Long-Term Growth Hormone Treatment of Children with PWS: The Earlier the Start, the Better the Outcomes?
Abstract
1. Introduction
2. Methods
2.1. Patients and Controls
2.2. Design
2.3. Dual Energy X-Ray Absorptiometry (DXA)
2.4. Anthropometrics
2.5. Cognition
2.6. Assay
2.7. Blood Pressure
2.8. Bone Maturation
2.9. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Results after 8 Years of GH Treatment Compared to Age-Matched Untreated Controls
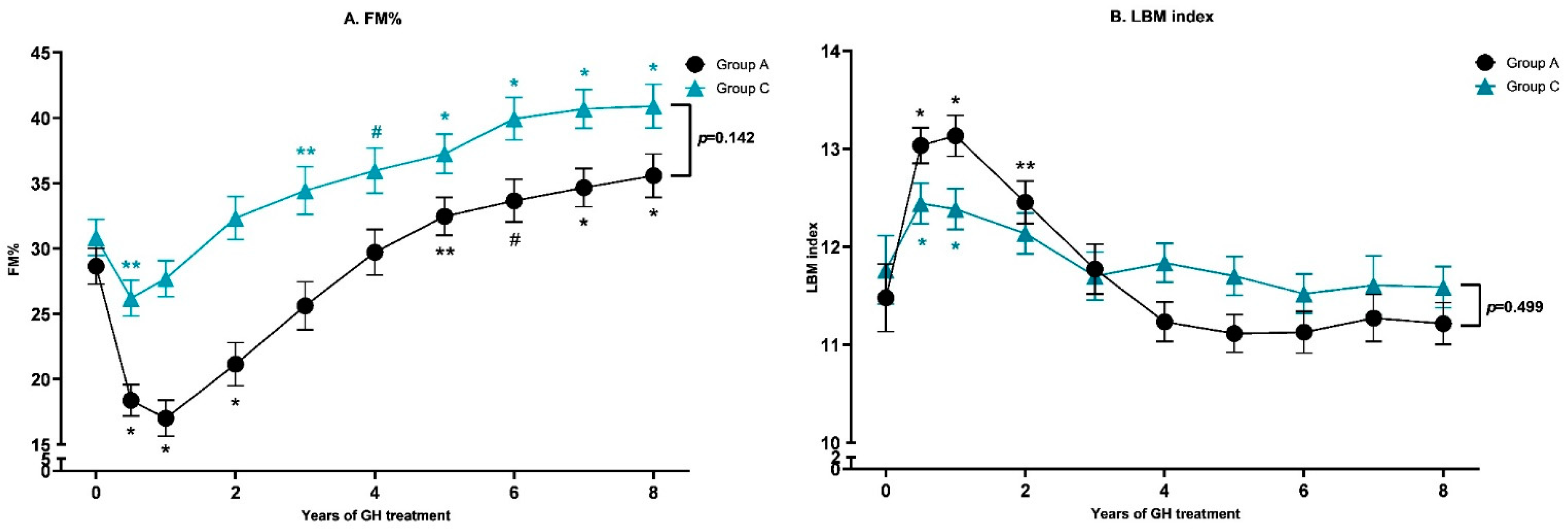
3.3. Results of 8 Years of GH Treatment in Subgroup A versus C
3.4. Body Composition
3.5. Anthropometry
3.6. Cognition
3.7. Safety Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Driscoll, D.J. Prader-Willi syndrome. Eur. J. Hum. Genet. 2009, 17, 3–13. [Google Scholar] [CrossRef]
- Deal, C.L.; Tony, M.; Hoÿbye, C.; Allen, D.B.; Tauber, M.; Christiansen, J.S.; the 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants. Growth hormone research society workshop summary: Consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1072–E1087. [Google Scholar] [CrossRef]
- Festen, D.A.M.; De Lind Van Wijngaarden, R.; Van Eekelen, M.; Otten, B.J.; Wit, J.M.; Duivenvoorden, H.J.; Hokken-Koelega, A.C.S. Randomized controlled GH trial: Effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin. Endocrinol. 2008, 69, 443–451. [Google Scholar] [CrossRef]
- Festen, D.A.M.; Wevers, M.; De Weerd, A.W.; van den Bossche, R.A.S.; Duivenvoorden, H.J.; Otten, B.J.; Wit, j.; Hokken-Koelega, A.C.S. Psychomotor development in infants with Prader-Willi syndrome and associations with sleep-related breathing disorders. Pediatr. Res. 2007, 62, 221–224. [Google Scholar] [CrossRef]
- Festen, D.A.M.; Wevers, M.; De Weerd, A.W.; Van Den Bossche, R.A.S.; Duivenvoorden, H.J.; Hokken-Koelega, A.C.S. Cognition and behavior in pre-pubertal children with Prader-Willi syndrome and associations with sleep-related breathing disorders. Am. J. Med. Genet. Part A 2008, 146, 3018–3025. [Google Scholar] [CrossRef]
- Bakker, N.E.; Kuppens, R.J.; Siemensma, E.P.C.; van Wijngaarden, R.F.A.T.L.; Festen, D.A.M.; Heus, G.C.B.B.; Bocca, G.; Haring, D.A.J.P.; Hoorweg-Nijman, J.J.G.; Houdijk, E.C.A.M.; et al. Eight years of growth hormone treatment in children with prader-willi syndrome: Maintaining the positive effects. J. Clin. Endocrinol. Metab. 2013, 98, 4013–4022. [Google Scholar] [CrossRef]
- Carrel, A.L.; Moerchen, V.; Myers, S.E.; Bekx, M.T.; Whitman, B.Y.; Allen, D.B. Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. J. Pediatr. 2004, 145, 744–749. [Google Scholar] [CrossRef]
