Body Mass Index and Mortality, Recurrence and Readmission after Myocardial Infarction: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Study Selection
2.5. Data Collection Process
2.6. Risk of Bias
2.7. Data Synthesis
2.8. Additional Analyses
3. Results
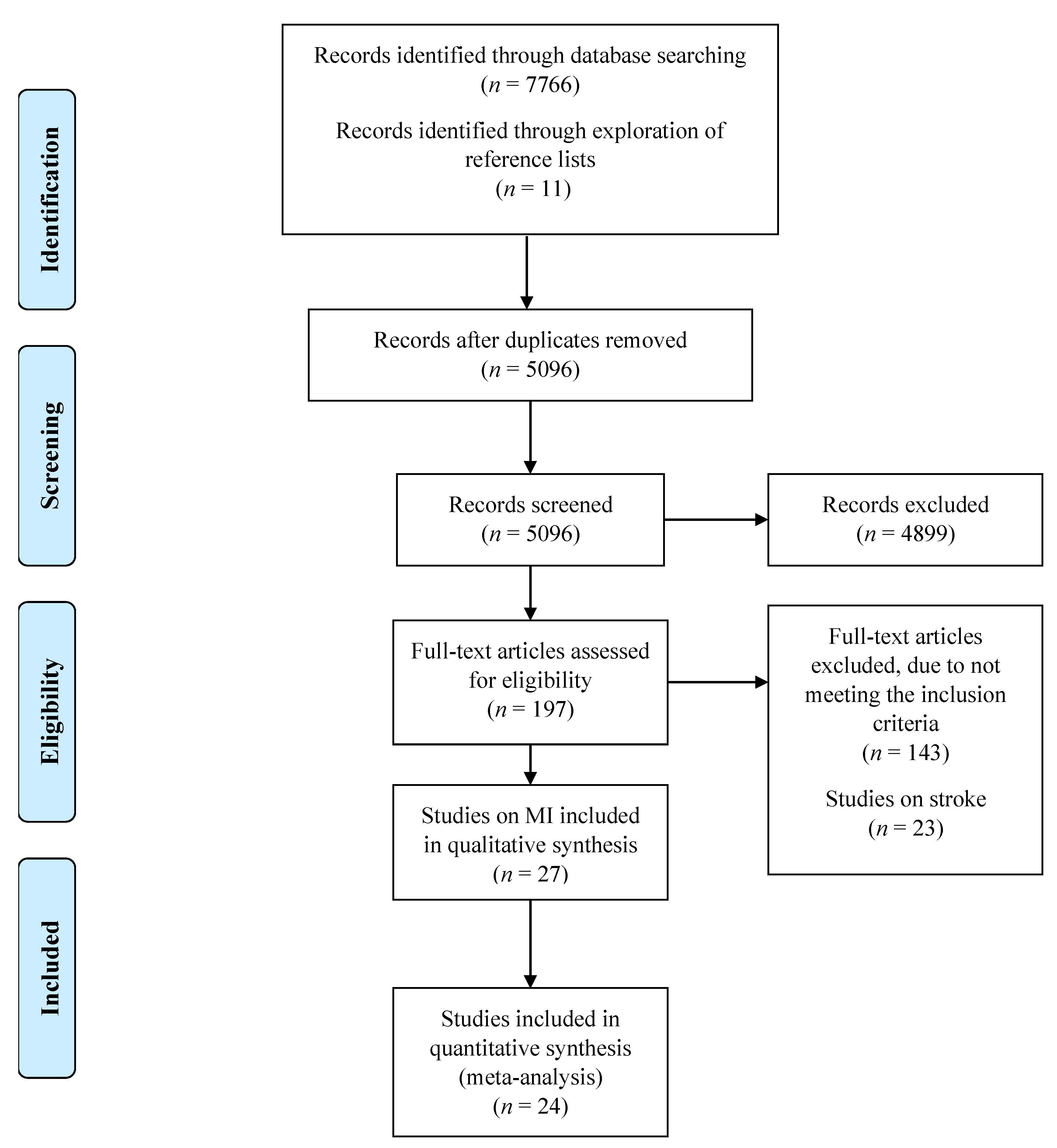
3.1. Study Selection
3.2. Study Characteristics
3.3. Critical Appraisal
3.4. Long-Term Mortality following MI in Overweight Patients Compared to Normal Weight
3.5. Long-Term Mortality Following MI in Obese and Morbidly Obese Patients Compared to Normal Weight
3.6. Long-Term Mortality following MI in Underweight Patients Compared to Normal Weight
3.7. Studies Not Included in Meta-Analysis
