Soluble Urokinase Plasminogen Activator Receptor Levels Are Associated with Severity of Fibrosis in Patients with Primary Sclerosing Cholangitis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Cohort
2.2. Measurement of Circulating suPAR Levels
2.3. Statistical Analysis
2.4. Consent
3. Results
3.1. Baseline Patient and Clinical Characteristics—PSC Cohort
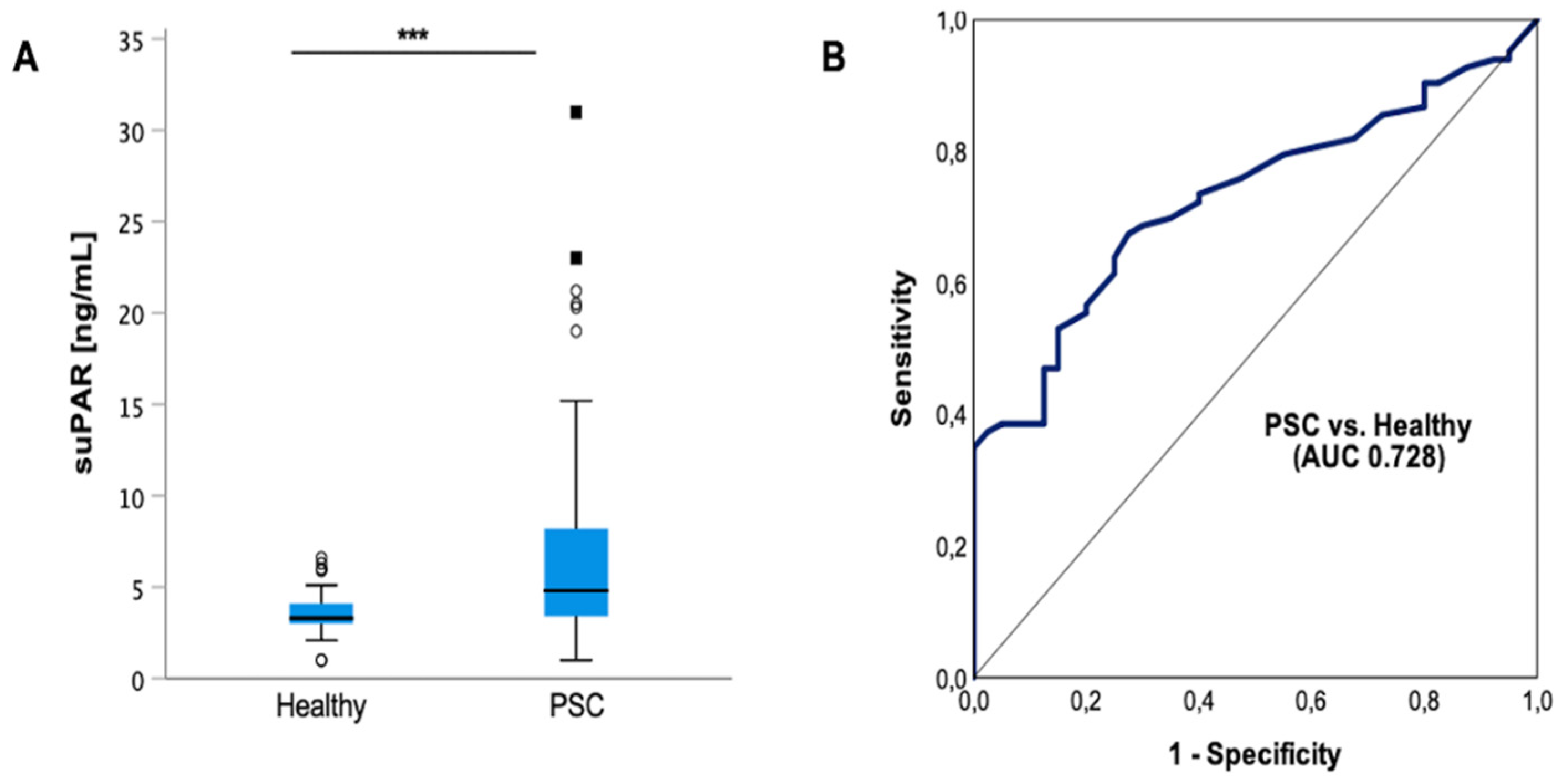
3.2. Circulating suPAR Is Elevated in Patients with PSC
3.3. suPAR Levels in PSC Patients Are not Associated with Metabolic Risk Factors or Comorbidities
3.4. Increased suPAR Levels in PSC Patients Are Associated with the Presence of Liver Cirrhosis
3.5. suPAR Is Elevated in PSC Patients with Acute Cholangitis but Does Not Indicate the Presence of Dominant Stenosis
3.6. suPAR Correlates with Prognostic Parameters in PSC Patients
3.7. suPAR Levels Are not Elevated in Patients with IBD and Do Not Reflect Disease Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| AUC | Area under the curve |
| AST | Aspartate aminotransferase |
| APRI | Aspartate transaminase-to-platelets ratio index |
| AIH | Autoimmune hepatitis |
| BMI | Body mass index |
| CRP | C-reactive protein |
| CHILD | Child–Pugh Score |
| CI | Confidence interval |
| CD | Crohn’s disease |
| ERCP | Endoscopic retrograde cholangiography–pancreatography |
| FIB4 index | Fibrosis-4 |
| GGT | Gamma-glutamyl transferase |
| IBD | Inflammatory bowel disease |
| INR | International normalized ratio |
| MRCP | Magnetic resonance cholangiopancreatography |
| MELD | Model of end-stage liver disease |
| NAFLD | Nonalcoholic fatty liver disease |
| PSC | Primary sclerosing cholangitis |
| ROC | Receiver operating characteristic |
| suPAR | Soluble urokinase-type plasminogen activator receptor |
| UC | Ulcerative Colitis |
| uPA | Urokinase plasminogen activator |
| uPAR | Urokinase plasminogen activator receptor |
References
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, J.E.; Talwalkar, J.A.; Lazaridis, K.N.; Gores, G.J.; Lindor, K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013, 145, 521–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary sclerosing cholangitis. Lancet 2013, 382, 1587–1599. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Vesterhus, M.; Boberg, K.M. Review article: Controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2014, 39, 282–301. [Google Scholar] [CrossRef]
- Chapman, M.H.; Thorburn, D.; Hirschfield, G.M.; Webster, G.G.J.; Rushbrook, S.M.; Alexander, G.; Collier, J.; Dyson, J.K.; Jones, D.E.; Patanwala, I.; et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019, 68, 1356–1378. [Google Scholar] [CrossRef] [Green Version]
- Rupp, C.; Rössler, A.; Halibasic, E.; Sauer, P.; Weiss, K.H.; Friedrich, K.; Wannhoff, A.; Stiehl, A.; Stremmel, W.; Trauner, M.; et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment. Pharmacol. Ther. 2014, 40, 1292–1301. [Google Scholar] [CrossRef]
- de Vries, E.M.; Wang, J.; Leeflang, M.M.; Boonstra, K.; Weersma, R.K.; Beuers, U.H.; Geskus, R.B.; Ponsioen, C.Y. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: Evaluation of prognostic value. Liver Int. 2016, 36, 1867–1875. [Google Scholar] [CrossRef]