- Magill, L.; Laemmer, C.; Woelfle, J.; Fimmers, R.; Gohlke, B. Early start of growth hormone is associated with positive effects on auxology and metabolism in Prader-Willi-syndrome. Orphanet. J. Rare Dis. 2020, 15, 283. [Google Scholar] [CrossRef]
- Angulo, M.; Abuzzahab, M.; Pietropoli, A.; Ostrow, V.; Tauber, M. SAT-282 Outcomes in Children Treated with Growth Hormone for Prader-Willi Syndrome: Data from the Answer® Program and Nordinet® International Outcome Study (IOS). J. Endocr. Soc. 2019, 3 (Suppl. S1), 1–8. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudional standers for height, weight, height velocity, weight velocity and stages of puberty. Arch. Dis. Child. 1976, 51, 170. [Google Scholar] [CrossRef]
- Guo, Y.; Franks, P.W.; Brookshire, T.; Antonio Tataranni, P. The intra- and inter-instrument reliability of DXA based on ex vivo soft tissue measurements. Obes. Res. 2004, 12, 1925–1929. [Google Scholar] [CrossRef]
- Fredriks, A.M.; Van Buuren, S.; Burgmeijer, R.J.F.; Meulmeester, J.F.; Beuker, R.J.; Brugman, E.; Roede, M.J.; Verloove-Vanhorick, S.P.; Wit, J. Continuing positive secular growth change in the Netherlands 1955–1997. Pediatr. Res. 2000, 47, 316–323. [Google Scholar] [CrossRef]
- Fredriks, A.M.; Van Buuren, S.; Wit, J.M.; Verloove-Vanhorick, S.P. Body index measurements in 1996-7 compared with 1980. Arch. Dis. Child. 2000, 82, 107–112. [Google Scholar] [CrossRef]
- van Haassen, P. Wechsler Intelligence Scale for Children-Revised (Dutch Version), Manual; Swets & Zetlinger BV: Lisse, The Netherlands, 1986. [Google Scholar]
- Tsushima, W.T. Short form of the WPPSI and WPPSI-R. J. Clin. Psychol. 1994, 50, 877–880. [Google Scholar] [CrossRef]
- Herrera-Graf, M.; Dipert, Z.J.; Hinton, R.N. Exploring the effective use of the Vocabulary/Block design short form with a special school population. Educ. Psychol. Meas. 1996, 56, 522–528. [Google Scholar] [CrossRef]
- Talkington, L.W.; Rieker, G.A. A Short Form of the WISC for Use with the Mentally Retarded. Psychol. Rep. 1969, 25, 461–462. [Google Scholar] [CrossRef]
- Hokken-Koelega, A.; van Pareren, Y.; Arends, N. Effects of growth hormone treatment on cognitive function and head circumference in children born small for gestational age. Horm. Res. 2005, 64 (Suppl. S3), 95–99. [Google Scholar] [CrossRef]
- Donze, S.H.; Damen, L.; Mahabier, E.F.; Hokken-Koelega, A.C.S. Cognitive functioning in children with Prader-Willi syndrome during 8 years of growth hormone treatment. Eur. J. Endocrinol. 2020, 182, 405–411. [Google Scholar] [CrossRef]