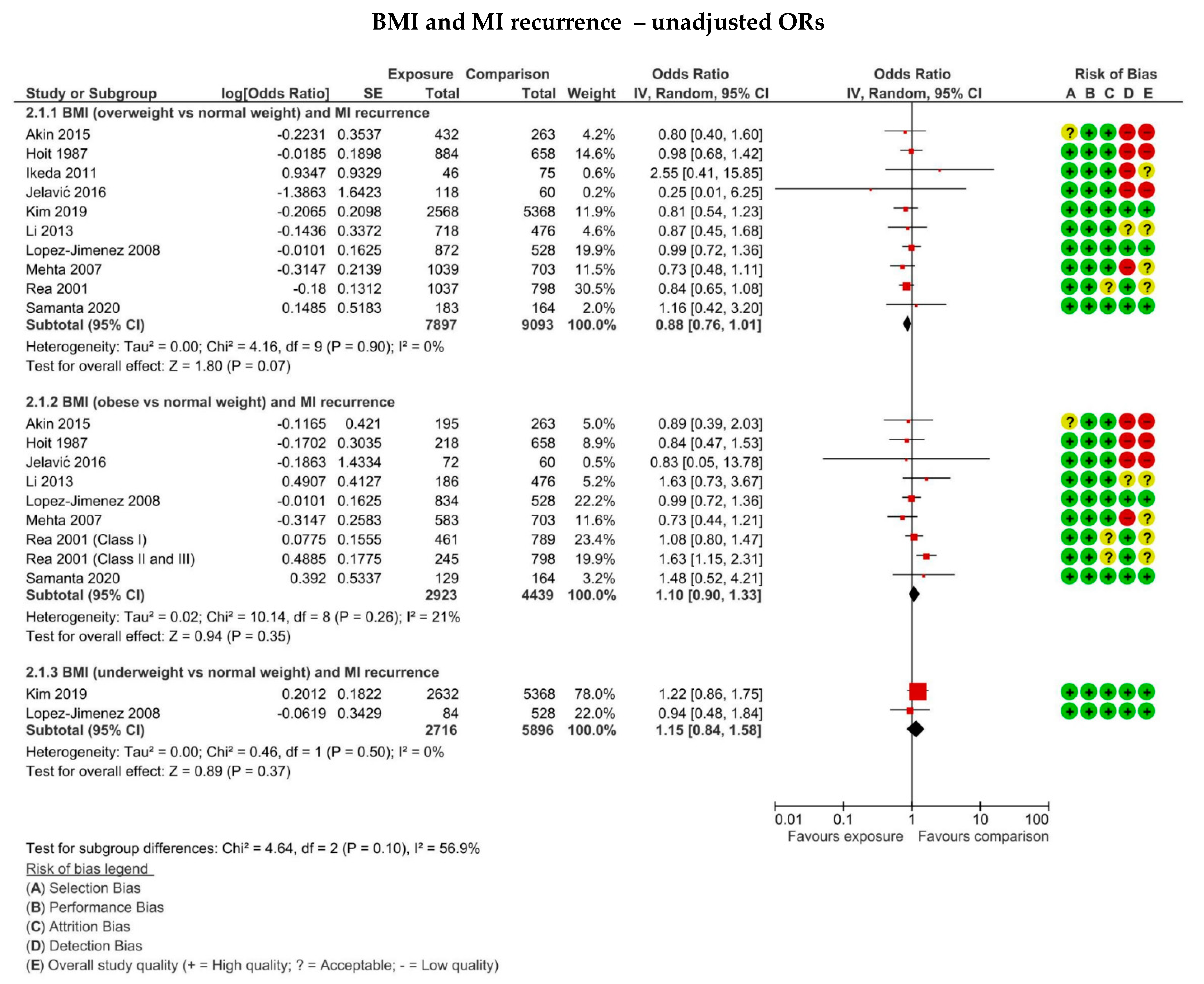
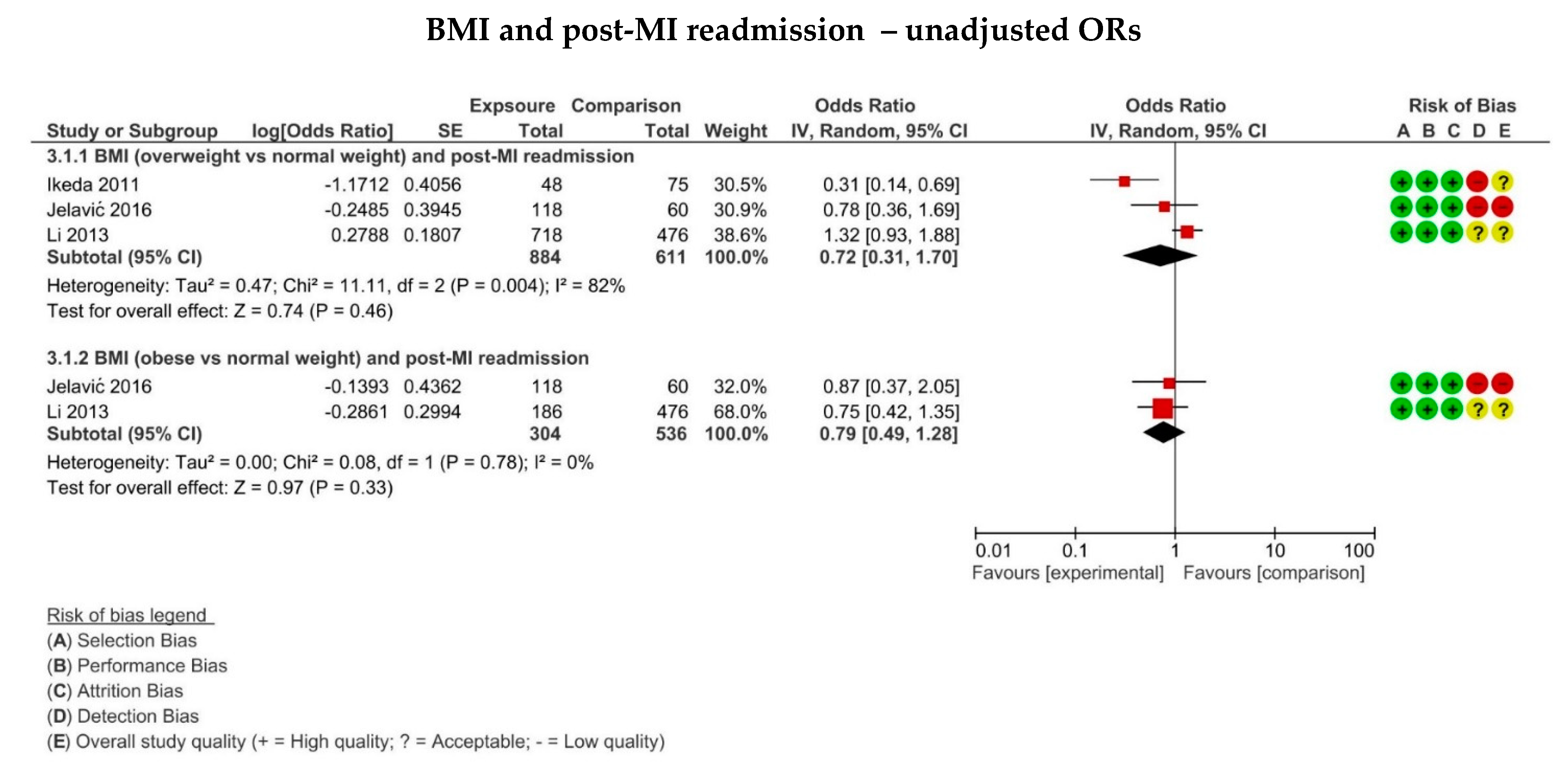
3.8. The Association between BMI and MI Recurrence on Hospital Readmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morse, S.A.; Gulati, R.; Reisin, E. The Obesity Paradox and Cardiovascular Disease. Curr. Hypertens. Rep. 2010, 12, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, V.W.; Langa, K.; Weir, D.; Iwashyna, T.J. The obesity paradox and incident cardiovascular disease: A population-based study. PLoS ONE 2017, 12, e0188636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oreopoulos, A.; Padwal, R.; Kalantar-Zadeh, K.; Fonarow, G.C.; Norris, C.M.; McAlister, F.A. Body mass index and mortality in heart failure: A meta-analysis. Am. Heart J. 2008, 156, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogue, C.W.; Stearns, J.D.; Colantuoni, E.; Robinson, K.A.; Stierer, T.; Mitter, N.; Pronovost, P.J.; Needham, D.M. The impact of obesity on outcomes after critical illness: A meta-analysis. Intensiv. Care Med. 2009, 35, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Martineau, J.; Bauer, J.D.; Isenring, E.; Cohen, S. Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin. Nutr. 2005, 24, 1073–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. 2021. Available online: https://rayyan.qcri.org/welcome (accessed on 10 February 2021).