- Bakhshi, Z.; Hilscher, M.B.; Gores, G.J.; Harmsen, W.S.; Viehman, J.K.; LaRusso, N.F.; Gossard, A.A.; Lazaridis, K.N.; Lindor, K.D.; Eaton, J.E. An update on primary sclerosing cholangitis epidemiology, outcomes and quantification of alkaline phosphatase variability in a population-based cohort. J. Gastroenterol. 2020, 55, 523–532. [Google Scholar] [CrossRef]
- Fossdal, G.; Mjelle, A.B.; Wiencke, K.; Bjørk, I.; Gilja, O.H.; Folseraas, T.; Karlsen, T.H.; Rosenberg, W.; Giil, L.M.; Vesterhus, M. Fluctuating biomarkers in primary sclerosing cholangitis: A longitudinal comparison of alkaline phosphatase, liver stiffness, and ELF. JHEP Rep. 2021, 3, 100328. [Google Scholar] [CrossRef]
- de Vries, E.M.; Beuers, U.; Ponsioen, C.Y. Biomarkers for disease progression of primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2015, 31, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.J.; Muir, A.J.; Levy, C.; Bowlus, C.L.; Manns, M.P.; Lu, X.; Crans, G.; Chung, C.; Subramanian, G.M.; Myers, R.P.; et al. Inter- and Intra-individual Variation, and Limited Prognostic Utility, of Serum Alkaline Phosphatase in a Trial of Patients with Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Möller, C.C.; Altintas, M.M.; Li, J.; Schwarz, K.; Zacchigna, S.; Xie, L.; Henger, A.; Schmid, H.; Rastaldi, M.P.; et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 2008, 14, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montuori, N.; Ragno, P. Multiple activities of a multifaceted receptor: Roles of cleaved and soluble uPAR. Front. Biosci. (Landmark Ed) 2009, 14, 2494–2503. [Google Scholar] [CrossRef] [Green Version]
- Guthaus, E.; Schmiedeberg, N.; Bürgle, M.; Magdolen, V.; Kessler, H.; Schmitt, M. The urokinase receptor (uPAR, CD87) as a target for tumor therapy: uPA-silica particles (SP-uPA) as a new tool for assessing synthetic peptides to interfere with uPA/uPA-receptor interaction. Recent Results Cancer Res. 2003, 162, 3–14. [Google Scholar] [CrossRef]
- Blasi, F.; Carmeliet, P. uPAR: A versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 2002, 3, 932–943. [Google Scholar] [CrossRef]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Carmeliet, P.; Schoonjans, L.; Kieckens, L.; Ream, B.; Degen, J.; Bronson, R.; De Vos, R.; van den Oord, J.J.; Collen, D.; Mulligan, R.C. Physiological consequences of loss of plasminogen activator gene function in mice. Nature 1994, 368, 419–424. [Google Scholar] [CrossRef]
- Carmeliet, P.; Moons, L.; Ploplis, V.; Plow, E.; Collen, D. Impaired arterial neointima formation in mice with disruption of the plasminogen gene. J. Clin. Investig. 1997, 99, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H.W.; Koch, A.; Seidler, S.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 2012, 32, 500–509. [Google Scholar] [CrossRef]
- Mazar, A.P. The urokinase plasminogen activator receptor (uPAR) as a target for the diagnosis and therapy of cancer. Anticancer Drugs 2001, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Han, Y.; Zhao, J.; Cui, J.; Wang, K.; Wang, R.; Liu, Y. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 39481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donadello, K.; Scolletta, S.; Covajes, C.; Vincent, J.L. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayek, S.S.; Leaf, D.E.; Samman Tahhan, A.; Raad, M.; Sharma, S.; Waikar, S.S.; Sever, S.; Camacho, A.; Wang, X.; Dande, R.R.; et al. Soluble Urokinase Receptor and Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 416–426. [Google Scholar] [CrossRef]
