Association between Metabolically Healthy Obesity and Subclinical Atherosclerosis in the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Anthropometry Measurements
2.3. Laboratory Assays
2.4. Definition of MH and OB Phenotype
2.5. Assessment of Carotid Atherosclerosis
2.6. Assessment of Nonalcoholic Fatty Liver Disease (NAFLD)
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Arnlöv, J.; Ingelsson, E.; Sundström, J.; Lind, L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010, 121, 230–236. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Zieske, A.W.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002, 105, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef]
- St-Pierre, A.C.; Cantin, B.; Mauriège, P.; Bergeron, J.; Dagenais, G.R.; Després, J.P.; Lamarche, B. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ 2005, 172, 1301–1305. [Google Scholar] [CrossRef] [Green Version]
- Meigs, J.B.; Wilson, P.W.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D’Agostino, R.B. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef]
- Kramer, C.K.; Zinman, B.; Retnakaran, R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 758–769. [Google Scholar] [CrossRef]
- Zheng, R.; Zhou, D.; Zhu, Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1024–1031. [Google Scholar] [CrossRef]
- Commodore-Mensah, Y.; Lazo, M.; Tang, O.; Echouffo-Tcheugui, J.B.; Ndumele, C.E.; Nambi, V.; Wang, D.; Ballantyne, C.; Selvin, E. High burden of subclinical and cardiovascular disease risk in adults with metabolically healthy obesity: The atherosclerosis risk in communities (ARIC) study. Diabetes Care 2021, 44, 1657–1663. [Google Scholar] [CrossRef]
- Rossello, X.; Fuster, V.; Oliva, B.; Sanz, J.; Friera, L.A.F.; López-Melgar, B.; Mendiguren, J.M.; Lara-Pezzi, E.; Bueno, H.; Fernández-Ortiz, A.; et al. Association between body size phenotypes and subclinical atherosclerosis. J. Clin. Endocrinol. Metab. 2020, 105, 3734–3744. [Google Scholar] [CrossRef]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 956–966. [Google Scholar] [CrossRef]
- Lassale, C.; Tzoulaki, I.; Moons, K.G.M.; Sweeting, M.; Boer, J.; Johnson, L.; Huerta, J.M.; Agnoli, C.; Freisling, H.; Weiderpass, E.; et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: A pan-European case-cohort analysis. Eur. Heart J. 2018, 39, 397–406. [Google Scholar] [CrossRef]
- Eckel, N.; Li, Y.; Kuxhaus, O.; Stefan, N.; Hu, F.B.; Schulze, M.B. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the nurses’ health study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 714–724. [Google Scholar] [CrossRef]
