, L.; Paruchuri, V.; Schnitzler, G.R.; Morine, K.J.; Annamalai, S.K.; Bogins, C.; Natov, P.S.; et al. Left Ventricular Unloading Before Reperfusion Promotes Functional Recovery after Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Møller-Helgestad, O.K.; Hyldebrandt, J.A.; Banke, A.; Rud, C.S.; Udesen, N.L.; Linde, L.; Okkels-Jensen, L.; Schmidt, H.; Ravn, H.B.; Møller, J.E. Impella CP or VA-ECMO in profound cardiogenic shock: Left ventricular unloading and organ perfusion in a large animal model. EuroIntervention 2019, 14, e1585–e1592. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.P.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Use of Mechanical Circulatory Support Devices among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Netw. Open 2021, 4, e2037748. [Google Scholar] [CrossRef]
- Broderick, T.M.; Bourdillon, P.D.; Ryan, T.; Feigenbaum, H.; Dillon, J.C.; Armstrong, W.F. Comparison of regional and global left ventricular function by serial echocardiograms after reperfusion in acute myocardial infarction. J. Am. Soc. Echocardiogr. 1989, 2, 315–323. [Google Scholar] [CrossRef]
- Kapur, N.K.; Qiao, X.; Paruchuri, V.; Morine, K.J.; Syed, W.; Dow, S.; Shah, N.; Pandian, N.; Karas, R.H. Mechanical Pre-Conditioning with Acute Circulatory Support before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015, 3, 873–882. [Google Scholar] [CrossRef]
- Meyns, B.; Stolinski, J.; Leunens, V.; Verbeken, E.; Flameng, W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J. Am. Coll. Cardiol. 2003, 41, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Udesen, N.L.J.; Helgestad, O.K.L.; Banke, A.B.S.; Frederiksen, P.H.; Josiassen, J.; Jensen, L.O.; Schmidt, H.; Edelman, E.R.; Chang, B.Y.; Ravn, H.B.; et al. Impact of concomitant vasoactive treatment and mechanical left ventricular unloading in a porcine model of profound cardiogenic shock. Crit. Care 2020, 24, 95. [Google Scholar] [CrossRef] [Green Version]
- Vallabhajosyula, S.; Dunlay, S.M.; Prasad, A.; Sangaralingham, L.R.; Kashani, K.; Shah, N.D.; Jentzer, J.C. Cardiogenic shock and cardiac arrest complicating ST-segment elevation myocardial infarction in the United States, 2000–2017. Resuscitation 2020, 155, 55–64. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar]
- FFrydland, M.; Møller, J.E.; Wiberg, S.; Lindholm, M.G.; Hansen, R.; Henriques, J.P.; Møller-Helgestad, O.K.; Bang, L.E.; Frikke-Schmidt, R.; Goetze, J.P.; et al. Lactate is a Prognostic Factor in Patients Admitted with Suspected ST-Elevation Myocardial Infarction. Shock 2019, 51, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Josiassen, J.; Helgestad, O.K.L.; Møller, J.E.; Kjaergaard, J.; Hoejgaard, H.F.; Schmidt, H.; Jensen, L.O.; Holmvang, L.; Ravn, H.B.; Hassager, C. Hemodynamic and metabolic recovery in acute myocardial infarction-related cardiogenic shock is more rapid among patients presenting with out-of-hospital cardiac arrest. PLoS ONE 2020, 15, e0244294. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Pöss, J.; de Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Feistritzer, H.-J.; Rubini, M.; Huber, K.; Windecker, S.; et al. Comparison of risk prediction models in infarct-related cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Lasarte, M.; Sans-Roselló, J.; Collado-Lledó, E.; González-Fernández, V.; Noriega, F.J.; Hernández-Pérez, F.J.; Fernández-Martínez, J.; Ariza, A.; Lidón, R.-M.; Viana-Tejedor, A.; et al. External validation and comparison of the CardShock and IABP-SHOCK II risk scores in real-world cardiogenic shock patients. Eur. Heart J. Acute Cardiovasc. Care 2020, 10, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Helgestad, O.K.L.; Povlsen, A.L.; Josiassen, J.; Möller, S.; Hassager, C.; Jensen, L.; Holmvang, L.; Schmidt, H.; Møller, J.; Ravn, H.B. Data-driven point-of-care risk model in patients with acute myocardial infarction and cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Fernandez-Gatta, M.; Merchan-Gomez, S.; Toranzo-Nieto, I.; Gonzalez-Cebrian, M.; Diego-Nieto, A.; Barrio, A.; Martin-Herrero, F.; Sanchez, P.L. Short-term mechanical circulatory support in elderly patients. Artif. Organs 2021, 46, 867–877. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Lassus, J.; Sionis, A.; Køber, L.; Tarvasmäki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef]