- van Pareren, Y.K.; Duivenvoorden, H.J.; Slijper, F.S.M.; Koot, H.M.; Hokken-Koelega, A.C.S. Intelligence and Psychosocial Functioning during Long-Term Growth Hormone Therapy in Children Born Small for Gestational Age. J. Clin. Endocrinol. Metab. 2004, 89, 5295–5302. [Google Scholar] [CrossRef][Green Version]
- De Lind Van Wijngaarden, R.F.A.; Siemensma, E.P.C.; Festen, D.A.M.; Otten, B.J.; van Mil, E.G.A.H.; Rotteveel, J.; Odink, R.J.H.; Heus, G.C.B.B.; van Leeuwen, M.; Haring, D.A.J.P.; et al. Efficacy and safety of long-term continuous growth hormone treatment in children with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 4205–4215. [Google Scholar] [CrossRef]
- Rikken, B.; Van Doorn, J.; Ringeling, A.; Van Den Brande, J.L.; Massa, G.; Wit, J.M. Plasma levels of insulin-like growth factor (IGF)-I, IGF-II and IGF-binding protein-3 in the evaluation of childhood growth hormone deficiency. Horm. Res. 1998, 50, 166–176. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Rosner, B.; Prineas, R.J.; Loggie, J.M.H.; Daniels, S.R. Blood pressure nomograms for children and adolescents, by height, sex, and age, in the United States. J. Pediatr. 1993, 123, 871–886. [Google Scholar] [CrossRef]
- Greulich, W.W.; Pyle, S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist; Stanford University Press: Stanford, CA, USA, 1959. [Google Scholar]
- Boot, A.M.; Bouquet, J.; De Ridder, M.A.J.; Krenning, E.P.; De Muinck Keizer-Schrama, S.M.P.F. Determinants of body composition measured by dual-energy X-ray absorptiometry in Dutch children and adolescents. Am. J. Clin. Nutr. 1997, 66, 232–238. [Google Scholar] [CrossRef]
- Carrel, A.L.; Myers, S.E.; Whitman, B.Y.; Allen, D.B. Benefits of long-term GH therapy in Prader-Willi Syndrome: A 4-year study. J. Clin. Endocrinol. Metab. 2002, 87, 1581–1585. [Google Scholar] [CrossRef]
- de Fluiter, K.S.; van Beijsterveldt, I.A.L.P.; Breij, L.M.; Acton, D.; Hokken-Koelega, A.C.S. Association Between Fat Mass in Early Life and Later Fat Mass Trajectories. JAMA Pediatr. 2020, 174, 1141–1148. [Google Scholar] [CrossRef]
- Lee, C.M.Y.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- He, F.; Rodriguez-Colon, S.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Bixler, E.O.; Berg, A.; Kawasawa, Y.I.; Sawyer, M.D.; Liao, D. Abdominal obesity and metabolic syndrome burden in adolescents-penn state children cohort study. J. Clin. Densitom. 2015, 18, 30–36. [Google Scholar] [CrossRef]