- Zembic, A.; Eckel, N.; Stefan, N.; Baudry, J.; Schulze, M.B. An empirically derived definition of metabolically healthy obesity based on risk of cardiovascular and total mortality. JAMA Netw. Open 2021, 4, e218505. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Song, B.M.; Lee, J.H.; Lee, S.W.; Park, J.H.; Choi, D.P.; Lee, M.H.; Ha, K.H.; Kim, D.J.; Park, S.; et al. Cardiovascular and metabolic diseases etiology research center (CMERC) cohort: Study protocol and results of the first 3 years of enrollment. Epidemiol. Health 2017, 39, e2017016. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.S.; Song, B.M.; Lee, J.H.; Lee, S.W.; Park, J.H.; Choi, D.P.; Lee, M.H.; Ha, K.H.; Kim, D.J.; Park, S.; et al. Cohort profile: The cardiovascular and metabolic diseases etiology research center cohort in Korea. Yonsei Med. J. 2019, 60, 804–810. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Cho, S.; Shin, A.; Choi, J.Y.; Park, S.M.; Kang, D.; Lee, J.K. Optimal cutoff values for anthropometric indices of obesity as discriminators of metabolic abnormalities in Korea: Results from a health examinees study. BMC Public Health 2021, 21, 459. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically healthy obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Seo, M.H.; Lee, W.-Y.; Kim, S.S.; Kang, J.-H.; Kang, J.-H.; Kim, K.K.; Kim, B.-Y.; Kim, Y.-H.; Kim, W.-J.; Kim, E.M.; et al. 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez, R.H.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European stroke conferences, Mannheim, Germany, 2004, brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakami, N.; Kaneto, H.; Shimomura, I. Carotid ultrasonography: A potent tool for better clinical practice in diagnosis of atherosclerosis in diabetic patients. J. Diabetes Investig. 2014, 5, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Fan, J.; Song, Y.; Chen, Y.; Hui, R.; Zhang, W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 168, 4761–4768. [Google Scholar] [CrossRef]
- Ortega, F.B.; Cadenas-Sanchez, C.; Migueles, J.H.; Labayen, I.; Ruiz, J.R.; Sui, X.; Blair, S.N.; Martínez-Vizcaino, V.; Lavie, C.J. Role of physical activity and fitness in the characterization and prognosis of the metabolically healthy obesity phenotype: A systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2018, 61, 190–205. [Google Scholar] [CrossRef]
- Roberson, L.L.; Aneni, E.C.; Maziak, W.; Agatston, A.; Feldman, T.; Rouseff, M.; Tran, T.; Blaha, M.J.; Santos, R.D.; Sposito, A.; et al. Beyond BMI: The metabolically healthy obese phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality—A systematic review. BMC Public Health 2014, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Opio, J.; Croker, E.; Odongo, G.S.; Attia, J.; Wynne, K.; McEvoy, M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: A systematic review and meta-analysis of prospective cohort studies. Obes. Rev. 2020, 21, e13127. [Google Scholar] [CrossRef]