- Gussen, H.; Hohlstein, P.; Bartneck, M.; Warzecha, K.T.; Buendgens, L.; Luedde, T.; Trautwein, C.; Koch, A.; Tacke, F. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J. Intensive Care 2019, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Özdirik, B.; Stueven, A.; Knorr, J.; Geisler, L.; Mohr, R.; Demir, M.; Hellberg, T.; Loosen, S.H.; Benz, F.; Wiedenmann, B.; et al. Soluble Urokinase Plasminogen Activator Receptor (suPAR) Concentrations are Elevated in Patients with Neuroendocrine Malignancies. J. Clin. Med. 2020, 9, 1647. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, X.; Lu, X.; Chen, G.; Ye, X.; Huang, J. Evaluation of plasma urokinase-type plasminogen activator and urokinase-type plasminogen-activator receptor in patients with acute and chronic hepatitis B. Thromb. Res. 2009, 123, 537–542. [Google Scholar] [CrossRef]
- Berres, M.L.; Schlosser, B.; Berg, T.; Trautwein, C.; Wasmuth, H.E. Soluble urokinase plasminogen activator receptor is associated with progressive liver fibrosis in hepatitis C infection. J. Clin. Gastroenterol. 2012, 46, 334–338. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Reuken, P.A.; Koch, A.; Bartneck, M.; Adams, D.H.; Trautwein, C.; Stallmach, A.; Tacke, F.; Bruns, T. Soluble urokinase plasminogen activator receptor is compartmentally regulated in decompensated cirrhosis and indicates immune activation and short-term mortality. J. Intern. Med. 2013, 274, 86–100. [Google Scholar] [CrossRef]
- Wei, C.; Zhu, K.; Reiser, J. Soluble Urokinase Receptor and Liver Disease. Clin. Liver Dis. 2019, 14, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Zimmermann, H.W.; Gassler, N.; Jochum, C.; Weiskirchen, R.; Bruensing, J.; Buendgens, L.; Dückers, H.; Bruns, T.; Gerken, G.; et al. Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int. 2014, 34, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Kowdley, K.V.; Harrison, M.E. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2015, 110, 646–659. [Google Scholar] [CrossRef] [PubMed]
- European Association for The Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis-A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef] [Green Version]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Krüger, M.; Trautwein, C.; Duckstein, N.; Schneider, A.; Manns, M.P.; Meier, P.N. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy 2006, 38, 665–669. [Google Scholar] [CrossRef]
- Stiehl, A.; Rudolph, G.; Klöters-Plachky, P.; Sauer, P.; Walker, S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: Outcome after endoscopic treatment. J. Hepatol. 2002, 36, 151–156. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis-2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Corpechot, C.; Gaouar, F.; El Naggar, A.; Kemgang, A.; Wendum, D.; Poupon, R.; Carrat, F.; Chazouillères, O. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 2014, 146, 970–979.e6. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, A.A.; Myers, R.P. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: A systematic review. Hepatology 2007, 46, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Therneau, T.M.; Wiesner, R.H.; Poterucha, J.J.; Benson, J.T.; Malinchoc, M.; LaRusso, N.F.; Lindor, K.D.; Dickson, E.R. A revised natural history model for primary sclerosing cholangitis. Mayo Clin. Proc. 2000, 75, 688–694. [Google Scholar] [CrossRef]