- Hofman, M.A. Evolution of the human brain: When bigger is better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef]
- Ayet-Roger, A.; Joga-Elvira, L.; Caixàs, A.; Corripio, R. Cognitive and Adaptive Effects of Early Growth Hormone Treatment in Prader–Willi Syndrome Patients: A Cohort Study. J. Clin. Med. 2022, 11, 1592. [Google Scholar] [CrossRef]
- Lindgren, A.C.; Lindberg, A. Growth hormone treatment completely normalizes adult height and improves body composition in Prader-Willi syndrome: Experience from KIGS (Pfizer international growth database). Horm. Res. 2008, 70, 182–187. [Google Scholar] [CrossRef]
- Bakker, N.E.; Lindberg, A.; Heissler, J.; Wollmann, H.A.; Camacho-Hü Bner, C.; Hokken-Koelega, A.C. Growth hormone treatment in children with prader-willi syndrome: Three years of longitudinal data in prepubertal children and adult height data from the KIGS database. J. Clin. Endocrinol. Metab. 2017, 102, 1702–1711. [Google Scholar] [CrossRef][Green Version]
- Lecka-Ambroziak, A.; Wysocka-Mincewicz, M.; Doleżal-Ołtarzewska, K.; Zygmunt-Górska, A.; Wędrychowicz, A.; Żak, T.; Noczyńska, A.; Birkholz-Walerzak, D.; Stawerska, R.; Hilczer, M.; et al. Effects of Recombinant Human Growth Hormone Treatment, Depending on the Therapy Start in Different Nutritional Phases in Paediatric Patients with Prader–Willi Syndrome: A Polish Multicentre Study. J. Clin. Med. 2021, 10, 3176. [Google Scholar] [CrossRef]
- Willemsen, R.H.; Van Dijk, M.; De Kort, S.W.K.; Van Toorenenbergen, A.W.; Hokken-Koelega, A.C.S. Plasma matrix metalloproteinase-9 levels and blood pressure in short children born small for gestational age and effects of growth hormone treatment. Clin. Endocrinol. 2008, 69, 264–268. [Google Scholar] [CrossRef]
| GH-Treated Group vs. GH-Untreated Controls | GH-Treated Subgroups | ||||||
|---|---|---|---|---|---|---|---|
| GH-Treated | GH-Untreated | p-Value b | A | B | C | p-Value # | |
| Number (boys) | 84 (44) | 22 (11) | 0.76 | 27 (11) | 28 (16) | 27 (17) | 0.102 |
| Genetic subtype N(%) | 0.086 | 0.273 | |||||
| Deletion | 44 (54.3) | 8 (40.0) | 13 (48.1) | 14 (51.9) | 17 (63.0) | ||
| mUPD | 36 (44.4) | 10 (50.0) | 14 (51.9) | 12 (44.4) | 10 (37.0) | ||
| ICD | 1 (1.2) | 2 (10) | 0 (0.0) | 1 (3.7) | 0 (0.0) | ||
| Age at start GH treatment (years) | 1.92 (1.20) | - | N.A. | 0.75 (0.13) | 1.64 (0.37) | 3.38 (1.20) | <0.001 |
| Fat mass percentage a | 29.7 (0.72) | - | N.A. | 29.4 (1.3) | 28.2 (1.2) | 31.5 (1.2) | 0.291 |
| Trunk/peripheral fat ratio a | 0.55 (0.02) | - | N.A. | 0.52 (0.02) | 0.50 (0.02) | 0.64 (0.02) | 0.001 |