- Zweck, E.; Thayer, K.L.; Helgestad, O.K.L.; Kanwar, M.; Ayouty, M.; Garan, A.R.; Hernandez-Montfort, J.; Mahr, C.; Wencker, D.; Sinha, S.S.; et al. Phenotyping Cardiogenic Shock. J. Am. Heart Assoc. 2021, 10, e020085. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Divchev, D.; Syntila, S.; Scheele, N.; Al Eryani, H.; Tousoulis, D.; Schieffer, B.; et al. Early Impella Support in Postcardiac Arrest Cardiogenic Shock Complicating Acute Myocardial Infarction Improves Short- and Long-Term Survival. Crit. Care Med. 2021, 49, 943–955. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Grines, C.; Schreiber, T.; Moses, J.; Maini, B.; Dixon, S.R.; Ohman, E.M. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am. Heart J. 2018, 202, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Masiero, G.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Timing of Impella implantation and outcomes in cardiogenic shock or high-risk percutaneous coronary revascularization. Catheter Cardiovasc. Interv. 2021, 98, E222–E234. [Google Scholar] [CrossRef]
- Kapur, N.K.; Alkhouli, M.; DeMartini, T.J.; Faraz, H.; George, Z.H.; Goodwin, M.J.; Hernandez-Montfort, J.A.; Iyer, V.S.; Josephy, N.; Kalra, S.; et al. Unloading the Left Ventricle before Reperfusion in Patients with Anterior ST-Segment-Elevation Myocardial Infarction. Circulation 2019, 139, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef] [Green Version]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock after Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.-M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs. Intra-aortic Balloon Pump with In-Hospital Mortality and Major Bleeding among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef]
- Ba, M.R.F.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar]
- Udesen, N.J.; Møller, J.E.; Lindholm, M.G.; Eiskjær, H.; Schäfer, A.; Werner, N.; Holmvang, L.; Terkelsen, C.J.; Jensen, L.O.; Junker, A.; et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am. Heart J. 2019, 214, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Syed, M.; Patel, B.; Munir, M.B.; Kheiri, B.; Caccamo, M.; Sokos, G.; Balla, S.; Basir, M.B.; Kapur, N.K.; et al. Invasive Hemodynamic Monitoring in Cardiogenic Shock Is Associated with Lower In-Hospital Mortality. J. Am. Heart Assoc. 2021, 10, e021808. [Google Scholar] [CrossRef]
- Ancona, M.B.; Montorfano, M.; Masiero, G.; Burzotta, F.; Briguori, C.; Pagnesi, M.; Pazzanese, V.; Trani, C.; Piva, T.; De Marco, F.; et al. Device-related complications after Impella mechanical circulatory support implantation: An IMP-IT observational multicentre registry substudy. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Gragnano, F.; Carrara, G.; Gargiulo, G.; Frigoli, E.; Vranckx, P.; Di Maio, D.; Spedicato, V.; Monda, E.; Fimiani, L.; et al. Prognostic Implications of Declining Hemoglobin Content in Patients Hospitalized with Acute Coronary Syndromes. J. Am. Coll. Cardiol.. 2021, 77, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Davodian, L.W.; Larsen, J.K.; Povlsen, A.L.; Josiassen, J.; Helgestad, O.K.; Udesen, N.L.; Hassager, C.; Schmidt, H.; Kjaergaard, J.; Holmvang, L.; et al. Timing and Causes of Death in Acute Myocardial Infarction Complicated by Cardiogenic Shock (from the RETROSHOCK Cohort). Am. J. Cardiol. 2022, 171, 25797. [Google Scholar] [CrossRef]

| Experimental data suggest axial flow pumps may lower wall stress, reduce myocardial oxygen consumption and reduce infarct size during coronary occlusion. |
| Candidacy for MCS including Impella should be decided when a shock is diagnosed and decided by the shock team. |
| Impella CP is likely best suited in the SCAI class C patient with predominantly LV failure and objective signs of hypoperfusion (elevated lactate). |
| Registry data are conflicting and available randomized trials are not adequately powered for mortality. Until adequately sized randomized trials are available, the use of the device should be based on shock team evaluation. |
| Pre-PCI placement of Impella should be considered in hemodynamically compromised patients, especially those with complex coronary anatomy. |
| Patients should be monitored with a pulmonary artery catheter in the intensive care unit combined with frequent lactate measurements and imaging to screen for device displacement, biventricular failure, and a need for escalation. |
| Most frequent complications are accessing site-related bleeding and limb ischemia that are more frequent than what is seen in patients supported by IABP. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Møller, J.E.; Kjaergaard, J.; Terkelsen, C.J.; Hassager, C. Impella to Treat Acute Myocardial Infarct-Related Cardiogenic Shock. J. Clin. Med. 2022, 11, 2427. https://doi.org/10.3390/jcm11092427
Møller JE, Kjaergaard J, Terkelsen CJ, Hassager C. Impella to Treat Acute Myocardial Infarct-Related Cardiogenic Shock. Journal of Clinical Medicine. 2022; 11(9):2427. https://doi.org/10.3390/jcm11092427
Chicago/Turabian StyleMøller, Jacob Eifer, Jesper Kjaergaard, Christian Juhl Terkelsen, and Christian Hassager. 2022. "Impella to Treat Acute Myocardial Infarct-Related Cardiogenic Shock" Journal of Clinical Medicine 11, no. 9: 2427. https://doi.org/10.3390/jcm11092427