- de Vries, E.M.; Wang, J.; Williamson, K.D.; Leeflang, M.M.; Boonstra, K.; Weersma, R.K.; Beuers, U.; Chapman, R.W.; Geskus, R.B.; Ponsioen, C.Y. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut 2018, 67, 1864–1869. [Google Scholar] [CrossRef] [Green Version]
- Cholongitas, E.; Papatheodoridis, G.V.; Vangeli, M.; Terreni, N.; Patch, D.; Burroughs, A.K. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment. Pharmacol. Ther. 2005, 22, 1079–1089. [Google Scholar] [CrossRef]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease (1). N. Engl. J. Med. 1991, 325, 928–937. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease (2). N. Engl. J. Med. 1991, 325, 1008–1016. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Bewtra, M.; Brensinger, C.M.; Tomov, V.T.; Hoang, T.B.; Sokach, C.E.; Siegel, C.A.; Lewis, J.D. An optimized patient-reported ulcerative colitis disease activity measure derived from the Mayo score and the simple clinical colitis activity index. Inflamm. Bowel Dis. 2014, 20, 1070–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Best, W.R. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm. Bowel Dis. 2006, 12, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Eugen-Olsen, J.; Andersen, O.; Linneberg, A.; Ladelund, S.; Hansen, T.W.; Langkilde, A.; Petersen, J.; Pielak, T.; Møller, L.N.; Jeppesen, J.; et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J. Intern. Med. 2010, 268, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Engström, G.; Björkbacka, H.; Hedblad, B. Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD. Results from the Malmö Diet and Cancer Study. Atherosclerosis 2012, 220, 502–505. [Google Scholar] [CrossRef] [Green Version]
- Hodges, G.W.; Bang, C.N.; Wachtell, K.; Eugen-Olsen, J.; Jeppesen, J.L. suPAR: A New Biomarker for Cardiovascular Disease? Can. J. Cardiol. 2015, 31, 1293–1302. [Google Scholar] [CrossRef]
- Saarinen, S.; Olerup, O.; Broomé, U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am. J. Gastroenterol. 2000, 95, 3195–3199. [Google Scholar] [CrossRef]
- Waldthaler, A.; Schramm, C.; Bergquist, A. Present and future role of endoscopic retrograde cholangiography in primary sclerosing cholangitis. Eur. J. Med. Genet. 2021, 64, 104231. [Google Scholar] [CrossRef]
- Aabakken, L.; Karlsen, T.H.; Albert, J.; Arvanitakis, M.; Chazouilleres, O.; Dumonceau, J.M.; Färkkilä, M.; Fickert, P.; Hirschfield, G.M.; Laghi, A.; et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy 2017, 49, 588–608. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, G.; Gotthardt, D.; Klöters-Plachky, P.; Kulaksiz, H.; Rost, D.; Stiehl, A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J. Hepatol. 2009, 51, 149–155. [Google Scholar] [CrossRef]
- Chapman, M.H.; Webster, G.J.; Bannoo, S.; Johnson, G.J.; Wittmann, J.; Pereira, S.P. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: A 25-year single-centre experience. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1051–1058. [Google Scholar] [CrossRef]
