Attenuated Amplitude of Pattern Electroretinogram in Glaucoma Patients with Choroidal Parapapillary Microvasculature Dropout
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurements
2.3. Optical Coherence Tomography Angiography
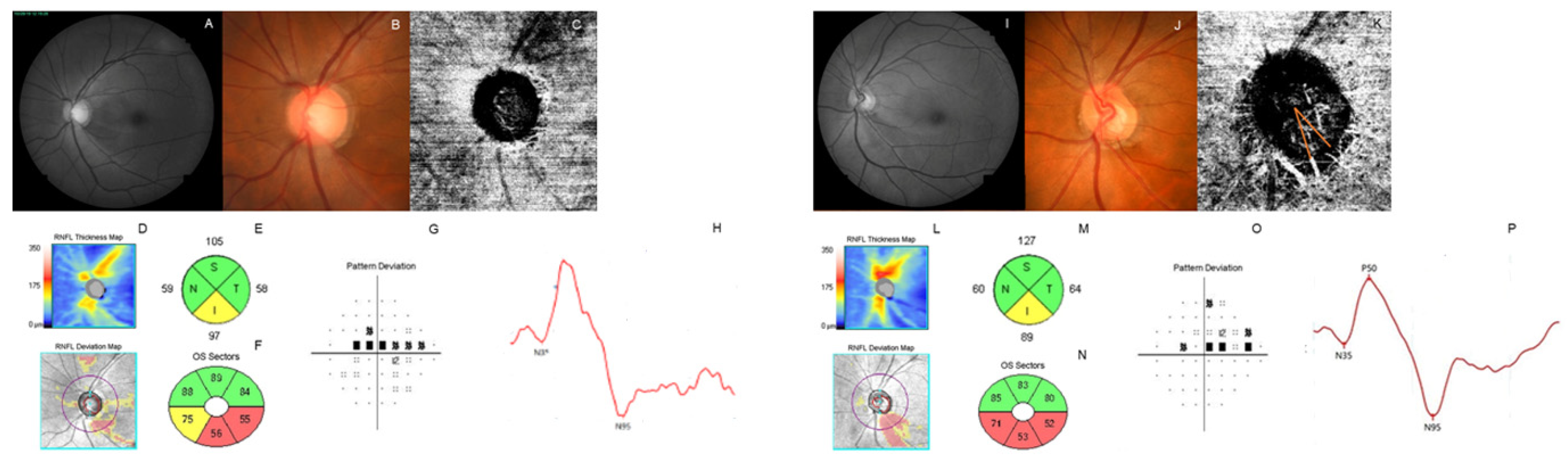
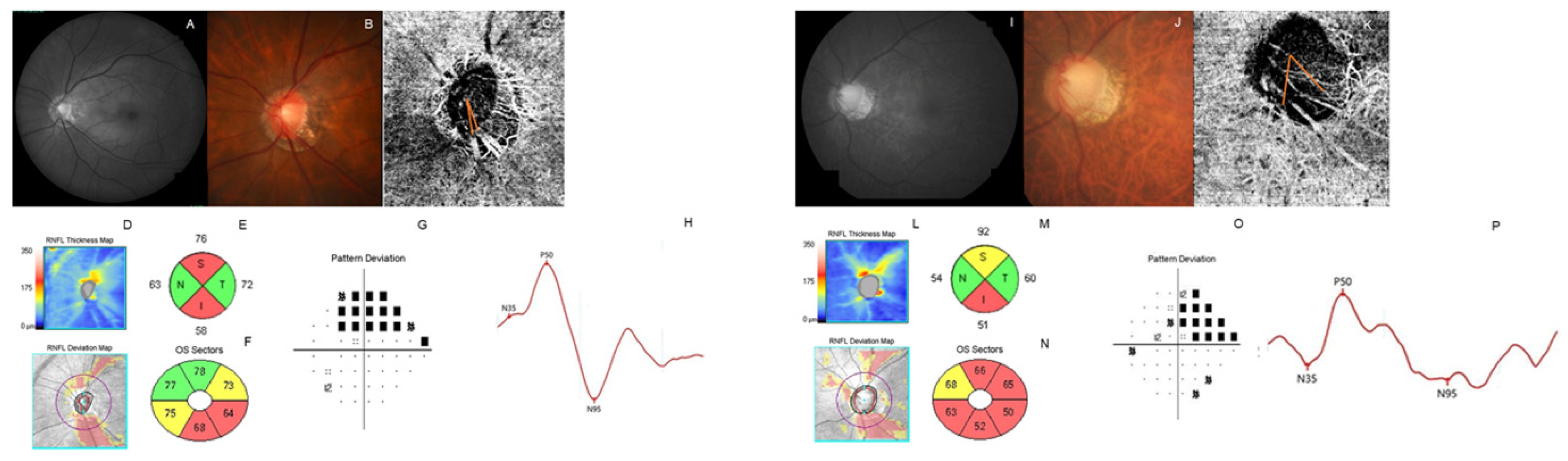
2.4. Measurement of Parapapillary Choroidal Microvasculature Dropout
2.5. Electroretinography
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W., Jr.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern® Guidelines. Ophthalmology 2016, 123, 41–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, P.J.; Mikelberg, F.S. Evaluating optic nerve damage: Pearls and pitfalls. Open Ophthalmol. J. 2009, 3, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwanza, J.C.; Budenz, D.L. Optical coherence tomography platforms and parameters for glaucoma diagnosis and progression. Curr. Opin. Ophthalmol. 2016, 27, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Grewal, D.S.; Tanna, A.P. Diagnosis of glaucoma and detection of glaucoma progression using spectral domain optical coherence tomography. Curr. Opin. Ophthalmol. 2013, 24, 150–161. [Google Scholar] [CrossRef]
- Asman, P.; Heijl, A. Glaucoma Hemifield Test. Automated visual field evaluation. Arch. Ophthalmol. 1992, 110, 812–819. [Google Scholar] [CrossRef]
- Yamashiro, H.; Tanaka, M.; Saito, M.; Shirato, S. The ability of frequency doubling technology to detect abnormality of visual function in early glaucoma. Nippon Ganka Gakkai Zasshi 2001, 105, 488–493. [Google Scholar] [CrossRef]
- Johnson, C.A. Recent developments in automated perimetry in glaucoma diagnosis and management. Curr. Opin. Ophthalmol. 2002, 13, 77–84. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef] [Green Version]
- Zangwill, L.M.; Bowd, C.; Berry, C.C.; Williams, J.; Blumenthal, E.Z.; Sánchez-Galeana, C.A.; Vasile, C.; Weinreb, R.N. Discriminating between normal and glaucomatous eyes using the Heidelberg Retina Tomograph, GDx Nerve Fiber Analyzer, and Optical Coherence Tomograph. Arch. Ophthalmol. 2001, 119, 985–993. [Google Scholar] [CrossRef]
- Greaney, M.J.; Hoffman, D.C.; Garway-Heath, D.F.; Nakla, M.; Coleman, A.L.; Caprioli, J. Comparison of optic nerve imaging methods to distinguish normal eyes from those with glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 140–145. [Google Scholar]
- Mwanza, J.C.; Budenz, D.L.; Warren, J.L.; Webel, A.D.; Reynolds, C.E.; Barbosa, D.T.; Lin, S. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br. J. Ophthalmol. 2015, 99, 732–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, D.C.; Kardon, R.H. A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijl, A.; Lindgren, A.; Lindgren, G. Test-retest variability in glaucomatous visual fields. Am. J. Ophthalmol. 1989, 108, 130–135. [Google Scholar] [CrossRef]