- Scottish Intercollegiate Guidelines Network (SIGN). Critical Appraisal Notes and Checklists. Available online: https://www.sign.ac.uk/checklists-and-notes.html (accessed on 10 February 2021).
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V. Chapter 9: Summarizing study characteristics and preparing for synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Version, 6.2; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Available online: https://www.training.cochrane.org/handbook (accessed on 10 February 2021).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Review Manager (RevMan). Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 10 February 2021).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Bucholz, E.M.; Rathore, S.S.; Reid, K.J.; Jones, P.G.; Chan, P.S.; Rich, M.W.; Spertus, J.A.; Krumholz, H.M. Body Mass Index and Mortality in Acute Myocardial Infarction Patients. Am. J. Med. 2012, 125, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Bucholz, E.M.; Beckman, A.L.; Krumholz, H.A.; Krumholz, H.M. Excess weight and life expectancy after acute myocardial infarction: The obesity paradox reexamined. Am. Heart J. 2016, 172, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucholz, E.M.; Krumholz, H.A.; Krumholz, H.M. Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries. PLoS Med. 2016, 19, e1001998. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D.; Gilpin, E.A.; Maisel, A.A.; Henning, H.; Carlisle, J.; Ross, J. Influence of obesity on morbidity and mortality after acute myocardial infarction. Am. Heart J. 1987, 114, 1334–1341. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Wu, C.O.; Tian, X.; O’Connor, C.; Rich, M.W.; Burg, M.M.; Sheps, D.; Raczynski, J.; Somers, V.K.; Jaffe, A.S. Weight Change after Myocardial Infarction—The Enhancing Recovery in Coronary Heart Disease patients (ENRICHD) Experience. Am. Heart. J. 2008, 155, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Mehta, L.; Devlin, W.; McCullough, P.A.; O’Neill, W.W.; Skelding, K.A.; Stone, G.W.; Boura, J.A.; Grines, C.L. Impact of Body Mass Index on Outcomes After Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. Am. J. Cardiol. 2007, 99, 906–910. [Google Scholar] [CrossRef]
- Neeland, I.J.; Das, S.R.; Simon, D.N.; Diercks, D.B.; Alexander, K.P.; Wang, T.Y.; de Lemos, J.A. The obesity paradox, extreme obesity, and long-term outcomes in older adults with ST-segment elevation myocardial infarction: Results from the NCDR. Eur. Heart J. Qual. Care Clin. Outcomes 2017, 3, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Nigam, A.; Wright, R.S.; Allison, T.G.; Williams, B.A.; Kopecky, S.L.; Reeder, G.S.; Murphy, J.G.; Jaffe, A.S. Excess weight at time of presentation of myocardial infarction is associated with lower initial mortality risks but higher long-term risks including recurrent re-infarction and cardiac death. Int. J. Cardiol. 2006, 110, 153–159. [Google Scholar] [CrossRef]
- Nikolsky, E.; Stone, G.W.; Grines, C.L.; Cox, D.A.; Garcia, E.; Tcheng, J.E.; Griffin, J.J.; Guagliumi, G.; Stuckey, T.; Turco, M.; et al. Impact of body mass index on outcomes after primary angioplasty in acute myocardial infarction. Am. Heart J. 2006, 151, 168–175. [Google Scholar] [CrossRef]
- O’Brien, E.C.; Fosbol, E.L.; Peng, S.A.; Alexander, K.P.; Roe, M.T.; Peterson, E.D. Association of body mass index and long-term outcomes in older patients with non-ST-segment-elevation myocardial infarction: Results from the CRUSADE Registry. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Rana, J.S.; Mukamal, K.J.; Morgan, J.P.; E Muller, J.; A Mittleman, M. Obesity and the risk of death after acute myocardial infarction. Am. Heart J. 2004, 147, 841–846. [Google Scholar] [CrossRef]