- Loosen, S.H.; Breuer, A.; Tacke, F.; Kather, J.N.; Gorgulho, J.; Alizai, P.H.; Bednarsch, J.; Roeth, A.A.; Lurje, G.; Schmitz, S.M.; et al. Circulating levels of soluble urokinase plasminogen activator receptor predict outcome after resection of biliary tract cancer. JHEP Rep. 2020, 2, 100080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöwall, C.; Martinsson, K.; Cardell, K.; Ekstedt, M.; Kechagias, S. Soluble urokinase plasminogen activator receptor levels are associated with severity of fibrosis in nonalcoholic fatty liver disease. Transl. Res. 2015, 165, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomi, H.; Kultti, J.; Danielsson, J.; Kangastupa, P.; Akerman, K.; Niemelä, O. Serum soluble urokinase plasminogen activator receptor in alcoholics: Relation to liver disease severity, fibrogenesis, and alcohol use. J. Gastroenterol. Hepatol. 2014, 29, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Cadranel, J.F.; Rufat, P.; Degos, F. Practices of liver biopsy in France: Results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000, 32, 477–481. [Google Scholar] [CrossRef]
- Pérez del Pulgar, S.; Pizcueta, P.; Engel, P.; Bosch, J. Enhanced monocyte activation and hepatotoxicity in response to endotoxin in portal hypertension. J. Hepatol. 2000, 32, 25–31. [Google Scholar] [CrossRef]
- May, A.E.; Kanse, S.M.; Lund, L.R.; Gisler, R.H.; Imhof, B.A.; Preissner, K.T. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J. Exp. Med. 1998, 188, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Higazi, A.A.; El-Haj, M.; Melhem, A.; Horani, A.; Pappo, O.; Alvarez, C.E.; Muhanna, N.; Friedman, S.L.; Safadi, R. Immunomodulatory effects of plasminogen activators on hepatic fibrogenesis. Clin. Exp. Immunol. 2008, 152, 163–173. [Google Scholar] [CrossRef]
- Sier, C.F.; Sidenius, N.; Mariani, A.; Aletti, G.; Agape, V.; Ferrari, A.; Casetta, G.; Stephens, R.W.; Brünner, N.; Blasi, F. Presence of urokinase-type plasminogen activator receptor in urine of cancer patients and its possible clinical relevance. Lab. Investig. 1999, 79, 717–722. [Google Scholar]
- Riisbro, R.; Christensen, I.J.; Høgdall, C.; Brünner, N.; Høgdall, E. Soluble urokinase plasminogen activator receptor measurements: Influence of sample handling. Int. J. Biol. Markers 2001, 16, 233–239. [Google Scholar] [CrossRef]
- Kofoed, K.; Schneider, U.V.; Scheel, T.; Andersen, O.; Eugen-Olsen, J. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin. Chem. 2006, 52, 1284–1293. [Google Scholar] [CrossRef]
- Koch, A.; Voigt, S.; Kruschinski, C.; Sanson, E.; Dückers, H.; Horn, A.; Yagmur, E.; Zimmermann, H.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit. Care 2011, 15, R63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolho, K.L.; Valtonen, E.; Rintamäki, H.; Savilahti, E. Soluble urokinase plasminogen activator receptor suPAR as a marker for inflammation in pediatric inflammatory bowel disease. Scand. J. Gastroenterol. 2012, 47, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Lönnkvist, M.H.; Theodorsson, E.; Holst, M.; Ljung, T.; Hellström, P.M. Blood chemistry markers for evaluation of inflammatory activity in Crohn’s disease during infliximab therapy. Scand. J. Gastroenterol. 2011, 46, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B.; Group, I.S. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| PSC Cohort n = 84 | |
|---|---|
| Gender | |
| 28 (33%) |
| 56 (67%) |
| Median current age (range) | 45 (20–71) |
| 71 (85%) |
| 13 (15%) |
| Median age at initial diagnosis (range) | 31 (9–67) |