- Zhou, Z.; Macpherson, J.; Gray, S.R.; Gill, J.M.R.; Welsh, P.; Celis-Morales, C.; Sattar, N.; Pell, J.P.; Ho, F.K. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381,363 UK biobank participants. Diabetologia 2021, 64, 1963–1972. [Google Scholar] [CrossRef]
- Price, G.M.; Uauy, R.; Breeze, E.; Bulpitt, C.J.; Fletcher, A.E. Weight, shape, and mortality risk in older persons: Elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am. J. Clin. Nutr. 2006, 84, 449–460. [Google Scholar] [CrossRef]
- Sims, E.A. Are there persons who are obese, but metabolically healthy? Metabolism 2001, 50, 1499–1504. [Google Scholar] [CrossRef]
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef] [Green Version]
- Kusminski, C.M.; Holland, W.L.; Sun, K.; Park, J.; Spurgin, S.B.; Lin, Y.; Askew, G.R.; Simcox, J.A.; McClain, D.A.; Li, C.; et al. Mitoneet-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 2012, 18, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Sung, K.C.; Cha, S.C.; Sung, J.W.; So, M.S.; Byrne, C.D. Metabolically healthy obese subjects are at risk of fatty liver but not of pre-clinical atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 256–262. [Google Scholar] [CrossRef]
- Vusirikala, A.; Thomas, T.; Bhala, N.; Tahrani, A.A.; Thomas, G.N.; Nirantharakumar, K. Impact of obesity and metabolic health status in the development of non-alcoholic fatty liver disease (NAFLD): A united kingdom population-based cohort study using the health improvement network (thin). BMC Endocr. Disord. 2020, 20, 96. [Google Scholar] [CrossRef]
- Chang, Y.; Jung, H.-S.; Cho, J.; Zhang, Y.; Yun, K.E.; Lazo, M.; Pastor-Barriuso, R.; Ahn, J.; Kim, C.-W.; Rampal, S.; et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Off. J. Am. Coll. Gastroenterol. ACG 2016, 111, 1133–1140. [Google Scholar] [CrossRef]
- Lutz, S.Z.; Peter, A.; Machicao, F.; Lamprinou, A.; Machann, J.; Schick, F.; Königsrainer, I.; Königsrainer, A.; Fritsche, A.; Staiger, H.; et al. Genetic variation in the 11β-hydroxysteroid-dehydrogenase 1 gene determines NAFLD and visceral obesity. J. Clin. Endocrinol. Metab. 2016, 101, 4743–4751. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xu, Y.; Hu, Y.; Wang, G. The role of fibroblast growth factor 21 in the pathogenesis of non-alcoholic fatty liver disease and implications for therapy. Metabolism 2015, 64, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Zaytseva, Y.Y.; Liu, Y.; Rychahou, P.; Jiang, K.; Starr, M.E.; Kim, J.T.; Harris, J.W.; Yiannikouris, F.B.; et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 2016, 533, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchetta, I.; Cimini, F.A.; Leonetti, F.; Capoccia, D.; di Cristofano, C.; Silecchia, G.; Orho-Melander, M.; Melander, O.; Cavallo, M.G. Increased plasma proneurotensin levels identify NAFLD in adults with and without type 2 diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann. Hepatol. 