- Wall, M.; Woodward, K.R.; Doyle, C.K.; Artes, P.H. Repeatability of automated perimetry: A comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Investig. Ophthalmol. Vis. Sci. 2009, 50, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Akil, H.; Huang, A.S.; Francis, B.A.; Sadda, S.R.; Chopra, V. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS ONE 2017, 12, e0170476. [Google Scholar] [CrossRef]
- Mansoori, T.; Sivaswamy, J.; Gamalapati, J.S.; Balakrishna, N. Radial Peripapillary Capillary Density Measurement Using Optical Coherence Tomography Angiography in Early Glaucoma. J. Glaucoma 2017, 26, 438–443. [Google Scholar] [CrossRef]
- Ghahari, E.; Bowd, C.; Zangwill, L.M.; Proudfoot, J.; Hasenstab, K.A.; Hou, H.; Penteado, R.C.; Manalastas, P.I.C.; Moghimi, S.; Shoji, T.; et al. Association of Macular and Circumpapillary Microvasculature with Visual Field Sensitivity in Advanced Glaucoma. Am. J. Ophthalmol. 2019, 204, 51–61. [Google Scholar] [CrossRef]
- Moghimi, S.; Bowd, C.; Zangwill, L.M.; Penteado, R.C.; Hasenstab, K.; Hou, H.; Ghahari, E.; Manalastas, P.I.C.; Proudfoot, J.; Weinreb, R.N. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019, 126, 980–988. [Google Scholar] [CrossRef]
- Porciatti, V.; Falsini, B.; Brunori, S.; Colotto, A.; Moretti, G. Pattern electroretinogram as a function of spatial frequency in ocular hypertension and early glaucoma. Doc. Ophthalmol. 1987, 65, 349–355. [Google Scholar] [CrossRef]
- Tafreshi, A.; Racette, L.; Weinreb, R.N.; Sample, P.A.; Zangwill, L.M.; Medeiros, F.A.; Bowd, C. Pattern electroretinogram and psychophysical tests of visual function for discriminating between healthy and glaucoma eyes. Am. J. Ophthalmol. 2010, 149, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Kim, J.; Lee, J. Measurement of macular structure-function relationships using spectral domain-optical coherence tomography (SD-OCT) and pattern electroretinograms (PERG). PLoS ONE 2017, 12, e0178004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Jia, Y.; Takusagawa, H.L.; Pechauer, A.D.; Edmunds, B.; Lombardi, L.; Davis, E.; Morrison, J.C.; Huang, D. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015, 133, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Kromer, R.; Glusa, P.; Framme, C.; Pielen, A.; Junker, B. Optical coherence tomography angiography analysis of macular flow density in glaucoma. Acta Ophthalmol. 2019, 97, e199–e206. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Shin, D.Y.; Park, H.L.; Park, C.K. Association of Retinal Blood Flow with Progression of Visual Field in Glaucoma. Sci. Rep. 2019, 9, 16813. [Google Scholar] [CrossRef] [PubMed]