- Rea, T.D.; Heckbert, S.R.; Kaplan, R.C.; Psaty, B.M.; Smith, N.L.; Lemaitre, R.N.; Lin, D. Body mass index and the risk of recurrent coronary events following acute myocardial infarction. Am. J. Cardiol. 2001, 88, 467–472. [Google Scholar] [CrossRef]
- Wu, A.H.; Pitt, B.; Anker, S.D.; Vincent, J.; Mujib, M.; Ahmed, A. Association of obesity and survival in systolic heart failure after acute myocardial infarction: Potential confounding by age. Eur. J. Heart Fail. 2010, 12, 566–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, I.; Schneider, H.; Nienaber, C.A.; Jung, W.; Lübke, M.; Rillig, A.; Ansari, U.; Wunderlich, N.; Birkemeyer, R. Lack of “obesity paradox” in patients presenting with ST-segment elevation myocardial infarction including cardiogenic shock: A multicenter German network registry analysis. BMC Cardiovasc. Disord. 2015, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.G.; Meisinger, C.; Amann, U.; Heier, M.; Von Scheidt, W.; Kuch, B.; Peters, A.; Kirchberger, I. Association of obesity and long-term mortality in patients with acute myocardial infarction with and without diabetes mellitus: Results from the MONICA/KORA myocardial infarction registry. Cardiovasc. Diabetol. 2015, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienbergen, H.; Gitt, A.K.; Juenger, C.; Schiele, R.; Heer, T.; Towae, F.; Gohlke, H.; Senges, J.; MITRA PLUS study group. Impact of the body mass index on occurrence and outcome of acute ST-elevation myocardial infarction. Clin. Res. Cardiol. 2007, 97, 83–88. [Google Scholar] [CrossRef]
- Ikeda, N.; Nakajima, R.; Utsunomiya, M.; Hori, M.; Itaya, H.; Makino, K.; Ono, T.; Yamamoto, M.; Nemoto, N.; Iijima, R.; et al. Higher body mass index at the time of acute myocardial infarction is associated with a favorable long-term prognosis (8-year follow-up). Heart Vessel. 2011, 26, 495–501. [Google Scholar] [CrossRef]
- Yokoyama, H.; Higuma, T.; Endo, T.; Nishizaki, F.; Hanada, K.; Yokota, T.; Yamada, M.; Okumura, K.; Tomita, H. Prognostic impact of body mass index and culprit lesion calcification in patients with acute myocardial infarction. Heart Vessel. 2019, 34, 1909–1916. [Google Scholar] [CrossRef]
- Fukuoka, S.; Kurita, T.; Dohi, K.; Masuda, J.; Seko, T.; Tanigawa, T.; Saito, Y.; Kakimoto, H.; Makino, K.; Ito, M. Untangling the obesity paradox in patients with acute myocardial infarction after primary percutaneous coronary intervention (detail analysis by age). Int. J. Cardiol. 2019, 289, 12–18. [Google Scholar] [CrossRef]
- Kang, W.Y.; Jeong, M.H.; Ahn, Y.K.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.-H.; Seong, I.-W.; Hong, T.J.; Choi, D.; et al. Obesity paradox in Korean patients undergoing primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. J. Cardiol. 2010, 55, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-W.; Her, S.-H.; Park, H.W.; Park, M.-W.; Chang, K.; Chung, W.S.; Seung, K.B.; Ahn, T.H.; Jeong, M.H.; Rha, S.-W.; et al. Association between body mass index and 1-year outcome after acute myocardial infarction. PLoS ONE 2019, 14, e0217525. [Google Scholar] [CrossRef] [Green Version]
- Samanta, R.; Narayan, A.; Kovoor, P.; Thiagalingam, A. Influence of BMI on Short and Long-Term Outcomes in Patients with STEMI and LV Dysfunction. Heart Lung Circ. 2020, 29, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jelavić, M.; Babić, Z.; Pintarić, H.; Mišigoj-Duraković, M. The Role of Anthropometry in Acute St-Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Acta Clin. Croat. 2016, 55, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, C.; Sun, Y.; Jiang, D.; Zhang, B.; Ren, L.; Gao, Y.; Yu, H.; Yang, G.; Guan, Q.; et al. Obesity paradox: Clinical benefits not observed in obese patients with ST-segment elevation myocardial infarction: A multicenter, prospective, cohort study of the northern region of China. Int. J. Cardiol. 2013, 168, 2949–2950. [Google Scholar] [CrossRef] [PubMed]