2020, 19, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Maurantonio, M.; Marrazzo, A.; Rinaldi, L.; Adinolfi, L.E. Nonalcoholic fatty liver disease: Evolving paradigms. World J. Gastroenterol. 2017, 23, 6571–6592. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, D.P.; Shim, J.-S.; Kim, D.J.; Park, S.-H.; Kim, H.C. Inter-rater reliability of carotid intima-media thickness measurements in a multicenter cohort study. J. Health Inform. Stat. 2016, 41, 49–56. [Google Scholar] [CrossRef]
- Hirata, T.; Arai, Y.; Takayama, M.; Abe, Y.; Ohkuma, K.; Takebayashi, T. Carotid plaque score and risk of cardiovascular mortality in the oldest old: Results from the tooth study. J. Atheroscler. Thromb. 2018, 25, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.; Papacosta, O.; Whincup, P.; Wannamethee, G.; Walker, M.; Nicolaides, A.N.; Dhanjil, S.; Griffin, M.; Belcaro, G.; Rumley, A.; et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women. Br. Reg. Heart Study Stroke 1999, 30, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Chambless, L.E.; Folsom, A.R.; Clegg, L.X.; Sharrett, A.R.; Shahar, E.; Nieto, F.J.; Rosamond, W.D.; Evans, G. Carotid wall thickness is predictive of incident clinical stroke: The atherosclerosis risk in communities (ARIC) study. Am. J. Epidemiol. 2000, 151, 478–487. [Google Scholar] [CrossRef]




| CMERC Cohort (n = 7824) | Normal Weight (n = 2934) | Overweight (n = 2068) | Obesity (n = 2822) | p | |
|---|---|---|---|---|---|
| Age (years) | 51.52 ± 8.63 | 50.64 ± 8.86 | 52.40 ± 8.30 | 51.78 ± 8.55 | <0.001 |
| Sex (Female) | 5095 (65.1%) | 2287 (77.9%) | 1336 (64.6%) | 1472 (52.2%) | <0.001 |
| BMI (kg/m2) | 24.2 ± 3.0 | 21.4 ± 1.1 | 24.0 ± 0.6 | 27.4 ± 2.2 | <0.001 |
| WHR | 0.67 ± 0.09 | 0.61 ± 0.06 | 0.66 ± 0.07 | 0.73 ± 0.08 | <0.001 |
| SBP (mmHg) | 119.9 ± 15.4 | 114.5 ± 14.8 | 120.5 ± 14.3 | 125.1 ± 14.9 | <0.001 |
| DBP (mmHg) | 76.8 ± 10.2 | 73.4 ± 9.5 | 76.9 ± 9.5 | 80.2 ± 10.1 | <0.001 |
| FPG (mg/dl) | 95.7 ± 20.7 | 90.9 ± 16.7 | 95.4 ± 20.2 | 100.9 ± 23.4 | <0.001 |
| HbA1c (%) | 5.7 ± 0.7 | 5.5 ± 0.6 | 5.7 ± 0.7 | 5.9 ± 0.8 | <0.001 |
| HOMA-IR | 1.9 [ 1.5; 2.6] | 1.60 [1.28; 2.00] | 1.92 [1.50; 2.50] | 2.45 [1.85; 3.36] | <0.001 |
| ALT (U/L) | 25.2 ± 18.5 | 20.6 ± 13.1 | 23.9 ± 13.3 | 31.0 ± 24.2 | <0.001 |
| AST (U/L) | 25.7 ± 13.5 | 24.3 ± 9.8 | 25.0 ± 7.6 | 27.7 ± 18.9 | <0.001 |
| γGTP (IU/L) | 31.3 ± 43.2 | 23.2 ± 26.8 | 29.4 ± 29.7 | 41.1 ± 60.1 | <0.001 |
| eGFR (mL/min/1.73 m2) | 85.1 ± 13.5 | 86.4 ± 13.2 | 84.5 ± 13.6 | 84.3 ± 13.6 | <0.001 |