- Yip, V.C.H.; Wong, H.T.; Yong, V.K.Y.; Lim, B.A.; Hee, O.K.; Cheng, J.; Fu, H.; Lim, C.; Tay, E.L.T.; Loo-Valdez, R.G.; et al. Optical Coherence Tomography Angiography of Optic Disc and Macula Vessel Density in Glaucoma and Healthy Eyes. J. Glaucoma 2019, 28, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, S.H.; Kim, J.A.; Kim, T.W. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association With Glaucomatous Damage. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3004–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, M.H.; Zangwill, L.M.; Manalastas, P.I.; Belghith, A.; Yarmohammadi, A.; Medeiros, F.A.; Diniz-Filho, A.; Saunders, L.J.; Weinreb, R.N. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2016, 123, 2509–2518. [Google Scholar] [CrossRef] [Green Version]
- Sihota, R.; Saxena, R.; Taneja, N.; Venkatesh, P.; Sinha, A. Topography and fluorescein angiography of the optic nerve head in primary open-angle and chronic primary angle closure glaucoma. Optom. Vis. Sci. 2006, 83, 520–526. [Google Scholar] [CrossRef]
- Plange, N.; Remky, A.; Arend, O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br. J. Ophthalmol. 2003, 87, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Suprasanna, K.; Shetty, C.M.; Charudutt, S.; Kadavigere, R. Doppler evaluation of ocular vessels in patients with primary open angle glaucoma. J. Clin. Ultrasound 2014, 42, 486–491. [Google Scholar] [CrossRef]
- Shin, D.Y.; Jeon, S.J.; Kim, E.K.; Jung, K.I.; Park, H.Y.L.; Park, C.K. Association between peripapillary scleral deformation and choroidal microvascular circulation in glaucoma. Sci. Rep. 2019, 9, 18503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.W.; Jo, Y.H.; Song, M.K.; Won, H.J.; Kook, M.S. Nocturnal blood pressure dip and parapapillary choroidal microvasculature dropout in normal-tension glaucoma. Sci. Rep. 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, E.J.; Kim, J.A.; Kim, H.; Kim, T.W. Progressive retinal nerve fibre layer thinning and choroidal microvasculature dropout at the location of disc haemorrhage in glaucoma. Br. J. Ophthalmol. 2021, 105, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.A.; Kim, T.W. Influence of Choroidal Microvasculature Dropout on the Rate of Glaucomatous Progression: A Prospective Study. Ophthalmol. Glaucoma 2020, 3, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mafei, L.; Fiorentini, A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science 1981, 211, 953–955. [Google Scholar] [CrossRef]
- Ventura, L.M.; Golubev, I.; Feuer, W.J.; Porciatti, V. Pattern electroretinogram progression in glaucoma suspects. J. Glaucoma 2013, 22, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Bach, M.; Unsoeld, A.S.; Philippin, H.; Staubach, F.; Maier, P.; Walter, H.S.; Bomer, T.G.; Funk, J. Pattern ERG as an early glaucoma indicator in ocular hypertension: A long-term, prospective study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4881–4887. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.I.; Jeon, S.; Shin, D.Y.; Lee, J.; Park, C.K. Pattern Electroretinograms in Preperimetric and Perimetric Glaucoma. Am. J. Ophthalmol. 2020, 215, 118–126. [Google Scholar] [CrossRef]