- Kragelund, C.; Hassager, C.; Hildebrandt, P.; Torp-Pedersen, C.; Køber, L.; TRACE study group. Impact of obesity on long-term prognosis following acute myocardial infarction. Int. J. Cardiol. 2005, 98, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Steg, P.G.; Ravisy, J.; Lorgis, L.; Laurent, Y.; Sicard, P.; Janin-Manificat, L.; Beer, J.-C.; Makki, H.; Lagrost, A.-C.; et al. Relation Between Body Mass Index, Waist Circumference, and Death After Acute Myocardial Infarction. Circulation 2008, 118, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, D.; Nassar, M.; Goldberg, T.; Kapeliovich, M.; Hammerman, H.; Azzam, Z.S. The impact of body mass index on clinical outcomes after acute myocardial infarction. Int. J. Cardiol. 2010, 145, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Sbraccia, P. Obesity phenotypes: Depot-differences in adipose tissue and their clinical implications. Eat. Weight Disord. 2017, 23, 3–14. [Google Scholar] [CrossRef]
- Cook, Z.; Kirk, S.; Lawrenson, S.; Sandford, S. Use of BMI in the assessment of undernutrition in older subjects: Reflecting on practice. Proc. Nutr. Soc. 2005, 64, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Zuccala, G.; Gambassi, G.; Incalzi, R.A.; Manigrasso, L.; Pagano, F.; Carbonin, P.; Bernabei, R. Body Mass Index and Mortality Among Older People Living in the Community. J. Am. Geriatr. Soc. 1999, 47, 1072–1076. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia and its implications for the elderly. Eur. J. Clin. Nutr. 2000, 54, S40–S47. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, S.-H.; Jung, K.-H.; Yu, K.-H.; Lee, B.-C.; Roh, J.-K.; For Korean Stroke Registry investigators. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology 2012, 79, 856–863. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalez, A.B.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Zhou, Y.J.; Galper, B.Z.; Gao, F.; Yeh, R.W.; Mauri, L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: A systematic review and meta-analysis. Heart 2015, 101, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Montori, V.; Somers, V.K.; Korinek, J.; Thomas, R.; Allison, T.G.; Mookadam, F.; Lopez-Jimenez, F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet 2006, 368, 666–678. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.H.; Han, S. Underweight: Another risk factor for cardiovascular disease? A cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine 2017, 96, e8769. [Google Scholar] [CrossRef]
- Izumida, T.; Nakamura, Y.; Ishikawa, S. Impact of body mass index and metabolically unhealthy status on mortality in the Japanese general population: The JMS cohort study. PLoS ONE 2019, 14, e0224802. [Google Scholar] [CrossRef] [Green Version]

| Study | Follow-up (months) | Females/ Males | Country | Exposure | Comparison | Outcome Assessed |
|---|---|---|---|---|---|---|
| Akin 2015 [27] | 12 | 205/ 685 | Germany | BMI > 30, 25–30 kg/m2 | BMI ≤ 24.9 kg/m2 | Mortality, recurrence |
| Aronson 2010 [40] | 26 | 459/ 1698 | Israel | BMI ≥ 35, 30–34.9, 25–29.9, < 18.5 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Bucholz 2012 [14] | 12 | 2076/ 4283 | USA | BMI ≥ 35, 30–34.9, 25–29.9 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Bucholz 2016 a [15] | 204 | 57,921/ 67,060 | USA | BMI ≥ 35, 30–34.9, 25–29.9 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Bucholz 2016 b [16] | 204 | 29,258/ 28316 | USA | BMI < 18.5 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Colombo 2015 [28] (Diabetes) | 120 | 337/ 853 | Germany | BMI ≥ 30, 25–29.9 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Colombo 2015 [28] (No diabetes) | 120 | 630/ 2234 | Germany | BMI > 30, 25–29.9 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Fukuoka 2019 [32] | 12 | 454/ 1666 | Japan | BMI ≥ 25, <20 kg/m2 | BMI 20–24.9 kg/m2 | Mortality |