| Mean BMI [kg/m2] (SD) | 24 ± 3.3 |
| 36/69 (52%) |
| 33/69 (48%) |
| Alcohol | |
| 7 (8%) |
| 77 (92%) |
| Smoking | |
| 7 (8%) |
| 77 (92%) |
| Comorbidities | |
| 16 (19%) |
| 2 (2%) |
| 3 (4%) |
| Overlap syndrome with AIH | |
| 14 (17%) |
| 70 (83%) |
| Presence of IBD | 60 (71%) |
| 50 (60%) |
| 6 (7%) |
| 4 (5%) |
| Presence of liver cirrhosis | |
| 27 (32%) |
| 57 (68%) |
| CHILD classification | |
| 16/27 (59%) |
| 8/27 (30%) |
| 3/27 (11%) |
| Portal hypertension | |
| 24 (29%) |
| 60 (71%) |
| Esophageal varices | |
| 67 (80%) |
| 10/17 (59%) |
| 3/17 (18%) |
| 4/17 (24%) |
| Ascites | |
| 11 (13%) |
| 73 (87%) |
| Acute Cholangitis | |
| 9 (11%) |
| 75 (89%) |
| Dominant Stenosis | |
| 53 (63%) |
| 31 (37%) |
| UDCA treatment | |
| 78 (93%) |
| 6 (7%) |
| All IBD Patients n = 68 | Crohn‘s Disease n = 39 | Ulcerative Colitis n = 29 | p-Value | |
|---|---|---|---|---|
| Gender | 0.555 | |||
| 24 (35%) | 14 (36%) | 10 (35%) | |
| 44 (65%) | 25 (64%) | 19 (66%) | |
| Median current age (range) | 42 (18–88) | 48 (18–86) | 38 (22–88) | 0.624 |
| Median age at initial diagnosis (range) | 30 (13–77) | 30 (13–77) | 27 (13–62) | 0.827 |
| Mean BMI (SD) | 24 ± 4,9 | 24 ± 5.3 | 24 (16–31) | 0.442 |
| Comorbidities | ||||
| 15 (21%) | 10 (26%) | 5 (17%) | 0.301 |
| 3 (4%) | 1 (3%) | 2 (7%) | 0.389 |
| 9 (13%) | 5 (13%) | 4 (14%) | 0.591 |
| Extraintestinal Manifestation | 0.373 | |||
| 19 (28%) | 12 (31%) | 7 (24%) | |
| 51 (73%) | 27 (69%) | 22 (76%) | |
| Disease activity at time of analysis | 0.169 | |||
| 36 (53%) | 20 (51%) | 16 (55%) | |
| 32 (47%) | 19 (49%) | 13 (45%) | |
| 18 (27%) | 14 (36%) | 4 (14%) | |
| 12 (18%) | 4 (10%) | 8 (28%) | |
| 2 (3%) | 1 (3%) | 1 (3%) | |
| IBD manifestation side | <0.001 | |||
| 1 (2%) | 1 (3%) | ||
| 3 (4%) | 3 (8%) | ||
| 19 (28%) | 19 (49%) | ||
| 6 (9%) | 5 (13%) | 1 (3%) | |
| 39 (57%) | 11 (28%) | 28 (97%) | |
| Surgery | 0.008 | |||
| 21 (31%) | 17 (44%) | 4 (14%) | |
| 47 (69%) | 22 (56%) | 25 (86%) | |
| Development of colorectal carcinoma | 0.325 | |||
| 2 (3%) | 2 (5%) | 0 (0%) | |
| 56 (97%) | 27 (95%) | 29 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdirik, B.; Maibier, M.; Scherf, M.; Nicklaus, J.M.; Frohme, J.; Puengel, T.; Meyer zum Büschenfelde, D.; Tacke, F.; Mueller, T.; Sigal, M. Soluble Urokinase Plasminogen Activator Receptor Levels Are Associated with Severity of Fibrosis in Patients with Primary Sclerosing Cholangitis. J. Clin. Med. 2022, 11, 2479. https://doi.org/10.3390/jcm11092479
Özdirik B, Maibier M, Scherf M, Nicklaus JM, Frohme J, Puengel T, Meyer zum Büschenfelde D, Tacke F, Mueller T, Sigal M. Soluble Urokinase Plasminogen Activator Receptor Levels Are Associated with Severity of Fibrosis in Patients with Primary Sclerosing Cholangitis. Journal of Clinical Medicine. 2022; 11(9):2479. https://doi.org/10.3390/jcm11092479
Chicago/Turabian StyleÖzdirik, Burcin, Martin Maibier, Maria Scherf, Jule Marie Nicklaus, Josephine Frohme, Tobias Puengel, Dirk Meyer zum Büschenfelde, Frank Tacke, Tobias Mueller, and Michael Sigal. 2022. "Soluble Urokinase Plasminogen Activator Receptor Levels Are Associated with Severity of Fibrosis in Patients with Primary Sclerosing Cholangitis" Journal of Clinical Medicine 11, no. 9: 2479. https://doi.org/10.3390/jcm11092479