- Kurysheva, N.I.; Maslova, E.V.; Trubilina, A.V.; Fomin, A.V.; Lagutin, M.B. Pattern electroretinogram and macular perfusion in glaucoma. Vestn. Oftalmol. 2018, 134, 34–40. [Google Scholar] [CrossRef]
- Lee, T.; Seo, D.R.; Kim, J.Y.; Choi, W.; Lee, S.Y.; Lee, J.M.; Seong, G.J.; Kim, C.Y.; Bae, H.W. Relationship between N95 Amplitude of Pattern Electroretinogram and Optical Coherence Tomography Angiography in Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3854. [Google Scholar] [CrossRef]
- Parodi, M.B.; Cicinelli, M.V.; Rabiolo, A.; Pierro, L.; Bolognesi, G.; Bandello, F. Vascular abnormalities in patients with Stargardt disease assessed with optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Park, H.L.; Park, C.K. Effect of Macular Vascular Density on Central Visual Function and Macular Structure in Glaucoma Patients. Sci. Rep. 2018, 8, 16009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.L.; Kim, J.W.; Park, C.K. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018, 125, 1003–1013. [Google Scholar] [CrossRef] [Green Version]
- Hodapp, E.; Parrish, R.K.; Anderson, D.R. Clinical Decisions in Glaucoma; Mosby: St Louis, MO, USA, 1993; pp. 52–61. [Google Scholar]
- Kim, J.A.; Lee, E.J.; Kim, T.W. Evaluation of Parapapillary Choroidal Microvasculature Dropout and Progressive Retinal Nerve Fiber Layer Thinning in Patients With Glaucoma. JAMA Ophthalmol. 2019, 137, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Ambrosio, L.; Bonavolontà, P.; Ambrosio, G. Pattern electroretinogram optimized for glaucoma screening (PERGLA) and retinal nerve fiber thickness in suspected glaucoma and ocular hypertension. Doc. Ophthalmol. 2010, 120, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kwon, J.; Shon, K.; Jeong, D.; Kook, M.S. Greater Severity of Glaucomatous Damage in Eyes With Than Without Choroidal Microvasculature Dropout in Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 901–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, H.M.; Lee, E.J.; Lee, S.H.; Kim, T.W. Evaluation of Peripapillary Choroidal Microvasculature to Detect Glaucomatous Damage in Eyes with High Myopia. J. Glaucoma 2020, 29, 39–45. [Google Scholar] [CrossRef]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Bach, M.; Cuno, A.K.; Hoffmann, M.B. Retinal conduction speed analysis reveals different origins of the P50 and N95 components of the (multifocal) pattern electroretinogram. Exp. Eye Res. 2018, 169, 48–53. [Google Scholar] [CrossRef]
- Bach, M.; Poloschek, C.M. Electrophysiology and glaucoma: Current status and future challenges. Cell Tissue Res. 2013, 353, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Weinreb, R.N.; Zangwill, L.M.; Suh, M.H. Parapapillary Deep-Layer Microvasculature Dropout and Visual Field Progression in Glaucoma. Am. J. Ophthalmol. 2019, 200, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kwon, J.; Jeong, D.; Shon, K.; Kook, M.S. Rapid Central Visual Field Progression Rate in Eyes with Open-Angle Glaucoma and Choroidal Microvasculature Dropout. Sci. Rep. 2019, 9, 8525. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Tepelus, T.C.; Nazikyan, T.; Sadda, S.R. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vigo, J.I.; Kudsieh, B.; Macarro-Merino, A.; Arriola-Villalobos, P.; Martínez-de-la-Casa, J.M.; García-Feijóo, J.; Fernández-Vigo, J. Reproducibility of macular and optic nerve head vessel density measurements by swept-source optical coherence tomography angiography. Eur. J. Ophthalmol. 2020, 30, 756–763. [Google Scholar] [CrossRef]

| Mean ± SD | |
|---|---|
| Age (Year) | 54.33 ± 12.27 |
| Sex (Male, %) | 46 (47.9%) |
| HTN (Yes, %) | 14 (16.9%) |
| DM (Yes, %) | 4 (4.8%) |
| CCT (μm) | 540.37 ± 39.85 |
| AL (mm) | 24.98 ± 1.25 |
| Pattern Electroretinography | |
| N35 implicit time (ms) | 22.75 ± 5.58 |
| P50 implicit time (ms) | 48.31 ± 4.29 |
| N95 implicit time (ms) | 101.72 ± 10.04 |
| P50 amplitude (mV) | 2.70 ± 0.99 |
| N95 amplitude (mV) | 4.67 ± 1.61 |
| Visual Field | |
| MD (dB) | −4.64 ± 6.53 |
| PSD (dB) | 4.97 ± 3.50 |
| OCT | |
| Average RNFLT (μm) | 74.51 ± 13.23 |
| Rim Area | 0.86 ± 0.22 |
| Disc Area | 1.97 ± 0.46 |
| Average CD ratio | 0.72 ± 0.11 |
| Average GCIPLT (μm) | 69.58 ± 8.76 |
| OCT Angiography | |
| Signal Strength | 65.92 ± 5.91 |
| Superficial VD | 39.36 ± 4.68 |
| Macular superficial VD | 34.26 ± 2.71 |
| Total PPA area (mm2) | 2.78 ± 4.64 |
| PPA VD | 49.77 ± 12.26 |
| Area of MvD (mm2) | 0.38 ± 0.40 * |
| PPA area except MvD (mm2) | 2.45 ± 4.60 * |
| Angle of MvD (°) | 52.21 ± 31.69 * |
| Angle of RNFLD (°) | 60.95 ± 56.68 * |
| MvD (−) (N, 33) | MvD (+) (N, 46) | p Value | ||
|---|---|---|---|---|
| Age (year) | 53.39 ± 13.01 | 55.93 ± 12.98 | 0.394 * | |
| Sex (male, %) | 15 (45.5%) | 23 (50%) | 0.820 # | |
| CCT (μm) | 531.79 ± 43.96 | 541.59 ± 32.65 | 0.275 * | |
| AL (mm) | 25.08 ± 1.32 | 25.37 ± 1.04 | 0.346 * | |
| Pattern Electroretinography | ||||
| N35 implicit time (ms) | 22.20 ± 5.71 | 23.48 ± 5.59 | 0.322 * | |
| P50 implicit time (ms) | 48.38 ± 4.38 | 48.57 ± 4.58 | 0.859 * | |
| N95 implicit time (ms) | 100.89 ± 9.12 | 103.00 ± 11.45 | 0.383 * | |
| P50 amplitude (mV) | 3.03 ± 1.10 | 2.42 ± 0.87 | 0.007 * | |
| N95 amplitude (mV) | 5.24 ± 1.76 | 4.14 ± 1.53 | 0.004 * | |
| Visual Field | ||||
| MD (dB) | −3.67 ± 5.20 | −6.09 ± 7.92 | 0.107 * | |
| PSD (dB) | 4.52 ± 3.55 | 5.37 ± 3.57 | 0.300 * | |
| OCT | ||||
| Optic disc parameter | Disc Area (mm2) | 1.92 ± 0.39 | 1.96 ± 0.52 | 0.729 * |
| Rim Area (mm2) | 0.85 ± 0.17 | 0.82 ± 0.23 | 0.532 * | |
| Average CD ratio | 0.72 ± 0.12 | 0.73 ± 0.12 | 0.600 * | |
| Average RNFLT (μm) | 75.06 ± 13.06 | 72.02 ± 13.25 | 0.319 * | |
| Average GCIPLT (μm) | 70.52 ± 8.70 | 67.82 ± 7.59 | 0.171 * | |
| OCT Angiography | ||||
| Signal Strength | 65.09 ± 6.73 | 65.37 ± 5.75 | 0.844 * | |
| Parapapillary VD | 38.89 ± 5.09 | 39.54 ± 4.13 | 0.565 * | |