| Height SDS | −2.01 (1.19) | - | N.A. | −1.82 (0.93) | −1.79 (1.17) | −2.42 (1.36) | 0.064 |
| Head circumference SDS | −1.05 (0.99) | - | N.A. | −1.42 (0.77) | −1.05 (1.08) | −0.73 (0.99) | 0.01 |
| Weight for height SDS | −0.13 (1.57) | - | N.A. | −0.41 (1.06) | −0.57 (1.49) | 0.60 (1.85) | 0.017 |
| BMI SDS | −0.07 (1.62) | - | N.A. | −0.74 (1.08) | 0.39 (1.54) | 0.93 (1.72) | <0.001 |
| Target height SDS | 0.42 (0.92) | 0.16 (0.71) | 0.233 | 0.59 (0.88) | 0.43 (0.92) | 0.23 (0.97) | 0.161 |
| GH-Treated Children | GH-Untreated Controls | p-Value | |
|---|---|---|---|
| (n = 82) | (n = 22) | ||
| Age (years) | 9.88 (1.19) | 10.35 (1.37) | 0.117 |
| Puberty (N, %) | 0.216 | ||
| Prepubertal | 55 (67.1) | 18 (81.8) | |
| Early pubertal | 18 (22.0) | 4 (18.2) | |
| Mid pubertal | 9 (11.1) | 0 | |
| Late pubertal | 0 | 0 | |
| Fat mass % SDS a | 1.90 (0.07) | 2.25 (0.15) | 0.036 |
| Fat mass percentage b | 38.0 (0.89) | 46.1 (1.76) | <0.001 |
| Trunk fat vs. peripheral fat ratio b | 0.82 (0.01) | 0.91 (0.02) | <0.001 |
| Lean body mass SDS a | −1.51 (0.10) | −1.92 (0.20) | 0.033 |
| Height SDS | 0.29 (1.19) | −2.01 (1.49) | <0.001 |
| Head circumference SDS | 0.60 (1.09) | −0.59 (0.75) | <0.001 |
| BMI SDS | 1.10 (1.50) | 1.63 (1.01) | 0.055 |
| Cognitive functioning (SS) | |||
| Block design | 4.29 (3.18) | 3.19 (2.66) | 0.217 |
| Vocabulary | 4.94 (2.82) | 4.81 (2.07) | 0.871 |
| Similarities | 6.16 (2.78) | 5.06 (2.93) | 0.179 |
| Estimated total IQ | 70.4 (14.1) | 67.3 (10.1) | 0.417 |
| A (n = 27) | B (n = 28) | C (n = 27) | p-Value # | |
|---|---|---|---|---|
| Age (years) | 8.75 (0.19) | 9.57 (0.35) | 11.33 (0.80) | <0.001 |
| Puberty (N (%)) | 0.002 | |||
| Prepubertal | 23 (85.2) | 20 (71.4) | 12 (44.4) | |
| Early pubertal | 4 (14.8) | 7 (25.0) | 7 (25.9) | |
| Mid pubertal | 0 | 1 (3.6) | 8 (29.6) | |
| Late pubertal | 0 | 0 | 0 | |
| Fat mass % SDS | 1.81 (0.54) | 2.07 (0.65) | 2.06 (0.87) | 0.212 |
| Fat mass percentage a | 36.0 (1.70) | 39.5 (1.62) | 40.7 (1.78) | 0.142 |
| Trunk fat vs. peripheral fat ratio a | 0.80 (0.02) | 0.82 (0.02) | 0.87 (0.02) | 0.043 |
| Lean body mass SDS | −1.49 (0.68) | −1.22 (1.19) | −1.33 (1.15) | 0.525 |
| Height SDS | −0.08 (0.86) | 0.61 (1.25) | 0.32 (1.34) | 0.199 |
| Head circumference SDS | 0.66 (0.89) | 0.63 (1.01) | 0.53 (1.29) | 0.131 |
| BMI SDS | 0.80 (1.09) | 1.10 (1.04) | 1.40 (1.26) | 0.064 |
| Cognitive functioning (SS) | ||||
| Block design | 5.29 (2.93) | 4.59 (3.19) | 3.58 (3.27) | 0.283 |