| Total cholesterol (mg/dL) | 195.2 ± 34.7 | 193.3 ± 33.4 | 195.2 ± 34.7 | 197.3 ± 36.0 | <0.001 |
| Triglyceride (mg/dL) | 132.5 ± 93.6 | 106.0 ± 61.5 | 133.0 ± 91.4 | 159.6 ± 113.0 | <0.001 |
| HDL-C (mg/dL) | 55.7 ± 14.0 | 60.7 ± 14.6 | 54.6 ± 13.3 | 51.3 ± 12.1 | <0.001 |
| LDL-C (mg/dL) | 115.3 ± 31.2 | 112.4 ± 29.7 | 116.1 ± 30.5 | 117.8 ± 33.0 | <0.001 |
| Hs-CRP (mg/L) | 0.58 [0.33; 1.19] | 0.42 [0.27; 0.77] | 0.57 [0.34; 1.10] | 0.82 [0.45; 1.66] | <0.001 |
| Current smoking (%) | 2416 (30.9%) | 629 (21.4%) | 634 (30.7%) | 1153 (40.9%) | <0.001 |
| Current drinking (%) | 5839 (74.6%) | 2186 (74.5%) | 1542 (74.6%) | 2111 (74.8%) | 0.964 |
| Physical activity (days/week) | <0.001 | ||||
| None | 4627 (59.1%) | 1656 (56.4%) | 1264 (61.1%) | 1707 (60.5%) | |
| <3 | 1375 (17.6%) | 498 (17.0%) | 336 (16.2%) | 541 (19.2%) | |
| ≥3 | 1822 (23.3%) | 780 (26.6%) | 468 (22.6%) | 574 (20.3%) | |
| Diabetes (%) | 237 (3.0%) | 7 (0.2%) | 20 (1.0%) | 210 (7.4%) | <0.001 |
| Hypertension (%) | 2703 (34.5%) | 601 (20.5%) | 737 (35.6%) | 1365 (48.4%) | <0.001 |
| Hyperlipidemia (%) | 1253 (35.8%) | 346 (31.0%) | 342 (36.0%) | 565 (39.3%) | <0.001 |
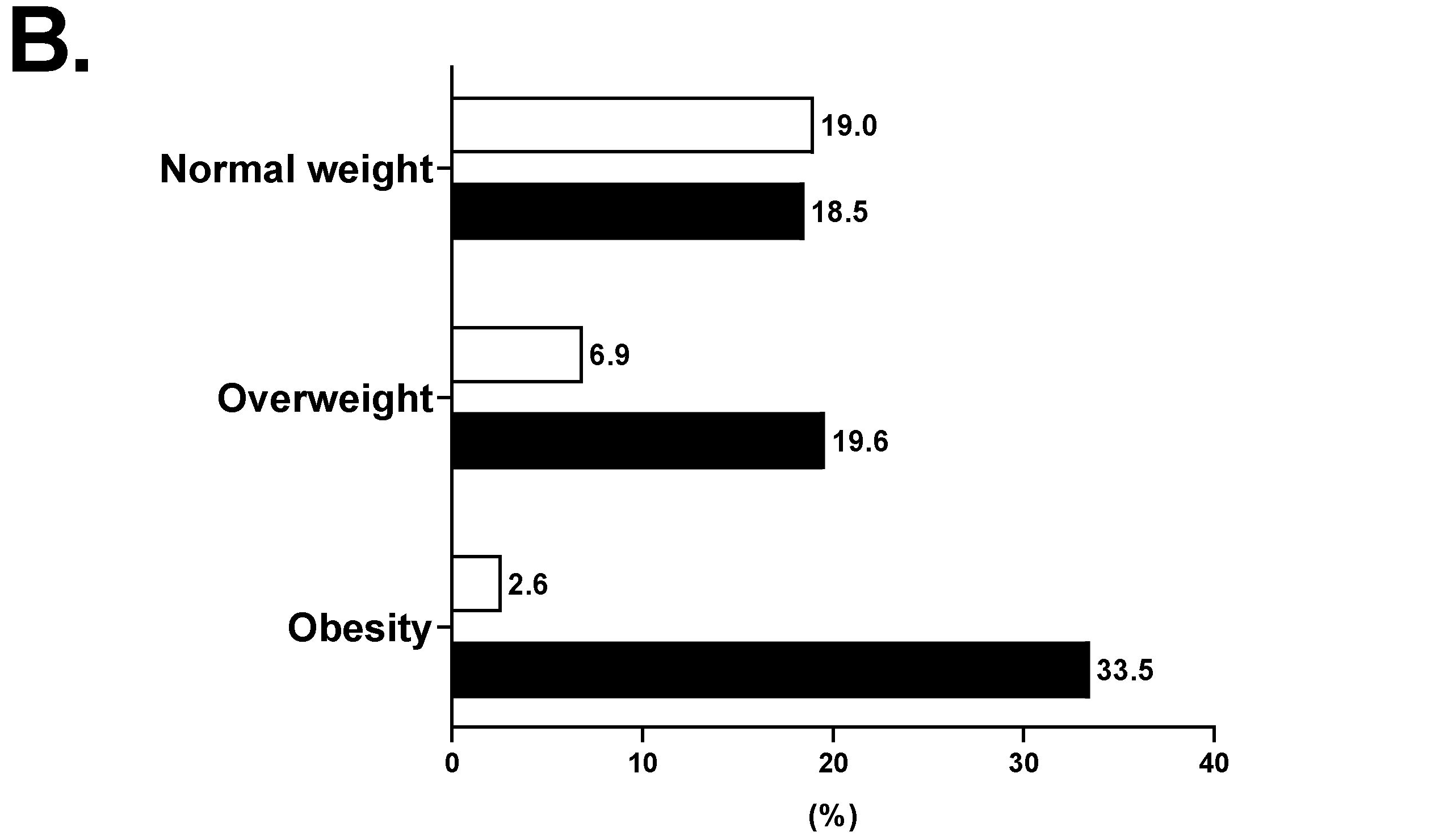
| NAFLD (%) | 1778 (22.7%) | 40 (1.4%) | 187 (9.0%) | 1551 (55.0%) | <0.001 |
| Mean IMT (mm) | 0.62 [0.55; 0.70] | 0.59 [0.54; 0.67] | 0.62 [0.56; 0.70] | 0.64 [0.57; 0.73] | <0.001 |
| Carotid atherosclerosis (%) | 653 (8.3%) | 172 (5.9%) | 179 (8.7%) | 302 (10.7%) | <0.001 |
| Metabolic phenotype | <0.001 | ||||
| Metabolically healthy | 4613 (59.0%) | 2249 (76.7%) | 1251 (60.5%) | 1113 (39.4%) | |
| Metabolically unhealthy | 3211 (41.0%) | 685 (23.3%) | 817 (39.5%) | 1709 (60.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.H.; Cho, Y.; Seo, S.; Ahn, S.H.; Hong, S.; Ha, K.H.; Shim, J.-S.; Kim, H.C.; Kim, D.J.; Kim, S.H. Association between Metabolically Healthy Obesity and Subclinical Atherosclerosis in the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) Cohort. J. Clin. Med. 2022, 11, 2440. https://doi.org/10.3390/jcm11092440
Seo DH, Cho Y, Seo S, Ahn SH, Hong S, Ha KH, Shim J-S, Kim HC, Kim DJ, Kim SH. Association between Metabolically Healthy Obesity and Subclinical Atherosclerosis in the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) Cohort. Journal of Clinical Medicine. 2022; 11(9):2440. https://doi.org/10.3390/jcm11092440
Chicago/Turabian StyleSeo, Da Hea, Yongin Cho, Seongha Seo, Seong Hee Ahn, Seongbin Hong, Kyung Hwa Ha, Jee-Seon Shim, Hyeon Chang Kim, Dae Jung Kim, and So Hun Kim. 2022. "Association between Metabolically Healthy Obesity and Subclinical Atherosclerosis in the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) Cohort" Journal of Clinical Medicine 11, no. 9: 2440. https://doi.org/10.3390/jcm11092440