| Macular VD | 33.63 ± 2.74 | 34.77 ± 2.65 | 0.193 * | |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Age (year) | 0.015 (0.980–1.051) | 0.416 | ||
| MD (dB) | −0.057 (0.873–1.023) | 0.164 | ||
| PSD (dB) | 0.075 (0.943–1.232) | 0.274 | ||
| N35 implicit time (ms) | 0.038 (0.958–1.126) | 0.354 | ||
| P50 implicit time (ms) | 0.018 (0.919–1.128) | 0.726 | ||
| N95 implicit time (ms) | 0.017 (0.973–1.063) | 0.451 | ||
| P50 amplitude (mV) | −0.620 (0.324–0.894) | 0.017 | ||
| N95 amplitude (mV) | −0.390 (0.502–0.913) | 0.011 | −0.668 (0.296–0.887) | 0.017 |
| Average RNFLT (μm) | −0.020 (0.946–1.015) | 0.260 | ||
| Average CD ratio | 1.311 (0.083–165.9) | 0.499 | ||
| Average GCIPLT (μm) | −0.041 (0.904–1.020) | 0.185 | ||
| Peripapillary VD | 0.029 (0.927–1.144) | 0.583 | ||
| Macular VD | 0.163 (0.974–1.423) | 0.091 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| B | p Value | B | p Value | |
| Age (year) | 0.354 | 0.347 | ||
| MD (dB) | −1.371 | 0.023 | ||
| PSD (dB) | 1.780 | 0.191 | ||
| N35 implicit time (ms) | 0.869 | 0.319 | ||
| P50 implicit time (ms) | −0.803 | 0.452 | ||
| N95 implicit time (ms) | 0.029 | 0.946 | ||
| P50 amplitude (mV) | −8.164 | 0.141 | ||
| N95 amplitude (mV) | −7.612 | 0.014 | −7.612 | 0.014 |
| Average RNFLT (μm) | −0.796 | 0.027 | ||
| Average CD ratio | 73.736 | 0.065 | ||
| Average GCIPLT (μm) | −0.965 | 0.188 |
| P50 Amplitude | N95 Amplitude | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | p Value | β | p Value | β | p Value | β | p Value | |
| Age (year) | −0.028 | 0.001 | −0.026 | 0.005 | −0.049 | <0.001 | −0.047 | 0.001 |
| MD (dB) | 0.025 | 0.106 | 0.095 | <0.001 | ||||
| PSD (dB) | 0.003 | 0.921 | −0.093 | 0.052 | ||||
| Average RNFLT (μm) | 0.013 | 0.093 | 0.046 | <0.001 | ||||
| Average CD ratio | −1.231 | 0.171 | −2.629 | 0.069 | ||||
| Average GCIPLT (μm) | 0.036 | 0.003 | 0.038 | 0.007 | 0.086 | <0.001 | 0.067 | 0.003 |
| Presence of MvD | −0.617 | 0.007 | −0.428 | 0.051 | −1.090 | 0.004 | −0.815 | 0.020 |
| Superficial VD | −0.015 | 0.553 | −0.007 | 0.854 | ||||
| Macular superficial VD | −0.034 | 0.421 | −0.018 | 0.782 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, C.K.; Jung, K.I. Attenuated Amplitude of Pattern Electroretinogram in Glaucoma Patients with Choroidal Parapapillary Microvasculature Dropout. J. Clin. Med. 2022, 11, 2478. https://doi.org/10.3390/jcm11092478
Lee J, Park CK, Jung KI. Attenuated Amplitude of Pattern Electroretinogram in Glaucoma Patients with Choroidal Parapapillary Microvasculature Dropout. Journal of Clinical Medicine. 2022; 11(9):2478. https://doi.org/10.3390/jcm11092478
Chicago/Turabian StyleLee, Jiyun, Chan Kee Park, and Kyoung In Jung. 2022. "Attenuated Amplitude of Pattern Electroretinogram in Glaucoma Patients with Choroidal Parapapillary Microvasculature Dropout" Journal of Clinical Medicine 11, no. 9: 2478. https://doi.org/10.3390/jcm11092478