| Vocabulary | 6.71 (2.56) | 5.50 (2.72) | 3.63 (2.54) | 0.012 |
| Similarities | 7.29 (2.36) | 6.57 (2.31) | 5.26 (3.26) | 0.148 |
| Estimated total IQ | 78.1 (12.6) | 72.8 (12.9) | 64.8 (14.5) | 0.043 |
| A (n = 27) | B (n = 28) | C (n = 27) | p-Value # | |
|---|---|---|---|---|
| IGF-1 SDS | 1.92 (0.57) | 2.13 (0.68) | 2.06 (0.94) | 0.516 |
| Average IGF-1 SDS during 8 years of GH treatment | 2.36 (0.58) | 2.59 (0.45) | 2.17 (0.89) | 0.349 |
| Fasting glucose a (mmol/L) | 4.94 (0.08) | 4.84 (0.08) | 4.84 (0.09) | 0.469 |
| Fasting insulin a (pmol/L) | 73.4 (10.1) | 73.1 (10.1) | 76.0 (10.3) | 0.979 |
| HOMA-IR a | 2.32 (0.34) | 2.33 (0.34) | 2.44 (0.35) | 0.961 |
| Triglycerides a (mmol/L) | 0.84 (0.07) | 0.91 (0.07) | 0.87 (0.07) | 0.440 |
| Total cholesterol a (mmol/L) | 4.13 (0.15) | 4.32 (0.15) | 4.55 (0.16) | 0.041 |
| HDL cholesterol a (mmol/L) | 1.58 (0.07) | 1.56 (0.07) | 1.60 (0.07) | 0.944 |
| LDL cholesterol a (mmol/L) | 2.49 (0.15) | 2.55 (0.15) | 2.74 (0.16) | 0.207 |
| Systolic blood pressure SDS | 0.72 (0.89) | 0.32 (1.05) | −0.06 (0.88) | 0.002 |
| Diastolic blood pressure SDS | 0.20 (0.51) | 0.33 (0.67) | 0.35 (0.80) | 0.395 |
| BA/CA ratio | 1.04 (0.10) | 1.08 (0.14) | 1.05 (0.11) | 0.731 |
| Scoliosis | 14 (51.9) | 20 (71.4) | 18 (66.7) | 0.668 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grootjen, L.N.; Trueba-Timmermans, D.J.; Damen, L.; Mahabier, E.F.; Kerkhof, G.F.; Hokken-Koelega, A.C.S. Long-Term Growth Hormone Treatment of Children with PWS: The Earlier the Start, the Better the Outcomes? J. Clin. Med. 2022, 11, 2496. https://doi.org/10.3390/jcm11092496
Grootjen LN, Trueba-Timmermans DJ, Damen L, Mahabier EF, Kerkhof GF, Hokken-Koelega ACS. Long-Term Growth Hormone Treatment of Children with PWS: The Earlier the Start, the Better the Outcomes? Journal of Clinical Medicine. 2022; 11(9):2496. https://doi.org/10.3390/jcm11092496
Chicago/Turabian StyleGrootjen, Lionne N., Demi J. Trueba-Timmermans, Layla Damen, Eva F. Mahabier, Gerthe F. Kerkhof, and Anita C. S. Hokken-Koelega. 2022. "Long-Term Growth Hormone Treatment of Children with PWS: The Earlier the Start, the Better the Outcomes?" Journal of Clinical Medicine 11, no. 9: 2496. https://doi.org/10.3390/jcm11092496
APA StyleGrootjen, L. N., Trueba-Timmermans, D. J., Damen, L., Mahabier, E. F., Kerkhof, G. F., & Hokken-Koelega, A. C. S. (2022). Long-Term Growth Hormone Treatment of Children with PWS: The Earlier the Start, the Better the Outcomes? Journal of Clinical Medicine, 11(9), 2496. https://doi.org/10.3390/jcm11092496