| Hoit 1987 [17] | 12 | 433/ 1327 | USA | BMI > 30, BMI 25–30 kg/m2 | BMI < 25 kg/m2 | Mortality, recurrence |
| Ikeda 2011 [30] | 60 | 21/ 100 | Japan | BMI ≥ 25 kg/m2 | BMI < 25 kg/m2 | Mortality, recurrence, readmission |
| Jelavić 2016 [36] | 12 | 73/ 177 | Croatia | BMI ≥ 30, 25–29.9 kg/m2 | BMI < 25 kg/m2 | Mortality, recurrence, readmission |
| Kang 2010 [33] | 12 | 928/ 2896 | South Korea | BMI ≥ 27.5, 23–27.5, <18.5 kg/m2 | BMI 18.5–23 kg/m2 | Mortality |
| Kim 2019 [34] | 12 | 2547/ 8021 | South Korea | BMI ≥ 26, <22 kg/m2 | BMI 22–26 kg/m2 | Mortality, recurrence |
| Kragelund 2005 [38] | 120 | 2172/ 4502 | Denmark | BMI ≥ 30, 25–29.9, <19 kg/m2 | BMI 20–25 kg/m2 | Mortality |
| Li 2013 [37] | 12 | 1380 (total) | China | BMI ≥ 28.0, 25–28 kg/m2 | BMI 18.5–24 kg/m2 | Mortality, recurrence, readmission |
| Lopez-Jimenez 2008 [18] | 6.2 | 1022/ 1296 | USA | BMI ≥ 30, 25–29.9, < 19 kg/m2 | BMI 20–25 kg/m2 | Mortality, recurrence |
| Mehta 2007 [19] | 12 | 606/ 1719 | USA | BM ≥ 30 kg/m2 | BMI < 25 kg/m2 | Mortality |
| Neeland 2017 [20] | 36 | 7397/ 12102 | USA | BMI ≥ 40, 35–39.9, 30–34.9, 25–29.9 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Nigam 2006 [21] | 12 | 278/ 616 | USA | BMI ≥ 30 kg/m2 | BMI < 25 kg/m2 | Mortality, recurrence |
| Nikolsky 2006 [22] | 12 | 542/ 1493 | USA | BMI ≥ 30, 25–30 kg/m2 | BMI < 25 kg/m2 | Mortality |
| O’Brien 2014 [23] | 36 | 16,351/ 18,114 | USA | BMI ≥ 40, 35–39.9, 30–34.9, 25–29.9, < 18.5 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality, readmission |
| Rana 2004 [24] | 45 | 1317/ 581 | USA | BMI ≥ 30, 25–29.9 kg/m2 | BMI 20–25 kg/m2 | Mortality |
| Rea 2001 [25] | 36 | 968/ 1573 | USA | BMI ≥ 30 kg/m2 | BMI < 25 kg/m2 | Recurrence |
| Samanta 2020 [35] | 12 | 77/ 399 | Australia | BMI > 30, 25–29.99 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality, recurrence |
| Wienbergen 2008 [29] | 14 | 3137/ 7397 | Germany | BMI ≥ 30, 25–29.9 kg/m2 | BMI 18.5–24.9 kg/m2 | Mortality |
| Wu 2010 [26] | 16 | 1885/ 4675 | USA | BMI ≥ 30, 25–29.9 kg/m2 | BMI < 25 kg/m2 | Mortality |
| Yokoyama 2019 [31] | 32.4 | 112/ 405 | Japan | BMI ≥ 26.0, ≥24- < 26, <21.9 kg/m2 | BMI ≥ 21.9- <24 kg/m2 | Mortality |
| Zeller 2008 [39] | 12 | 593/ 1636 | France | BMI ≥ 30, 25–29.9 kg/m2 | BMI < 25 kg/m2 | Mortality |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Paola, L.; Mehta, A.; Pana, T.A.; Carter, B.; Soiza, R.L.; Kafri, M.W.; Potter, J.F.; Mamas, M.A.; Myint, P.K. Body Mass Index and Mortality, Recurrence and Readmission after Myocardial Infarction: Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2581. https://doi.org/10.3390/jcm11092581
De Paola L, Mehta A, Pana TA, Carter B, Soiza RL, Kafri MW, Potter JF, Mamas MA, Myint PK. Body Mass Index and Mortality, Recurrence and Readmission after Myocardial Infarction: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(9):2581. https://doi.org/10.3390/jcm11092581
Chicago/Turabian StyleDe Paola, Lorenzo, Arnav Mehta, Tiberiu A. Pana, Ben Carter, Roy L. Soiza, Mohannad W. Kafri, John F. Potter, Mamas A. Mamas, and Phyo K. Myint. 2022. "Body Mass Index and Mortality, Recurrence and Readmission after Myocardial Infarction: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 9: 2581. https://doi.org/10.3390/jcm11092581







